Corrected Axial Length and Choroidal Thickness: A Correlation Analysis for Scientific Purposes
Abstract
:1. Introduction
- (1)
- A change in the relationship between AL and ChT measurements could occur;
- (2)
- More important, the choice of AL cut-off values in these types of studies could change accordingly.
2. Materials and Methods
2.1. Participants
2.2. Clinical and Instrumental Examination
- -
- slit lamp inspection;
- -
- visual acuity test;
- -
- fundus examination.
2.2.1. OCT Analysis
2.2.2. AL Measurements
2.3. Statistical Analysis
3. Results
- -
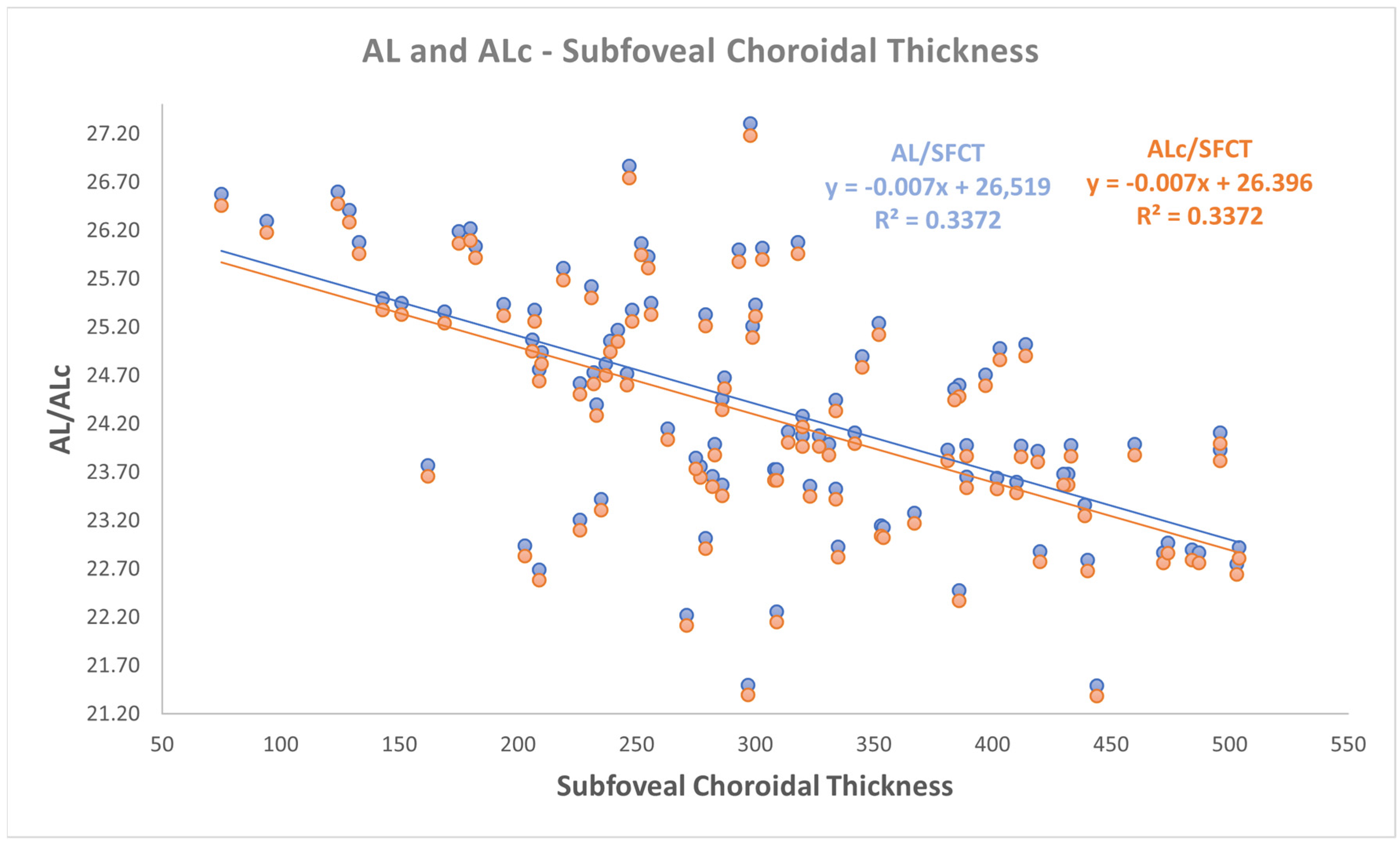
- a negative correlation was found between both AL and ALc with subfoveal ChT (r: −0.581, p < 0.001);
- -
