Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events
Abstract
1. Introduction
2. Methods
3. Ocular Involvement of COVID-19
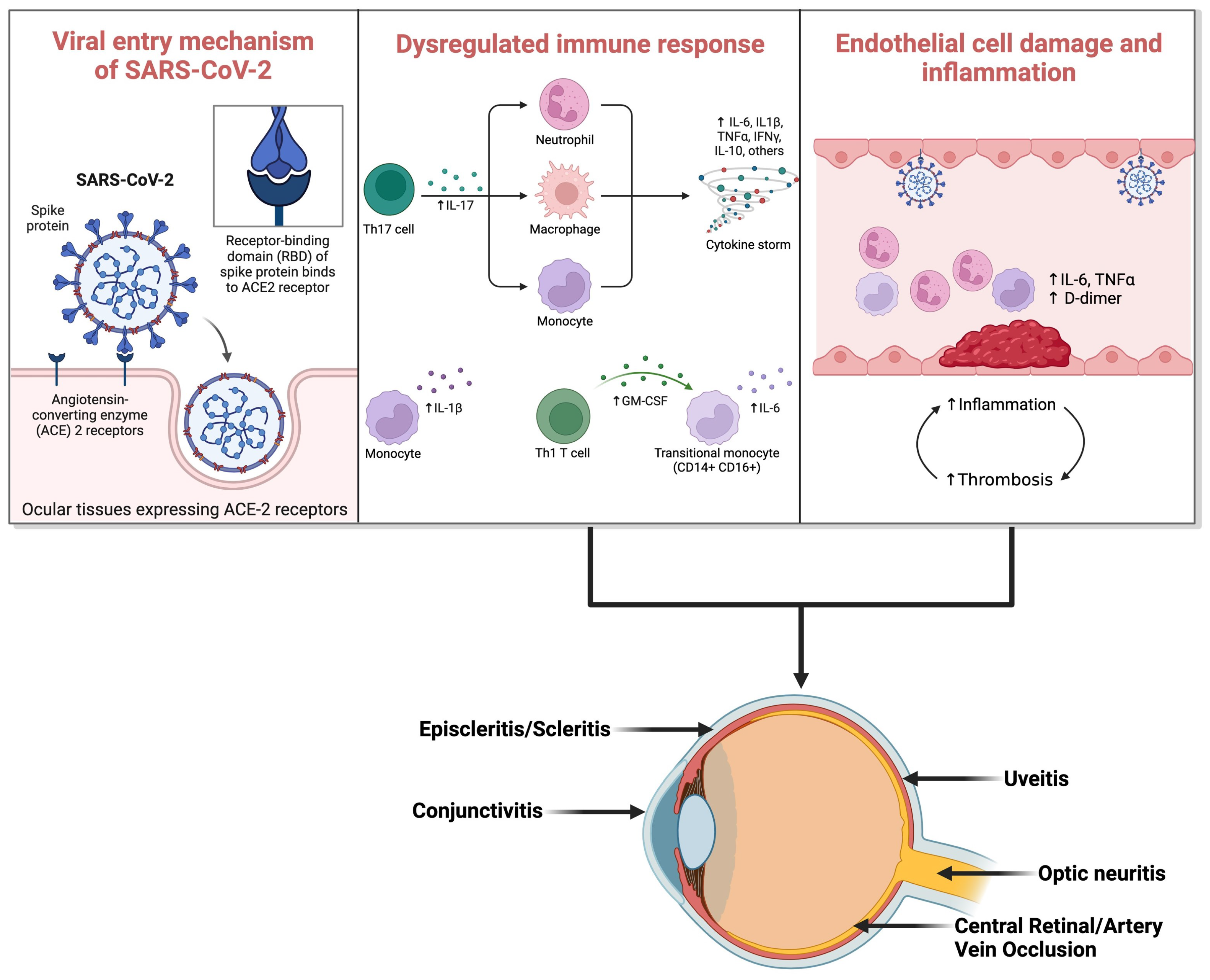
3.1. Overview of SARS-CoV-2 Viruses
3.2. Viral Transmission
3.3. Susceptibility to Ocular Involvement
3.4. Prevalence and Incidence of Ocular Manifestations
3.5. Types of Ocular Manifestations
3.5.1. Conjunctivitis
3.5.2. Scleritis and Episcleritis
3.5.3. Uveitis
3.5.4. Retinopathy
3.5.5. Other Ocular Complications
4. Clinical Practice and Diagnostic Considerations
5. Mechanisms of Ocular Infection by SARS-CoV-2
5.1. Entry Routes of the Virus into Ocular Tissues
5.2. Underlying Pathophysiology and Immune Response in Ocular Tissues
5.3. Association between Ocular Involvement and Disease Severity
6. Ocular Side Effects of COVID-19 Vaccines
6.1. Overview of COVID-19 Vaccine Types
6.2. Reported Ocular Side Effects
6.2.1. Cornea
6.2.2. Uvea
6.2.3. Retina
6.2.4. Other Reported Ocular Side Effects
7. Mechanisms of Ocular Side Effects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loon, S.C.; Lun, K. SARS: A Timely Reminder. Br. J. Ophthalmol. 2013, 97, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Novel Coronavirus (2019-NCoV): Situation Report, 11. World Health Organization. 2020. Available online: https://iris.who.int/handle/10665/330776 (accessed on 12 May 2024).
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Martin, D.; Bremond-Gignac, D. Ocular Manifestation as First Sign of Coronavirus Disease 2019 (COVID-19): Interest of Telemedicine during the Pandemic Context. J. Fr. Ophtalmol. 2020, 43, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Ichhpujani, P.; Parmar, U.P.S.; Duggal, S.; Kumar, S. COVID-19 Vaccine-Associated Ocular Adverse Effects: An Overview. Vaccines 2022, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation and Characterization of 2019-NCoV-like Coronavirus from Malayan Pangolins. bioRxiv 2020. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, C.-B.; Jhanji, V.; Xu, C.; Yuan, X.-L.; Liang, J.-J.; Huang, Y.; Cen, L.-P.; Ng, T.K. Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Human Primary Conjunctival and Pterygium Cell Lines and in Mouse Cornea. Eye 2020, 34, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Holappa, M.; Vapaatalo, H.; Vaajanen, A. Many Faces of Renin-Angiotensin System—Focus on Eye. Open Ophthalmol. J. 2017, 11, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 Are Expressed on the Human Ocular Surface, Suggesting Susceptibility to SARS-CoV-2 Infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Rota, P.A.; Tumpey, T.M. Ocular Tropism of Respiratory Viruses. Microbiol. Mol. Biol. Rev. 2013, 77, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Sharifi, H.; Bazrafshan, A.; Noori, A.; Karamouzian, M.; Sharifi, A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-Analysis. J. Ophthalmic Vis. Res. 2021, 16, 103. [Google Scholar] [CrossRef]
- Doherty, M.J. Ocular Manifestations of Feline Infectious Peritonitis. J. Am. Vet. Med. Assoc. 1971, 159, 417–424. [Google Scholar] [PubMed]
