Abstract
Background: This study evaluated the clinical outcomes of applying a 68 Gy EQD2(α/β=3) dose constraint to the most exposed 2 cm3 area of the vagina in post-operative endometrial cancer patients treated with vaginal-cuff brachytherapy after external beam irradiation and the impact of vaginal dilator use on late vaginal complications. Material and methods: We analyzed 131 patients treated with vaginal-cuff brachytherapy after external beam irradiation. Group-1 (65 patients) received one fraction of 7 Gy, and Group-2 (66 patients) received one fraction of between 5.5 and 7.0 Gy after applying a 68 Gy EQD2(α/β=3) dose constraint. Vaginal-cuff relapse, late toxicity, clinical target volume, vaginal dilator use, D90, and EQD2(α/β=3) at 2 cm3 of the most exposed part of the clinical target volume were evaluated. Descriptive analysis, the chi-squared test, Student’s t-test, and the Cox proportional and Kaplan–Meier models were used for the statistical analysis. Results: With a median follow-up of 60 months, the vaginal-cuff relapse rate was 1/131 (0.8%). Late vaginal complications appeared in 36/65 (55.4%) Group-1 patients and 17/66 (25.8%) Group-2 patients (p = 0.003). Multivariate analysis showed that belonging to Group-1 and vaginal dilator use of <9 months were independent prognostic factors of late vaginal complications with hazard ratios of 1.99 (p = 0.021) and 3.07 (p = 0.010), respectively. Conclusions: A 68 Gy EQD2(α/β=3) constraint at 2 cm3 of clinical target volume and vaginal dilator use of ≥9 months were independent prognostic factors, having protective effects on late vaginal complications.
1. Introduction
Endometrial cancer is the sixth most common malignant disorder worldwide and the most frequent gynecological cancer. The treatment of endometrial cancer involves total hysterectomy and bilateral salpingo-oophorectomy with or without lymph node assessment. The indication for adjuvant radiation therapy is based on stage, tumor type, and the presence of risk factors including molecular factors. Most recurrences (65–85%) are diagnosed within 3 years of primary treatment, and 40–75% of recurrences are in the vagina [1,2,3].
According to the data from randomized trials, no adjuvant treatment is recommended in patients with low-risk FIGO Stage IA grade 1 or 2 endometrial carcinoma. Adjuvant vaginal-cuff brachytherapy (VCB) is recommended to decrease vaginal recurrence in patients with intermediate or high-intermediate risk. For high-intermediate risk patients, external beam radiation therapy (EBRT) can be considered in cases of substantial lymphovascular space invasion (LVSI), p53 mutation, and for stage II endometrial cancer. Adjuvant brachytherapy can be recommended to reduce vaginal recurrence. Adjuvant chemotherapy can be considered, especially in high-grade endometrial cancer and/or cases with substantial LVSI. In high-risk patients, EBRT with concurrent and adjuvant chemotherapy or, alternatively, sequential chemotherapy and radiotherapy is recommended. Nevertheless, the administration of VCB varies among centers and some recent studies have shown the improvements in local vaginal control with the use of VCB + EBRT in the advanced stages [3,4,5,6,7].
The most common EBRT doses range from 45 to 50.4 Gy delivered in 25 to 28 fractions over 5 to 6 weeks. Since 2019, the gold standard has been the intensity-modulated radiation therapy and volumetric-modulated arc therapy (VMAT) techniques [8]. The American Brachytherapy Society (ABS) has reported a wide variation in VCB dose schedules, including 22 regimens used as a boost after EBRT [9].
The organs at risk (OAR) in pelvic radiation therapy include the bladder, rectum, and vagina. The incidence of late rectal and bladder complications is more closely associated with EBRT, while VCB is more often related to the development of late vaginal complications (LVCs), which mostly occur after combined treatment with VCB and EBRT. A commonly observed LVC is Grade-1 (G1) and Grade-2 (G2) radiation therapy (RT)-induced vaginal stenosis (VS), defined as the abnormal tightening and shortening of the vagina due to the formation of adherences and fibrosis. It is well recognized that RT-induced advanced VS may have a negative impact on patient well-being, especially in relation to sexual dysfunction and dyspareunia and limiting physical examination in post-treatment follow-up. The main factors associated with the development of LVCs, mainly stenosis, are vaginal surface doses, cylinder diameter, high dose per fraction, active source length, and vaginal dilator (VD) use. However, there is no consensus on the ideal length of time of VD use and dose-fractionation schedules [10,11,12,13,14,15].