- a negative correlation was found between both AL and ALc with nasal ChT (r: −0.527, p < 0.001);
- -
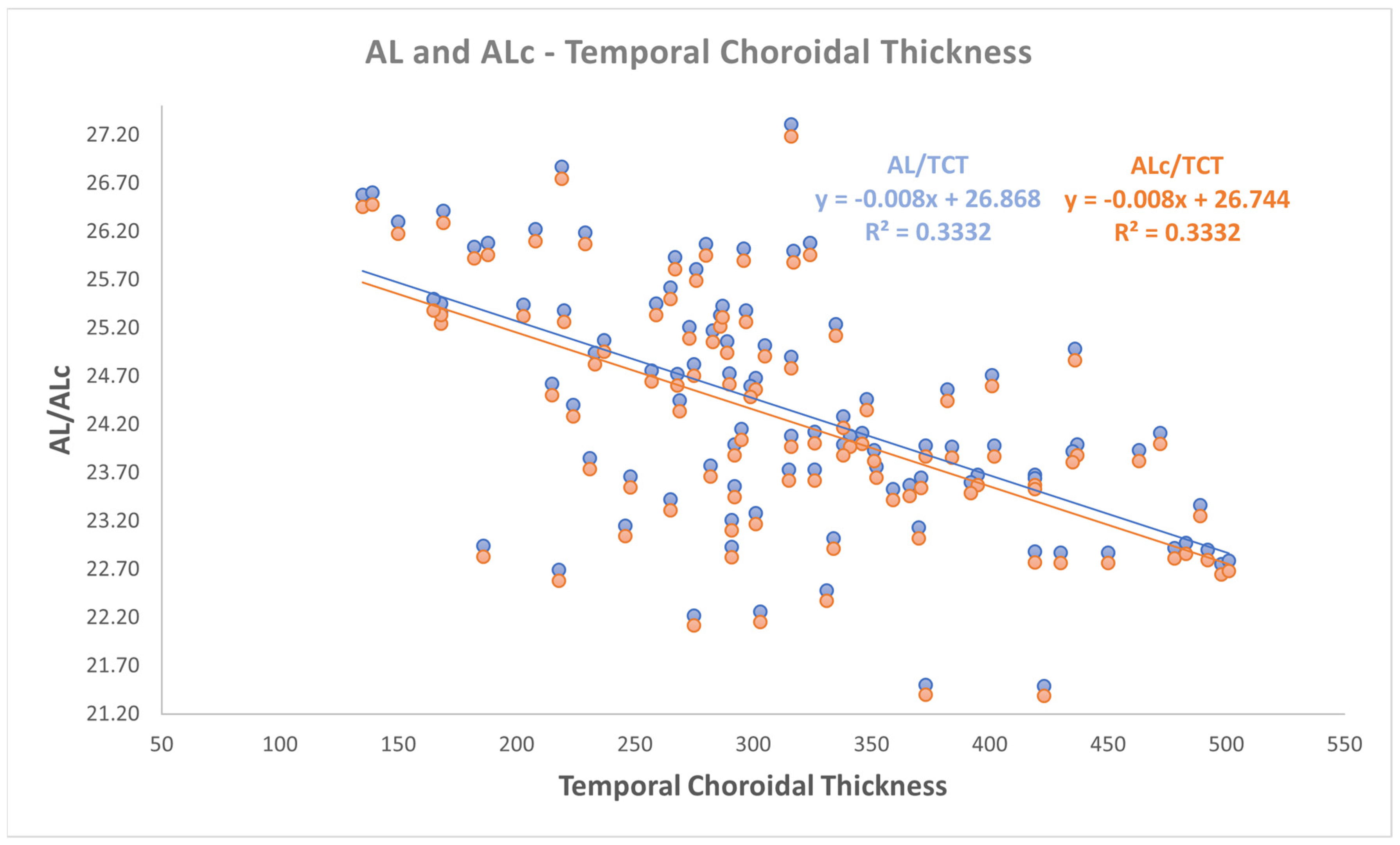
- a negative correlation was found between both AL and ALc with temporal ChT (r: −0.577, p < 0.001).
4. Discussion
- -
- Inaccurate measurement technique: for example, contact ultrasound biometry is known to be unreliable;
- -
- Principle of function of optical biometer: GRI-based biometers are more sensitive to AL measurement error, rather than sum-of-segments biometers;
- -
- Eye length, with less reliable AL values with GRI-based biometers in case of long eyes.
- -
- Lens opacity, that could affect reliability of all types of optical biometers, but especially GRI-based biometers.