- Scalinci, S.Z.; Trovato Battagliola, E. Conjunctivitis Can Be the Only Presenting Sign and Symptom of COVID-19. IDCases 2020, 20, e00774. [Google Scholar] [CrossRef] [PubMed]
- Danthuluri, V.; Grant, M.B. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmol. Ther. 2020, 9, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Meshksar, A.; Naderi, A.; Esfandiari, H. Anterior Scleritis Manifesting After Coronavirus Disease 2019: A Report of Two Cases. Cornea 2021, 40, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Otaif, W.; Al Somali, A.I.; Al Habash, A. Episcleritis as a Possible Presenting Sign of the Novel Coronavirus Disease: A Case Report. Am. J. Ophthalmol. Case Rep. 2020, 20, 100917. [Google Scholar] [CrossRef] [PubMed]
- Méndez Mangana, C.; Barraquer Kargacin, A.; Barraquer, R.I. Episcleritis as an Ocular Manifestation in a Patient with COVID-19. Acta Ophthalmol. 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhao, M.; Mo, J.; Cao, X.; Chen, W.; Wang, H. New Onset or Recurrence of Uveitis Following COVID-19 Infection. BMC Ophthalmol. 2024, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Murthy, S.I.; Annum, S. Bilateral Multifocal Choroiditis Following COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Walinjkar, J.; Makhija, S.; Sharma, H.; Morekar, S.; Natarajan, S. Central Retinal Vein Occlusion with COVID-19 Infection as the Presumptive Etiology. Indian J. Ophthalmol. 2020, 68, 2572. [Google Scholar] [CrossRef] [PubMed]
- Gaba, W.H.; Ahmed, D.; Al Nuaimi, R.K.; Dhanhani, A.A.; Eatamadi, H. Bilateral Central Retinal Vein Occlusion in a 40-Year-Old Man with Severe Coronavirus Disease 2019 (COVID-19) Pneumonia. Am. J. Case Rep. 2020, 21, e927691-1. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Messenio, D.; Cereda, M.; Olivieri, P.; Brambilla, A.M.; Staurenghi, G. Impending Central Retinal Vein Occlusion in a Patient with Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, H.R.; Abrishami, M.; Motamed Shariati, M.; Ghavami Shahri, S.H.; Ansari Astaneh, M.R. Atypical Central Retinal Artery Occlusion Following COVID-19 Infection: A Case Report. Case Rep. Ophthalmol. 2023, 14, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal Findings in Patients with COVID-19: Results from the SERPICO-19 Study. EClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef] [PubMed]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal Findings in Patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; Soares, L.C.M.; Nascimento, P.A.; Cirillo, L.R.N.; Sakuma, H.T.; da Veiga, G.L.; Fonseca, F.L.A.; Lima, V.L.; Abucham-Neto, J.Z. Retinal Findings in Hospitalised Patients with Severe COVID-19. Br. J. Ophthalmol. 2022, 106, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Narayanan, R.; Tyagi, M.; Belenje, A.; Basu, S. Acute Retinal Necrosis as a Presenting Ophthalmic Manifestation in COVID-19 Recovered Patients. Ocul. Immunol. Inflamm. 2021, 29, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Verkuil, L.D.; Liu, G.T.; Brahma, V.L.; Avery, R.A. Pseudotumor Cerebri Syndrome Associated with MIS-C: A Case Report. Lancet 2020, 396, 532. [Google Scholar] [CrossRef] [PubMed]