The Groupe Européen de Curiethérapie of the European Society for Radiotherapy and Oncology (GEC-ESTRO) and other groups recommend EQD2(α/β=3) at the most exposed 2 cm3 of normal tissue as a limit for the bladder, rectum, and sigmoid. Hence, several years ago, we hypothesized that EQD2(α/β=3) at the most exposed 2 cm3 of the vagina (which we also consider as an OAR) could be a predictor of vaginal toxicity. On analyzing the correlation of dose with post-operative toxicity in patients treated with high-dose rate (HDR) VCB, we found that a dose constraint of 68 Gy EQD2(α/β=3) at 2 cm3 was associated with vaginal toxicity ≥ G2. Therefore, this dose limit has been applied for reducing vaginal toxicity since 2017 [13,16,17,18].
Prior to 2017, patients would receive a single dose of 7 Gy after EBRT. However, from 2017 to 2022, we prospectively applied the restriction of the EQD2(α/β=3) to 68 Gy at 2 cm3 of the most exposed vaginal clinical target volume (CTV) in 79 patients treated with EBRT + VCB. Preliminary results showed a reduction in LVCs in patients when the constraint was applied [13].
In a recent study on exclusive VCB using 7.5 Gy × 2 fractions in 110 patients, the use of the 68 Gy constraint did not seem useful (only four patients received more than this constraint) [19]. Therefore, the aim of this study was to retrospectively compare vaginal control and LVCs in a group of patients receiving one dose of 7 Gy of VCB after EBRT, with a second group receiving a 68 Gy EQD2(α/β=3) dose constraint to the most exposed 2 cm3 of the vagina. In addition, we evaluated the impact of vaginal dilator use greater or less than 9 months on the development of vaginal complications. This is the first study evaluating these data using 3D treatment planning in VCB.
2. Materials and Methods
The present study was approved by the Institutional Ethical Review Board of our center (HCB 2022/0379), and patient consent for study participation was obtained. We retrospectively analyzed 159 post-operative endometrial cancer (PEC) patients treated with EBRT followed by VCB from 2014 to 2022. Among these 159 patients, 28 (14 patients from each group) were excluded due to a lack of at least 12 months of follow-up or related assessments, and thus a total of 131 patients were analyzed.
From 2014 to 2017, 65 patients were treated with EBRT + VCB using 1 fraction of 7 Gy after EBRT and underwent adequate follow-up (Group-1). Subsequently, from 2017 to 2022, 66 patients were treated with EBRT+ VCB, receiving one fraction of 5.5 to 7 Gy while ensuring a constraint of EQD2(α/β=3) < 68 Gy at 2 cm3 of the most exposed part of the vaginal CTV before receiving adequate follow-up (Group-2). Figure 1 shows the patient selection process and the treatment received.
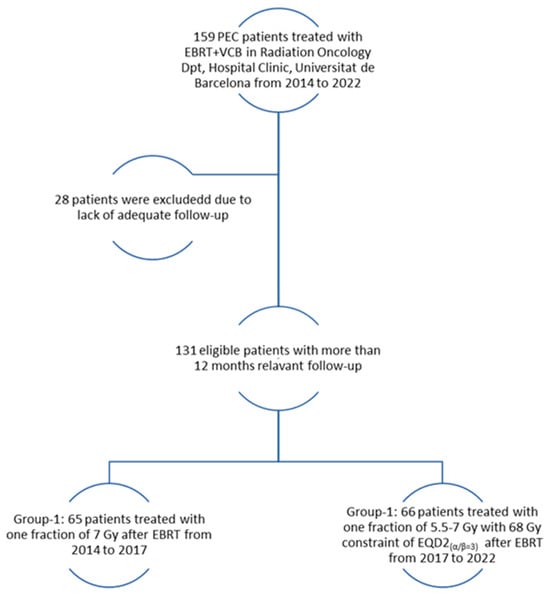
Figure 1.
Inclusion and exclusion flowchart.
Following the diagnosis of endometrial cancer, patients underwent imaging workup studies, including magnetic resonance imaging, positron emission tomography, computerized tomography, and/or ultrasonography. Subsequently, all patients underwent surgery involving the following surgical approaches: laparoscopic-assisted vaginal hysterectomy and bilateral salpingo-oophorectomy (LAVH-BSO) with pelvic ± para-aortic lymphadenectomy in 50 (38.2%) patients, LAVH-BSO with pelvic ± para-aortic lymphadenectomy in 30 (22.9%) patients, vaginal hysterectomy in 5 (3.8%) patients, abdominal hysterectomy in 3 (2.3%) patients, and an omentectomy in 13 (9.9%) patients. Thirty (22.9%) patients were treated using other methods, such as a LAVH-BSO by robotic surgery.
Following pathological analysis, all patients received EBRT + VCB, and 4–6 cycles of chemotherapy (carboplatin/paclitaxel) were administered to 54 patients (41.2%), according to their general status, age, and comorbidities.