- -
- In all these cases, both clinicians and researchers should pay attention in both AL and ChT evaluation.
- (1)
- Ignoring AL correction could introduce bias to the ChT evaluation of patients grouped by AL. It results in an unreliable estimation of ChT of patients with macular edema and subretinal fluid [23] and in a difficult comparison between these patients and their healthy controls. Grading and staging different ocular and systemic pathologies through ChT could be an interesting clinical and therapeutic skill, so its trustworthy use is decisive.
- (2)
- AL correction is essential for both patients with cataracts and subjects that underwent GRI-based biometry, as discussed in previous paragraphs.
- (3)
- (4)
- (5)
- In addition, relationships between ChT and retinal diseases [34] should be analyzed with ALc values.
- (6)
- In conclusion, patients’ stratification according to AL should be performed with ALc in ChT studies [26].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Bernardo, M.; Altieri, V.; Coppola, A.; Gioia, M.; Rosa, N. Choroidal evaluation in patients under alpha-lytic therapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- de Bernardo, M.; Vitiello, L.; Battipaglia, M.; Mascolo, F.; Iovino, C.; Capasso, L.; Ciacci, C.; Rosa, N. Choroidal structural evaluation in celiac disease. Sci. Rep. 2020, 11, 16398. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moreno, I.; Lugo, F.; Duker, J.S.; Ruiz-Moreno, J.M. The relationship between axial length and choroidal thickness in eyes with high myopia. Am. J. Ophthalmol. 2013, 155, 314–319. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500, Erratum in Am. J. Ophthalmol. 2009, 148, 325. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Imamura, Y.; Margolis, R.; Slakter, J.S.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am. J. Ophthalmol. 2009, 148, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Spaide, R.F.; Ostrin, L.A.; Logan, N.S.; Flitcroft, I.; Panda-Jonas, S. IMI-Nonpathological Human Ocular Tissue Changes With Axial Myopia. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5. [Google Scholar] [CrossRef] [PubMed]
- Bulut, A.; Oner, V.; Buyuktarakcı, Ş.; Kaim, M. Associations between choroidal thickness, axial length and spherical equivalent in a paediatric population. Clin. Exp. Optom. 2016, 99, 356–359. [Google Scholar] [CrossRef]
- Gupta, P.; Cheung, C.Y.; Saw, S.M.; Bhargava, M.; Tan, C.S.; Tan, M.; Yang, A.; Tey, F.; Nah, G.; Zhao, P.; et al. Peripapillary choroidal thickness in young Asians with high myopia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1475–1481. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 2015, 159, 1123–1131. [Google Scholar] [CrossRef]
- El-Shazly, A.A.; Farweez, Y.A.; ElSebaay, M.E.; El-Zawahry, W.M.A. Correlation between choroidal thickness and degree of myopia assessed with enhanced depth imaging optical coherence tomography. Eur. J. Ophthalmol. 2017, 27, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Li, T.; Lin, Y.; Ma, W.; Chen, X.; Lyu, C.; Li, Y.; Lu, L. Correlation of subfoveal choroidal thickness with axial length, refractive error, and age in adult highly myopic eyes. BMC Ophthalmol. 2018, 18, 127. [Google Scholar] [CrossRef]
- Atchison, D.A.; Smith, G. Possible errors in determining axial length changes during accommodation with the IOL Master. Optom. Cision Sci. 2004, 81, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Woodman, E.C.; Read, S.A.; Collins, M.J. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vision. Res. 2012, 72, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kaphle, D.; Schmid, K.L.; Suheimat, M.; Read, S.A.; Atchison, D.A. Central and peripheral choroidal thickness and eye length changes during accommodation. Ophthalmic Physiol. Opt. 2023, 43, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.; Lütjen-Drecoll, E.; Jungkunz, W.; Rohen, J.W. Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1678–1692. [Google Scholar]