- Belghmaidi, S.; Nassih, H.; Boutgayout, S.; El Fakiri, K.; El Qadiry, R.; Hajji, I.; Bourrahouat, A.; Moutaouakil, A. Third Cranial Nerve Palsy Presenting with Unilateral Diplopia and Strabismus in a 24-Year-Old Woman with COVID-19. Am. J. Case Rep. 2020, 21, e925897-1. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.D.M.C.D.; Santos, D.H.; Olivetti, B.C.; Takahashi, J.T. Bilateral Trochlear Nerve Palsy Due to Cerebral Vasculitis Related to COVID-19 Infection. Arq. Neuropsiquiatr. 2020, 78, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.M.; Díaz-Maroto, I.; Segura, T. Multiple Sclerosis Following SARS-CoV-2 Infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef] [PubMed]
- Malayala, S.V.; Raza, A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus 2020, 12, e8918. [Google Scholar] [CrossRef]
- Llorente Ayuso, L.; Torres Rubio, P.; Beijinho do Rosário, R.F.; Giganto Arroyo, M.L.; Sierra-Hidalgo, F. Bickerstaff Encephalitis after COVID-19. J. Neurol. 2021, 268, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Patel, J.; Swiston, C.; Patel, B.C. Ophthalmic Manifestations of Coronavirus (COVID-19); StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Raony, Í.; de Figueiredo, C.S.; Pandolfo, P.; Giestal-de-Araujo, E.; Oliveira-Silva Bomfim, P.; Savino, W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front. Immunol. 2020, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the Human Corneal Innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; García-Feijoó, J.; Sáenz-Francés, F.; Arriola-Villalobos, P.; Martinez-de-la-Casa, J.M.; Benítez-del-Castillo, J.M.; Herrera de la Muela, M. Conjunctivitis in COVID-19 Patients: Frequency and Clinical Presentation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, C.S.; Raony, Í.; Giestal-de-Araujo, E. SARS-CoV-2 Targeting the Retina: Host–Virus Interaction and Possible Mechanisms of Viral Tropism. Ocul. Immunol. Inflamm. 2020, 28, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, L.E.; Binkhorst, M.; Bourgonje, A.R.; Offringa, A.K.; Mulder, D.J.; Bos, E.M.; Kolundzic, N.; Abdulle, A.E.; van der Voort, P.H.; Olde Rikkert, M.G.; et al. COVID-19: Immunopathology, Pathophysiological Mechanisms, and Treatment Options. J. Pathol. 2021, 254, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ge, Y.; Sun, J. IL-33 in COVID-19: Friend or Foe? Cell. Mol. Immunol. 2021, 18, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Cron, R.Q. The Genetics of Macrophage Activation Syndrome. Genes Immun. 2020, 21, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hooks, J.J.; Percopo, C.; Wang, Y.; Detrick, B. Retina and Retinal Pigment Epithelial Cell Autoantibodies Are Produced during Murine Coronavirus Retinopathy. J. Immunol. 1993, 151, 3381–3389. [Google Scholar] [CrossRef]