EBRT was delivered with 6 or 18 MV photons to 127/131 (96.9%) patients after 3D treatment planning, while 4 (3.1%) patients received EBRT by VMAT. The delineation of the CTV and the planning target volume were performed following the Radiation Therapy Oncology Group (RTOG) protocols (20). The dose per fractionation ranged from 1.8 to 2 Gy per day, administered as 5 fractions per week. When positive lymph nodes were present, a dose of up to 65 Gy was used.
After finishing EBRT, VCB was performed. Applicators were placed in the operating room, where the patients were first examined, to confirm the type and diameter of the applicator to use. A colpostat is preferred in patients with a small introitus and wide vagina, while a vaginal cylinder is commonly used for uniform and adequate anatomy.
The Oncentra Brachy planning system (Elekta®, Nucletron BV, Veenendaal, The Netherlands) was used for treatment planning. The vaginal CTV was delineated at 2.5 cm along the first cylinder. The VCB dose was prescribed at 0.5 cm from the applicator surface with optimization to points. A 90% isodose was considered to cover all the CTV of the vagina. The brachytherapy planning technique has been described elsewhere (13).
Follow-up was carried out 15 days after brachytherapy and then every 3–4 months during the first 2 years and every 6 months thereafter up to 5 years. The patients were evaluated in terms of recurrence and side effects by clinical and gynecological examination and imaging studies. All patients were visited by the same radiation oncologist during and after EBRT, before VCB, and during follow-up. All patients were advised to use VDs, adapted to their vaginal size. The follow-up period for the analysis was defined from the date of VCB until the last visit of the patient, and the LVC-free time was defined as the time between VCB and the appearance of LVCs.
We analyzed these two VCB dose fractionation groups in relation to various factors, including vaginal-cuff relapse (VCR), late toxicities in the vagina, rectum, and bladder, CTV, the use of VDs ≥ 9 months versus no usage or <9 months, D90, EQD2(α/β=3) at 2 cm3 of the most exposed part of CTV of VCB, with the corresponding overall value representing the cumulative dose of VBT + EBRT.
Late toxicity of the rectum and bladder was assessed using the RTOG scores, and LVCs were evaluated with the objective criteria of LENT-SOMA [20,21].
Statistical analysis: Categorical variables were expressed using frequencies and percentages, while continuous data were described using mean and standard deviation (SD) or median and interquartile range. The homogeneity study between dose regimen groups was performed using the chi-squared test or Fisher’s Exact Test for the categorical variables, or Student’s t-test in the case of continuous variables. The mean, median, or proportion differences between dose regimen groups were estimated with a 95% confidence interval (CI).
We identified potential prognostic factors by univariate analysis. Subsequently, variables that showed significance in the univariate analysis were included in the multivariable Cox proportional hazards regression model. This model adjusts for the simultaneous influence of multiple factors on the hazard of developing an LVC over time, and hazard ratios (HR) were estimated with a 95% CI.
Due to varying follow-up times, we adopted a Kaplan–Meier survival analysis approach to effectively capture the time-to-event outcome of interest, which, in this case, was the occurrence of LVCs. Additionally, we employed the Kaplan–Meier estimator to graphically depict the probability of remaining free from LVCs at each month. The effect of the dose regimen and other prognostic factors on the probability of LVC-free time was investigated using the Cox proportional hazards model. This model is particularly suited for survival data as it allows for an assessment of the relative hazards (risks) of developing LVCs between groups, while accounting for varying follow-up durations and censoring. The analyses were performed using R software version 4.2.2 package (R project for statistical computing; Vienna, Austria).
3. Results
We analyzed 131 eligible patients with a median follow-up of 60 months (15–60) in Group-1 and 46 months (14–60) in Group-2. Among the entire series, 16 patients died. Of these, one patient in each group died as a consequence of endometrial cancer within 14–15 months of follow-up.
Table 1 shows the comparison of prognostic factors of local recurrence between the two study groups, which were homogenous except for histologic grade and focal LVSI.

Table 1.
The clinical characteristics of the entire sample of patients and by study group.
In the present study, VCR was observed in only one patient (0.8%) in Group-1, who had received 7 Gy and died 7 months after relapse due to causes unrelated to cancer. In 3/66 (4.5%) patients in Group-2, VCR occurred in the middle or outer third of the vagina, outside the field of brachytherapy. Two of these patients received 6.2 Gy and one received 6.5 Gy. These three patients remained alive after treatment for VCR.
Table 2 shows the dosimetry parameters and characteristics of the brachytherapy administered. The median EQD2(α/β=3) of EBRT was 45 Gy (40;50) in both groups. The mean VCB EQD2(α/β=3) at 2 cm3 of CTV was 27.4 Gy in Group-1 and 22.9 Gy in Group-2 (p < 0.001). All the Group-1 patients received more than 68 Gy overall EQD2(α/β=3), and all the patients in Group-2 received less than this dose.