- Woodman-Pieterse, E.C.; Read, S.A.; Collins, M.J.; Alonso-Caneiro, D. Regional changes in choroidal thickness associated with accommodation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Yazdi, H.; Read, S.A.; Alonso-Caneiro, D.; Collins, M.J. Retinal OFF-pathway overstimulation leads to greater accommodation-induced choroidal thinning. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- de Bernardo, M.; Cione, F.; Capasso, L.; Coppola, A.; Rosa, N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci. Rep. 2022, 12, 18845. [Google Scholar] [CrossRef]
- Wang, L.; Shirayama, M.; Ma, X.J.; Kohnen, T.; Koch, D.D. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J. Cataract. Refract. Surg. 2011, 37, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Koch, D.D. Modified axial length adjustment formulas in long eyes. J. Cataract. Refract. Surg. 2018, 44, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Holladay, J.T.; Koch, D.D. Wang-Koch axial length adjustment for the Holladay 2 formula in long eyes. J. Cataract. Refract. Surg. 2019, 45, 117. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.L.; Cooke, T.L. Approximating sum-of-segments axial length from a traditional optical low-coherence reflectometry measurement. J. Cataract. Refract. Surg. 2019, 45, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; de Bernardo, M.; Pagliarulo, S.; Cione, F.; Mottola, F.F.; Marca, A.L.; de Pascale, I.; Albano, G.; Rosa, N. Evaluation of Tropicamide-Phenylephrine Mydriatic Eye Drop Instillation on Choroidal Thickness. J. Clin. Med. 2023, 12, 6355. [Google Scholar] [CrossRef]
- Hansen, M.H.; Kessel, L.; Li, X.Q.; Skovgaard, A.M.; Larsen, M.; Munch, I.C. Axial length change and its relationship with baseline choroidal thickness—A five-year longitudinal study in Danish adolescents: The CCC2000 eye study. BMC Ophthalmol. 2020, 20, 152. [Google Scholar] [CrossRef] [PubMed]
- Ulaganathan, S.; Read, S.A.; Collins, M.J.; Vincent, S.J. Daily axial length and choroidal thickness variations in young adults: Associations with light exposure and longitudinal axial length and choroid changes. Exp. Eye Res. 2019, 189, 107850. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Wang, D.; Xiao, O.; Guo, X.; Zhang, J.; Yin, Q.; Wang, W.; He, M.; Li, Z. Choroidal Vascularity and Axial Length Elongation in Highly Myopic Children: A 2-Year Longitudinal Investigation. Investig. Ophthalmol. Vis. Sci. 2024, 65, 7. [Google Scholar] [CrossRef]
- Zeppieri, M.; Marsili, S.; Enaholo, E.S.; Shuaibu, A.O.; Uwagboe, N.; Salati, C.; Spadea, L.; Musa, M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina 2023, 59, 2114. [Google Scholar] [CrossRef]
- Pathak, M.; Sahu, V.; Kumar, A.; Kaur, K.; Gurnani, B. Current Concepts and Recent Updates of Optical Biometry—A Comprehensive Review. Clin. Ophthalmol. 2024, 18, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Cione, F.; de Bernardo, M.; Di Paola, I.; Caputo, A.; Graziano, M.; Rosa, N. IOL power calculation in long eyes: Selection of the best axial length adjustement factor using the most common formulas. Heliyon 2024, 10, e36609. [Google Scholar] [CrossRef] [PubMed]
- Stopyra, W.; Langenbucher, A.; Grzybowski, A. Intraocular Lens Power Calculation Formulas—A Systematic Review. Ophthalmol. Ther. 2023, 12, 2881–2902. [Google Scholar] [CrossRef] [PubMed]
- Voytsekhivskyy, O.; Tutchenko, L. Comparison of the prediction accuracy of 13 formulas in long eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, D.; Hu, Y.; Ao, S.; Zeng, J.; Yang, X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont. Lens Anterior Eye. 2017, 40, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Kang, J.; Niu, T.; Guo, L.; Fan, L. Axial Length Control Is Associated with a Choroidal Thickness Increase in Myopic Adolescents After Orthokeratology. Eye Contact Lens. 2023, 49, 512–520. [Google Scholar] [CrossRef]