- Hooper, L.C.; Chin, M.S.; Detrick, B.; Hooks, J.J. Retinal Degeneration in Experimental Coronavirus Retinopathy (ECOR) Is Associated with Increased TNF-α, Soluble TNFR2 and Altered TNF-α Signaling. J. Neuroimmunol. 2005, 166, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Moriki, T.; Igari, A.; Matsubara, Y.; Ohnishi, T.; Hosokawa, K.; Murata, M. Studies of a Microchip Flow-Chamber System to Characterize Whole Blood Thrombogenicity in Healthy Individuals. Thromb. Res. 2013, 132, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Levy, J.H.; Iba, T. The Influence of Hyperglycemia on Neutrophil Extracellular Trap Formation and Endothelial Glycocalyx Damage in a Mouse Model of Type 2 Diabetes. Microcirculation 2020, 27, e12617. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High Risk of Thrombosis in Patients with Severe SARS-CoV-2 Infection: A Multicenter Prospective Cohort Study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Swinnen, T.; Kampmann, B.; Parker, E.P.K. An Interactive Website Tracking COVID-19 Vaccine Development. Lancet Glob. Health 2021, 9, e590–e592. [Google Scholar] [CrossRef] [PubMed]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA 2021, 325, 1575. [Google Scholar] [CrossRef] [PubMed]
- The Vaccine Adverse Event Reporting System (VAERS) about. Available online: https://wonder.cdc.gov/controller/datarequest/D8 (accessed on 13 May 2024).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and Reactogenic Efficacy of Covaxin and Covishield: A Comparative Review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef]
- Soheili, M.; Khateri, S.; Moradpour, F.; Mohammadzedeh, P.; Zareie, M.; Mortazavi, S.M.M.; Manifar, S.; Kohan, H.G.; Moradi, Y. The Efficacy and Effectiveness of COVID-19 Vaccines around the World: A Mini-Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 Vaccine Efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.-Y.; Lu, Q.-B.; Cui, F. Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Protection against SARS-CoV-2 Related Disease. Vaccines 2022, 10, 920. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Zhang, X.; Li, J.; Zhu, F. CoronaVac: A Review of Efficacy, Safety, and Immunogenicity of the Inactivated Vaccine against SARS-CoV-2. Hum. Vaccines Immunother. 2022, 18, 2096970. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.; Kalita, C.; Neog, N.; Goswami, C.; Sarma, M.K.; Hazarika, I. A Comparative Analysis on the Safety and Efficacy of Covaxin versus Other Vaccines against COVID-19: A Review. Z. Naturforschung C 2022, 77, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Fujio, K.; Sung, J.; Nakatani, S.; Yamamoto, K.; Iwagami, M.; Fujimoto, K.; Shokirova, H.; Okumura, Y.; Akasaki, Y.; Nagino, K.; et al. Characteristics and Clinical Ocular Manifestations in Patients with Acute Corneal Graft Rejection after Receiving the COVID-19 Vaccine: A Systematic Review. J. Clin. Med. 2022, 11, 4500. [Google Scholar] [CrossRef] [PubMed]