Table 2.
Brachytherapy characteristics of the entire sample of patients and by study group.
The dose per fraction in Group-2 was as follows: 5.5 Gy 1/66 (1.5%), 5.7 Gy 1/66 (1.5%), 6.0 Gy 3/66 (4.5%), 6.2 Gy 13/66 (19.7%), 6.5 Gy 37/66 (56.1%), 6.75 Gy 6/66 (9.1%), and 7.0 Gy 5/66(7.6%). The mean dose per fraction in Group-2 was 6.5 Gy (SD 0.3) with a median of 6.5 Gy (5.5;7).
In Group-1, 2/65 (3.1%) patients presented late rectal complications; one G1 and one G2. In Group-2, 2/66 (3.0%) patients developed G1 late rectal complications (p = 1.0). Late bladder complications were observed in 2/66 (3.0%) in Group-2; one G1 and one G2 (p = 0.5).
Considering LVCs, 7/65 (10.8%) Group-1 patients presented Grade-1 (G1-LVC) as small adhesions, while 11/65 (16.9%) presented adhesions or simultaneous small dog ear (a retraction of <1 cm located in the vaginal cuff corners on vaginal digital examination) and telangiectasia, and 3/65 (4.6%) presented a small dog ear alone while 8/65 (12.3%) presented telangiectasia. Grade-2 LVCs (G2-LVCs) appeared as vaginal shortening or cleisis in 3/65 (4.6%) patients and bleeding adhesion in 4/65 (6.2%) patients. Group-2 presented G1-LVCs as small adhesions in 5/66 (7.6%) patients, small dog ears in 2/66 (3.0%), telangiectasia in 3/66 (4.5%), adhesions or small dog ears and telangiectasia at the same time in 3/66 (4.5%) patients, and vaginal shortening of less than one-third in 1/66 (1.5%). G2-LVCs were observed in 3/66 (4.5%) patients, all in the form of vaginal shortening of between 1/3 and 2/3.
In the whole series, 112/131 (85.5%) patients received more than 6.5 Gy; among these, 39/112 (34.8%) patients presented G1-LVCs and 10/112 (8.9%) G2-LVCs. Up to 19 of the 131 (14.5%) patients received < 6.5 Gy per fraction, with only 4/19 (21.1%) presenting G1-LVCs, and no G2-LVCs were observed (p-value = 0.06). Among the Group-2 patients who received more than 6.5 Gy per fraction, 10/47 (21.3%) presented G1-LVCs and 3/47 (6.4%) patients had G2-LVCs (p-value = 0.78).
Table 3 shows the univariate analysis of possible prognostic factors associated with the appearance of LVCs. The median time to LVC appearance was 12.9 (0.4; 60.0) months in the whole series; however, the mean time to G2-LVC development was longer in Group-2, with 22.1 (SD 13.6) months in Group-1 and 39.2 (SD4.5) in Group-2 (p-value < 0.01). Table 3 shows that, in the present study, VD use of ≥9 months, a lower VCB dose per fraction, and consequently belonging to treatment Group-2 demonstrated a protective effect against the onset of LVCs over time.

Table 3.
Univariate analysis of prognostic factors of the appearance of late vaginal toxicity.
Table 4 shows the results of the multivariable analysis of prognostic factors of time to the appearance of the LVCs. This analysis revealed that VD use of <9 months was associated with a HR of 3.07 (95% CI 1.30, 7.23; p = 0.010), indicating a significant three times higher risk of developing LVCs compared to VD use ≥ 9 months. Furthermore, the multivariate model identified group assignment as an independent prognostic factor, with Group-1 demonstrating a HR of 1.99 (95% CI 1.11, 3.55; p = 0.021) compared to Group-2.

Table 4.
Multivariable analysis of prognostic factors of the appearance of late vaginal toxicity.
Figure 2 and Figure 3 illustrate the Kaplan–Meier survival curves for the probability of LVC-free time based on the duration of VD use and treatment group. At the beginning of the study, the probability of remaining LVC-free was high in all patients. However, over time, the probability of remaining LVC-free decreased with the appearance of complications. The curves for each group slope downward at different rates, reflecting the monthly probability of LVC-free survival in each group. Note that the curve for VD use of <9 months and the curve for Group-1 fell more steeply.

Figure 2.
Probability of late vaginal complication-free time according to vaginal dilator use.