- Sato, M.; Minami, S.; Nagai, N.; Suzuki, M.; Kurihara, T.; Shinojima, A.; Sonobe, H.; Akino, K.; Ban, N.; Watanabe, K.; et al. Association between axial length and choroidal thickness in early age-related macular degeneration. PLoS ONE 2020, 15, e0240357. [Google Scholar] [CrossRef]
- Rosa, N.; Gioia, M.; Orlando, R.; De Luca, M.; D’Aniello, E.; Fioretto, I.; Sannino, C.; De Bernardo, M. Impact of Brightness on Choroidal Vascularity Index. J. Clin. Med. 2024, 13, 1020. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Aramberri, J.; Haigis, W.; Olsen, T.; Savini, G.; Shammas, H.J.; Bentow, S. Protocols for studies of intraocular lens formula accuracy. Am. J. Ophthalmol. 2015, 160, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Anders, P.; Anders, L.M.; Barbara, A.; Szentmary, N.; Langenbucher, A.; Gatzioufas, Z. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Ther. Adv. Ophthalmol. 2022, 14, 25158414221118524. [Google Scholar] [CrossRef] [PubMed]
- Anter, A.M.; Bleeker, A.R.; Shammas, H.J.; Suraneni, S.; Kingrey, B.; Murphy, D.A.; Leal, S.; Ghalibafan, S.; Tonk, R.S.; Cooke, D.L.; et al. Comparison of Legacy and New No-History IOL Power Calculation Formulas in Postmyopic Laser Vision Correction Eyes. Am. J. Ophthalmol. 2024, 264, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Albis-Donado, O.; Ramirez-Neria, P.; Rios-Acosta, N.; Stalmans, I. The influence of altitude on the differences between Goldmann tonometry and Pascal dynamic contour tonometry: An ecological meta-analysis. Indian. J. Ophthalmol. 2024, 72 (Suppl. S3), S398–S403. [Google Scholar] [CrossRef] [PubMed]

| Parameter | AL | ALc | ChT Nasal | ChT Subfoveal | ChT Temporal |
|---|---|---|---|---|---|
| MEAN | 24.36 mm | 24.25 mm | 250.57 µm | 307.18 µm | 313.72 µm |
| SD | 1.23 mm | 1.22 mm | 93.03 µm | 101.66 µm | 88.86 µm |
| MEDIAN | 24.12 mm | 24.00 mm | 250.00 µm | 299.50 µm | 301.00 µm |
| MIN | 21.49 mm | 21.39 mm | 73.00 µm | 75.00 µm | 135.00 µm |
| MAX | 27.31 mm | 27.18 mm | 507.00 µm | 504.00 µm | 501.00 µm |
| Correlation | r | 95% CI | R2 | p-Value |
|---|---|---|---|---|
| AL—Subfoveal ChT | −0.527 | −0.669 −0.383 | 0.3372 | 0.000 |
| AL—Nasal ChT | −0.581 | −0.705 −0.449 | 0.2779 | 0.000 |
| AL—Temporal ChT | −0.577 | −0.700 −0.451 | 0.3332 | 0.000 |
| ALc—Subfoveal ChT | −0.527 | −0.669 −0.383 | 0.3372 | 0.000 |
| ALc—Nasal ChT | −0.581 | −0.705 −0.449 | 0.2779 | 0.000 |
| ALc—Temporal ChT | −0.577 | −0.700 −0.451 | 0.3332 | 0.000 |
| Correlation | r—RE (p-Value) | 95% CI—RE | r—LE (p-Value) | 95% CI—LE |
|---|---|---|---|---|
| AL—Subfoveal ChT | −0.548 (0.000) | −0.727 −0.352 | −0.621 (0.000) | −0.774 −0.412 |
| AL—Nasal ChT | −0.546 (0.000) | −0.719 −0.334 | −0.507 (0.000) | −0.697 −0.285 |
| AL—Temporal ChT | −0.533 (0.000) | −0.711 −0.312 | −0.624 (0.000) | −0.782 −0.421 |
| Alc—Subfoveal ChT | −0.548 (0.000) | −0.727 −0.352 | −0.621 (0.000) | −0.774 −0.412 |
| ALc—Nasal ChT | −0.546 (0.000) | −0.719 −0.334 | −0.507 (0.000) | −0.697 −0.285 |
| Alc—Temporal ChT | −0.533 (0.000) | −0.712 −0.312 | −0.624 (0.000) | −0.782 −0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioia, M.; De Bernardo, M.; Cione, F.; De Luca, M.; Rosa, N. Corrected Axial Length and Choroidal Thickness: A Correlation Analysis for Scientific Purposes. J. Pers. Med. 2025, 15, 15. https://doi.org/10.3390/jpm15010015
Gioia M, De Bernardo M, Cione F, De Luca M, Rosa N. Corrected Axial Length and Choroidal Thickness: A Correlation Analysis for Scientific Purposes. Journal of Personalized Medicine. 2025; 15(1):15. https://doi.org/10.3390/jpm15010015
Chicago/Turabian StyleGioia, Marco, Maddalena De Bernardo, Ferdinando Cione, Martina De Luca, and Nicola Rosa. 2025. "Corrected Axial Length and Choroidal Thickness: A Correlation Analysis for Scientific Purposes" Journal of Personalized Medicine 15, no. 1: 15. https://doi.org/10.3390/jpm15010015
APA StyleGioia, M., De Bernardo, M., Cione, F., De Luca, M., & Rosa, N. (2025). Corrected Axial Length and Choroidal Thickness: A Correlation Analysis for Scientific Purposes. Journal of Personalized Medicine, 15(1), 15. https://doi.org/10.3390/jpm15010015