- Wasser, L.M.; Roditi, E.; Zadok, D.; Berkowitz, L.; Weill, Y. Keratoplasty Rejection After the BNT162b2 Messenger RNA Vaccine. Cornea 2021, 40, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, T.R.J.; Jørgensen, C.; Kyhn, M.C.; Cabrerizo, J. Acute Bilateral Descemet Membrane Endothelial Keratoplasty Graft Rejection After the BNT162b2 MRNA COVID-19 Vaccine. Int. Med. Case Rep. J. 2022, 15, 201–204. [Google Scholar] [CrossRef]
- Crnej, A.; Khoueir, Z.; Cherfan, G.; Saad, A. Acute Corneal Endothelial Graft Rejection Following COVID-19 Vaccination. J. Fr. Ophtalmol. 2021, 44, e445–e447. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.I.; Ting, D.S.J.; Said, D.G.; Dua, H.S. Corneal Graft Rejection Following COVID-19 Vaccine. Eye 2022, 36, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Molero-Senosiain, M.; Houben, I.; Savant, S.; Savant, V. Five Cases of Corneal Graft Rejection after Recent COVID-19 Vaccinations and a Review of the Literature. Cornea 2022, 41, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Phylactou, M.; Li, J.-P.O.; Larkin, D.F.P. Characteristics of Endothelial Corneal Transplant Rejection Following Immunisation with SARS-CoV-2 Messenger RNA Vaccine. Br. J. Ophthalmol. 2021, 105, 893–896. [Google Scholar] [CrossRef]
- Renisi, G.; Lombardi, A.; Stanzione, M.; Invernizzi, A.; Bandera, A.; Gori, A. Anterior Uveitis Onset after Bnt162b2 Vaccination: Is This Just a Coincidence? Int. J. Infect. Dis. 2021, 110, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, A.-W.; Razok, A.; Al-Allaf, Y.; Aker, L. Post-COVID-19 Vaccine Medium-Vessel Vasculitis and Acute Anterior Uveitis, Causation vs. Temporal Relation; Case Report and Literature Review. Ann. Med. Surg. 2022, 75. [Google Scholar] [CrossRef]
- Ortiz-Egea, J.M.; Sánchez, C.G.; López-Jiménez, A.; Navarro, O.D. Herpetic Anterior Uveitis Following Pfizer–BioNTech Coronavirus Disease 2019 Vaccine: Two Case Reports. J. Med. Case Rep. 2022, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Seo, M.-H.; Choi, K.-E.; Lee, S.; Choi, B.; Yun, C.; Kim, S.-W.; Kim, Y.Y. Vision-Threatening Ocular Adverse Events after Vaccination against Coronavirus Disease 2019. J. Clin. Med. 2022, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Mudie, L.I.; Zick, J.D.; Dacey, M.S.; Palestine, A.G. Panuveitis Following Vaccination for COVID-19. Ocul. Immunol. Inflamm. 2021, 29, 741–742. [Google Scholar] [CrossRef] [PubMed]
- van Dam, C.S.; Lede, I.; Schaar, J.; Al-Dulaimy, M.; Rösken, R.; Smits, M. Herpes Zoster after COVID Vaccination. Int. J. Infect. Dis. 2021, 111, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, P.; Chicharro, P.; Cabrera, L.-M.; Seguí, M.; Morales-Caballero, Á.; Llamas-Velasco, M.; Sánchez-Pérez, J. Varicella-Zoster Virus Reactivation after SARS-CoV-2 BNT162b2 MRNA Vaccination: Report of 5 Cases. JAAD Case Rep. 2021, 12, 58–59. [Google Scholar] [CrossRef]
- Aksu, S.B.; Öztürk, G.Z. A Rare Case of Shingles after COVID-19 Vaccine: Is It a Possible Adverse Effect? Clin. Exp. Vaccine Res. 2021, 10, 198. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Mambretti, M.; Huemer, J.; Torregrossa, G.; Ullrich, M.; Findl, O.; Casalino, G. Acute Macular Neuroretinopathy Following Coronavirus Disease 2019 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Book, B.A.J.; Schmidt, B.; Foerster, A.M.H. Bilateral Acute Macular Neuroretinopathy After Vaccination Against SARS-CoV-2. JAMA Ophthalmol. 2021, 139, e212471. [Google Scholar] [CrossRef]