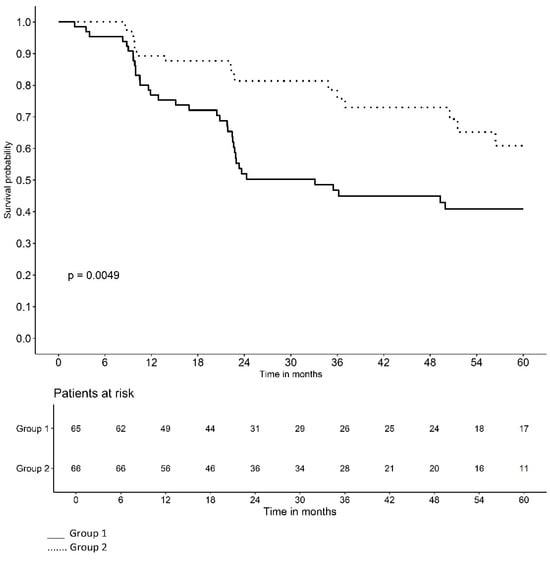
Figure 3.
Probability of late vaginal complication-free time according to treatment group.
4. Discussion
The present study analyzed the prognostic factors for LVCs in patients with PEC receiving EBRT + VBT. The aim of the study was not to establish the role of VBT in advanced stages of PEC, since there is no agreement between centers and some guidelines and authors have reported advantages in combining VBT with EBRT to enhance local vaginal control as in the present series [1,2,3,4].
The rate of VCR reported in the literature varies from 1.5% to 7% [2,10,14,19]. In the present study, the VCR rate was 0.8% in the entire series, with one patient in Group-1. Due to the low VCR rate, the difference between the two groups was not statistically significant.
The data available regarding the incidence and grading of LVCs are based on retrospective evidence and small cohorts, with a wide variation in the LVC measurement techniques and fractionation schedules as shown in the literature, and the reported rates of VS ranging from 1.3% to 88.0% based on the CTCAE or LENT-SOMA scores. In our previous studies, the incidence of G1-LVCs varied from 13% to 30%, while that of G2-LVCs was from 13% to 15% in patients receiving VCB after EBRT, and the incidence of VS was 10%, being very similar to our current study [11,21,22].
In our previous retrospective studies, univariate analysis showed that in patients > 55 years of age, an EQD2(α/β=3) of more than 68 Gy at the most exposed 2 cm3 of the CTV and the use of VD for < 9 months were associated with LVCs and, in multivariate analysis, VD use of ≥9 months was an independent prognostic factor for G2-LVCs in patients receiving VCB ± EBRT [23]. However, in a recent study by our group on exclusive VCB in PEC patients, the application of a 68 Gy EQD2(α/β=3) constraint in exclusive VCB did not seem necessary, probably due to the lower VCB dose received by the group [19].
In the group receiving 7 Gy after EBRT in the present study, there was a higher incidence of complications, and all these patients received > 68 Gy EQD2(α/β=3) to the most exposed 2 cm3 of the vagina. The present study highlights that a 68 Gy EQD2(α/β=3) vaginal dose constraint and VD use of ≥9 months in PEC patients treated with EBRT + VCB are independent prognostic factors of LVCs.
It is assumed that a higher volume of vagina treated is associated with an increased risk of VS. In the EMBRACE I study on cervical cancer, the 24-month actuarial estimate for symptomatic VS was 21%, and the vaginal tumor invasion into the vagina was reported to be a considerable risk factor [24]. Nevertheless, the EMBRACE I study included cervical tumors treated with a curative aim and with vaginal invasion in some of the cases, and thus higher doses were administered to the vagina. In the present series of PEC treatment, all the patients were treated with same vaginal CTV longitude and the mean CTV was similar in both groups, and therefore the results were not affected. Moreover, in the univariate analysis, there was no correlation between the CTV and LVCs. Hintz et al. suggested that high vaginal toxicity observed in their study may also have been due to the irradiation of the lower third of the vagina, which is more sensitive to radiotherapy, but this was not the case in the present study [25]. Glatzer et al. reported that 71% of the experts in their study administered the treatment to the upper 3 cm of the vagina, which could be considered as a reference in vaginal CTV delineation for reducing vaginal complications associated with higher vaginal-treated volumes [6].
The use of an applicator diameter of <2.5 cm has been associated with a higher incidence of G1–2 VS [26]. In the present study, univariate analysis did not show a correlation between the applicator diameter and the LVCs (Table 3). Nonetheless, the number of patients treated with an applicator diameter of 2.5 cm or less was too low to establish conclusions (13/131). Moreover, it should be noted that the use of the constraint of 68 Gy of the applicator diameter rules out an association between the applicator diameter and the LVCs.
The EBRT dose used is commonly 45/0–50.4 Gy in 25–28 fractions over 5–6 weeks. Radiation doses to vaginal surface > 80 Gy have been associated with a 10% to 15% increased risk of G2-LVCs, including VS. Maintaining an EBRT dose of 45 Gy/25 fractions and decreasing the brachytherapy dose to the vagina reduces the risk of VS [27]. In our study, it is unlikely that the EBRT dose affected the comparison of the outcomes of the two groups, considering that there was no significant difference between the mean EBRT dose between the two groups. The addition of a VCB boost to EBRT should generally result in a vaginal surface low-dose rate (EBRT and brachytherapy) equivalent to 65–70 Gy. The HDR VCB fractionation recommended by the ABS is 5–6 Gy in three fractions or 6 Gy in two fractions prescribed to the surface after 45 Gy or 50.4 Gy fractions of EBRT [2,14,24,28].