- Bøhler, A.D.; Strøm, M.E.; Sandvig, K.U.; Moe, M.C.; Jørstad, Ø.K. Acute Macular Neuroretinopathy Following COVID-19 Vaccination. Eye 2022, 36, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Subramony, R.; Lin, L.C.; Knight, D.K.; Aminlari, A.; Belovarski, I. Bilateral Retinal Detachments in a Healthy 22-Year-Old Woman After Moderna SARS-CoV-2 Vaccination. J. Emerg. Med. 2021, 61, e146–e150. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, C.; Gómez-Figueroa, E.; Alban, L.; Arias-Cárdenas, A. Optic Neuritis after COVID-19 Vaccine Application. Clin. Exp. Neuroimmunol. 2022, 13, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nagasato, D.; Nakakura, S.; Nagasawa, T.; Wakuda, H.; Kurusu, A.; Mitamura, Y.; Tabuchi, H. Branch Retinal Vein Occlusion Post Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination. Taiwan J. Ophthalmol. 2022, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Cheng, S.S.H.; Sharma, A.; Moloney, T.P. Retinal Vein Occlusion Following COVID-19 Vaccination. Clin. Exp. Ophthalmol. 2022, 50, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, N.; Yadav, D.; Kota, A.; Singh, H. Central Retinal Vein Occlusion Post-COVID-19 Vaccination. Indian J. Ophthalmol. 2022, 70, 308. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.S.C.; Finamor, L.P.S.; Andrade, G.C.; Lima, L.H.; Zett, C.; Muccioli, C.; Sarraff, E.P.; Marinho, P.M.; Peruchi, J.; Oliveira, R.D.d.L.; et al. Vascular Retinal Findings after COVID-19 Vaccination in 11 Cases: A Coincidence or Consequence? Arq. Bras. Oftalmol. 2022, 85, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nagasato, D.; Nakakura, S.; Tanabe, H.; Nagasawa, T.; Wakuda, H.; Imada, Y.; Mitamura, Y.; Tabuchi, H. Exacerbation of Branch Retinal Vein Occlusion Post SARS-CoV2 Vaccination. Medicine 2021, 100, e28236. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Li, J.; Parmar, U.P.S.; Jeng, B.H.; Jhanji, V. Vaccine-Associated Corneal Graft Rejection Following SARS-CoV-2 Vaccination: A CDC-VAERS Database Analysis. Br. J. Ophthalmol. 2024, 108, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ritterband, D.C.; Mehta, I. Acute Corneal Transplant Rejection After Severe Acute Respiratory Syndrome Coronavirus 2 MRNA-1273 Vaccination. Cornea 2022, 41, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Kahale, F.; Agarwal, A.; Tsui, E. Vaccine-Associated Uveitis after COVID-19 Vaccination. Ophthalmology 2023, 130, 179–186. [Google Scholar] [CrossRef]