This study has shown that higher doses to the most exposed 2 cm3 of the CTV are associated with an increase in complications as suggested in previous studies by our group. Although G1 complications have little clinical impact, the mentioned constraint would likely reduce G2-LVCs. Thus, the use of this constraint seems to have a protective effect against G1- and G2-LVCs, especially in G2 vaginal shortening. Nonetheless, studies with more participants are necessary to confirm these findings.
The mean dose per fraction was higher in patients showing G1- or G2-LVCs at 6.9 Gy (SD 0.3) compared to 6.6 Gy (SD 0.3) in those without LVCs. Despite the lack of statistical differences in the incidence of LVCs between patients receiving ≥ or <6.5 Gy (p = 0.10), only 4 out of 19 (21.1%) patients showed G1-LVCs, and no G2-LVCs were observed among 19/131 (14.5%) patients who received < 6.5 Gy per fraction. Considering the LVCs and the EQD2, we can hypothesize that an exclusive fraction of between 6.0 Gy and 6.5 Gy could be enough to reduce complications in these patients.
Following vaginal or pelvic radiotherapy VD use is recommended to prevent VS. However, the time between the development of VS and the optimal length of time of VD use remain unknown. Stahl et al. proposed that the risk of VS persists beyond 1 year after brachytherapy. VD compliance beyond 1 year may mitigate this risk. The Delphi method encourages women to begin using a VD four weeks after completing RT treatment, 1–3 min, 2–3 times per week, and for 9 to 12 months. The Brazilian consensus recommended that VD use last at least 5–10 min, 2–3 times per week for an indefinite time [12,29,30,31,32,33,34]. After our previous multivariate analysis showing that VD use > 9 months reduced G2-LVCs in patients who complied with usage 2–3 days a week, our patients were encouraged to use VD daily during the 5-year follow-up. In the current study, only one patient with G1-VS and no patient with G2-VS reported VD use of ≥9 months. This might confirm the protective role of VD use of ≥9 months in the appearance and severity of LVCs and VS. The present analysis confirmed the results of our previous retrospective study. Moreover, the EMBRACE II study reported the benefits of VD use in cervical cancer [35].
Univariate analysis of possible prognostic factors of possible LVC-free time, comparing patients with and without LVCs, showed that belonging to treatment Group-2 and VD use ≥ 9 months have protective effects against the onset of LVCs over time. Considering the fact that all the patients in Group-2 received < 68 Gy overall EQD2(α/β=3) at the most exposed 2 cm3 of vaginal CTV, it could be concluded that a 68 Gy constraint has a positive effect on LVC-free time as well as LVC severity.
Multivariate analysis revealed that belonging to the treatment group and VD use are independent prognostic factors. Patients in Group-1 had nearly a 2-fold greater risk of developing LVCs compared to Group-2. By analyzing the interaction between VD use ≥ 9 months and treatment group, it was observed that, depending on the dose group, there were no differences in the effect of VD use ≥ 9 months, showing that VD use and dose group are independent prognostic factors with an impact on LVCs (Table 4). This reinforces the importance of considering both the treatment regimen and the duration of VD use in the clinical management and risk assessment of LVCs.
The Kaplan–Meier survival curves for the probability of LVC-free time based on the duration of VD use and treatment group illustrate that the curve for VD use < 9 months and the curve for Group-1 fell more steeply, indicating a lower monthly probability of remaining LVC-free (Figure 2 and Figure 3). In other words, belonging to Group-2 and VD use ≥ 9 months have a protective effect on the time of LVC development.
Considering the present results, the effect of VD use remains consistent in PEC treated with EBRT + VCB regardless of the dose received and treatment group.
The limitations of this study were the number of patients included with a low number of VCR, LVC, and VS cases available for analysis. Nevertheless, the results are promising enough to initiate a prospective study to further analyze this topic. Additionally, future studies should consider quality of life metrics, sexual function, and variations in patterns of VD use, including the number of days and other associated medical treatments.
5. Conclusions
Considering that all the patients in Group-2 received less than 68 Gy EQD2(α/β=3) at 2 cm3 of CTV and had a higher probability of LVC-free time, it can be concluded that this constraint could prevent the development of LVCs while maintaining the same vaginal control. Since the mean dose per fraction in Group-2 was 6.5 Gy, it could be hypothesized that one fraction of 6.0–6.5 Gy is an effective VCB boost after EBRT in patients with PEC. Moreover, VD use ≥ 9 months has an independent protective effect on the incidence of LVCs. In order to confirm these results, studies with a larger number of participants are needed.
Author Contributions
Conceptualization, F.N., R.A. and A.R.; methodology, F.N., R.A. and A.R.; validation, A.H. and A.R.; formal analysis, R.A.; investigation, F.N.; resources, F.N. and A.R.; data curation, F.N.; writing—original draft preparation, F.N.; writing—review and editing, A.R., Y.Z., A.H., V.L., S.S., A.T., E.A.-C. and L.T.; visualization, F.N. and R.A.; supervision, A.R.; project administration, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study was approved by the Institutional Ethical Review Board of Hospital Clinic, Universitat de Barcelona (HCB 2022/0379, approved on 26 April 2022).
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data are not publicly available due to data protections regulations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 45–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harkenrider, M.M.; Abu-Rustum, N.; Albuquerque, K.; Bradfield, L.; Bradley, K.; Dolinar, E.; Doll, C.M.; Elshaikh, M.; Frick, M.A.; Gehrig, P.A.; et al. Radiation Therapy for Endometrial Cancer: An American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2023, 13, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Giustozzi, A.; Salutari, V.; Giudice, E.; Musacchio, L.; Ricci, C.; Landolfo, C.; Perri, M.T.; Scambia, G.; Lorusso, D. Refining Adjuvant Therapy for Endometrial Cancer: New Standards and Perspectives. Biology 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bingham, B.; Orton, A.; Boothe, D.; Stoddard, G.; Huang, Y.J.; Gaffney DKPoppe, M.M. Brachytherapy Improves Survival in Stage III Endometrial Cancer With Cervical Involvement. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Glatzer, M.; Tanderup, K.; Rovirosa, A.; Fokdal, L.; Ordeanu, C.; Tagliaferri, L.; Chargari, C.; Strnad, V.; Dimopoulos, J.A.; Šegedin, B.; et al. Role of Brachytherapy in the Postoperative Management of Endometrial Cancer: Decision-Making Analysis among Experienced European Radiation Oncologists. Cancers 2022, 14, 906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serkies, K.; Baczkowska-Waliszewska, Z. Adjuvant vaginal cuff brachytherapy in surgically treated endometrial carcinoma patients—In view of the recent evidence. J. Contemp. Brachytherapy 2021, 13, 221–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albuquerque, K.; Hrycushko, B.A.; Harkenrider, M.M.; Mayadev, J.; Klopp, A.; Beriwal, S.; Petereit, D.G.; Scanderbeg, D.J.; Yashar, C. Compendium of fractionation choices for gynecologic HDR brachytherapy-An American Brachytherapy Society Task Group Report. Brachytherapy 2019, 18, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Stenfert Kroese, M.C.; Nijman, H.W.; et al. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur. J. Cancer 2012, 48, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.; Do, V.; Chard, J.; Brand, A.H. Radiation-induced vaginal stenosis: Current perspectives. Int. J. Womens Health 2017, 9, 273–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haddad, N.C.; Soares Brollo, L.C.; Pinho Oliveira, M.A.; Bernardo-Filho, M. Diagnostic Methods for Vaginal Stenosis and Compliance to Vaginal Dilator Use: A Systematic Review. J. Sex. Med. 2021, 18, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gomez, G.; Ascaso, C.; Herreros, A.; Fornes, B.; Mases, J.; Rochera, J.; Tagliaferri, L.; Sabater, S.; Torne, A.; et al. Preliminary results of a vaginal constraint for reducing G2 late vaginal complications after postoperative brachytherapy in endometrial cancer: A prospective analysis. Clin. Transl. Oncol. 2022, 24, 875–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, L.A.; Bohlke, K.; Powell, M.A.; Fader, A.N.; Franklin, G.E.; Lee, L.J.; Matei, D.; Coallier, L.; Wright, A.A. Postoperative radiation therapy for endometrial cancer: American society of clinical oncology clinical practice guideline endorsement of the American society for radiation oncology evidence-based guideline. J. Clin. Oncol. 2015, 33, 2908–2913. [Google Scholar] [CrossRef] [PubMed]
- Varytė, G.; Bartkevičienė, D. Pelvic Radiation Therapy Induced Vaginal Stenosis: A Review of Current Modalities and Recent Treatment Advances. Medicina 2021, 57, 336. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Haie-Meder, C.; Limbergen, E.V.; Barillot, I.; Brabandere, M.D.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics. Radiobiol. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.D.V.; Rovirosa, Á.; Ascaso, C.; Herreros, A.; Sánchez, J.; Garcia-Migue, J.; Cortes, S.; Agusti, E.; Camacho, C.; Zhang, Y.; et al. Late G2 vagina toxicity in post-operative endometrial carcinoma is associated with a 68 Gy dose equivalent to 2 Gy per fraction (α/β = 3Gy) at 2 cm3 of vagina. J. Contemp. Brachytherapy 2018, 10, 40–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Ascaso, C.; Herreros, A.; Sánchez, J.; Sabater, S.; Pino, M.D.; Li, Y.; Gómez, G.; Torné, A.; Biete, A.; et al. Postoperative endometrial carcinoma treated with external beam irradiation plus vaginal-cuff brachytherapy. Is there a dose relationship with G2 vaginal complications? Rep. Pract. Oncol. Radiother. 2020, 25, 227–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noorian, F.; Abellana, R.; Zhang, Y.; Herreros, A.; Baltrons, C.; Lancellota, V.; Tagliaferri, L.; Sabater, S.; Torne, A.; Rovirosa, A. Are 7.5 Gy × 2 fractions more efficient than 6 Gy × 3 in exclusive postoperative endometrial cancer brachytherapy? A clinical and dosimetrical analysis. Radiother. Oncol. 2023, 189, 109909. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Routledge, J.A.; Burns, M.P.; Swindell, R.; Khoo, V.S.; West, C.M.; Davidson, S.E. Evaluation of the LENT-SOMA scales for the prospective assessment of treatment morbidity in cervical carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Noorian, F.; Abellana, R.; Rochera, J.; Herreros, A.; Antelo, G.; Lancellotta, V.; Tagliaferri, L.; Han, Q.; Torne, A.; et al. Vaginal dilator use more than 9 months is a main prognostic factor for reducing G2-late vaginal complications in 3D-vaginal-cuff brachytherapy (interventional radiotherapy)? Clin. Transl. Oncol. 2023, 25, 1748–1755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirchheiner, K.; Nout, R.A.; Lindegaard, J.C.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; Jürgenliemk-Schulz, I.M.; Hoskin, P.J.; Rai, B.; Dörr, W.; et al. Dose–Effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother. Oncol. 2016, 118, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hintz, B.L.; Kagan, A.R.; Chan, P.; Gilbert, H.A.; Nussbaum, H.; Rao, A.R.; Wollin, M. Radiation tolerance of the vaginal mucosa. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.M.; Stahl, J.M.; Young, M.R.; Ratner, E.; Damast, S. Impact of vaginal cylinder diameter on outcomes following brachytherapy for early stage endometrial cancer. J. Gynecol. Oncol. 2017, 28, e84. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Lee, L.J.; Eswara, J.R.; Horowitz, N.S.; Konstantinopoulos, P.A.; Mirabeau-Beale, K.L.; Rose, B.S.; von Keudell, A.G.; Wo, J.Y. Complications of pelvic radiation in patients treated for gynecologic malignancies. Cancer 2014, 120, 3870–3883. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Ratner, E.S.; Lucarelli, L.; Polizzi, S.; Higgins, S.A.; Damast, S. Predictors of vaginal stenosis after intravaginal high-dose-rate brachytherapy for endometrial carcinoma. Brachytherapy 2015, 14, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Bahng, A.Y.; Dagan, A.; Bruner, D.W.; Lin, L.L. Determination of Prognostic Factors for Vaginal Mucosal Toxicity Associated with Intravaginal High-Dose Rate Brachytherapy in Patients with Endometrial Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 484–490. [Google Scholar] [CrossRef]
- Brand, A.H.; Bull, C.A.; Cakir, B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int. J. Gynecol. Cancer 2006, 16, 288–293. [Google Scholar] [CrossRef]
- Hartman, P.; Diddle, A.W. Vaginal stenosis following irradiation therapy for carcinoma of the cervix uteri. Cancer 1972, 30, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.M.; Qian, J.M.; Tien, C.J.; Carlson, D.J.; Chen, Z.; Ratner, E.S.; Park, H.S.; Damast, S. Extended duration of dilator use beyond 1 year may reduce vaginal stenosis after intravaginal high-dose-rate brachytherapy. Support. Care Cancer 2019, 27, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.M.; ter Kuile, M.M.; Vermeer, W.M.; Nout, R.A.; Mens, J.W.; van Doorn, L.C.; de Kroon, C.D.; Hompus, W.C.; Braat, C.; Creutzberg, C.L. Sexual rehabilitation after pelvic radiotherapy and vaginal dilator use: Consensus using the Delphi method. Int. J. Gynecol. Cancer 2014, 24, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Matos, S.R.L.; Lucas Rocha Cunha, M.; Podgaec, S.; Weltman, E.; Yamazaki Centrone, A.F.; Cintra Nunes Mafra, A.C. Consensus for vaginal stenosis prevention in patients submitted to pelvic radiotherapy. PLoS ONE 2019, 14, e0221054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).