- Cafiero, C.; Re, A.; Micera, A.; Palmirotta, R.; Monaco, D.; Romano, F.; Fabrizio, C.; Di Francia, R.; Cacciamani, A.; Surico, P.L.; et al. Pharmacogenomics and Pharmacogenetics: In Silico Prediction of Drug Effects in Treatments for Novel Coronavirus SARS-CoV-2 Disease. Pharmacogenom. Pers. Med. 2020, 13, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Wu, H.; Cheng, K.; Chang, Y. Disc Edema in One Eye and Central Serous Chorioretinopathy in the Other Eye Shortly after AstraZeneca COVID-19 Vaccination. Kaohsiung J. Med. Sci. 2022, 38, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Gupta, R.; Garcia, A.J.V.; Cho, W.; Singh, K.P.; Agarwal, A. Retinal Vascular Occlusion after Severe Acute Respiratory Syndrome Coronavirus Vaccination. Ophthalmol. Sci. 2024, 4, 100354. [Google Scholar] [CrossRef] [PubMed]
- Pawar, N.; Ravindran, M.; Padmavathy, S.; Chakrabarty, S. Acute Abducens Nerve Palsy after COVID-19 Vaccination in a Young Adult. Indian J. Ophthalmol. 2021, 69, 3764. [Google Scholar] [CrossRef]
- Colella, G.; Orlandi, M.; Cirillo, N. Bell’s Palsy Following COVID-19 Vaccination. J. Neurol. 2021, 268, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, T.J. Thyroid Eye Disease Following COVID-19 Vaccine in a Patient With a History Graves’ Disease: A Case Report. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e221–e223. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Ichhpujani, P.; Jeng, B.H.; Jhanji, V. Herpetic Eye Disease After SARS-CoV-2 Vaccination: A CDC-VAERS Database Analysis. Cornea 2023, 42, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Abičić, A.; Adamec, I.; Habek, M. Miller Fisher Syndrome Following Pfizer COVID-19 Vaccine. Neurol. Sci. 2022, 43, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Cho, W.; Ichhpujani, P. Glaucoma Cases Following SARS-CoV-2 Vaccination: A VAERS Database Analysis. Vaccines 2022, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Menacher, M.; Christ, M.; Behrens, L.; Rank, A.; Naumann, M. Bilateral Superior Ophthalmic Vein Thrombosis, Ischaemic Stroke, and Immune Thrombocytopenia after ChAdOx1 NCoV-19 Vaccination. Lancet 2021, 397, e11. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.T.; Moorthy, R.S.; Fraunfelder, F.W.; Zierhut, M. Vaccine-Associated Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, T.; Ben-Arie-Weintrob, Y.; Hareuveni-Blum, T.; Shaer, B.; Vishnevskia-Dai, V.; Shulman, S.; Newman, H.; Biadsy, M.; Masarwa, D.; Fischer, N.; et al. Uveitis after the BNT162b2 mRNA Vaccination against SARS-CoV-2 Infection. Retina 2021, 41, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.A.; Solyman, O.; Abushanab, M.M.; Abo Obaia, A.S.; Elhusseiny, A.M. Ocular Complications Following Vaccination for COVID-19: A One-Year Retrospective. Vaccines 2022, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Sawant, O.B.; Singh, S.; Wright, R.E.; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in Human Post-Mortem Ocular Tissues. Ocul. Surf. 2021, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Sheth, J.; Narayanan, R.; Goyal, J.; Goyal, V. Retinal Vein Occlusion in COVID-19: A Novel Entity. Indian J. Ophthalmol. 2020, 68, 2291. [Google Scholar] [CrossRef] [PubMed]
| Ocular Complication | Age and Sex | Ocular Presentation | Systemic Symptoms | Diagnostic Test for COVID-19 | Symptom Timeline | Study |
|---|---|---|---|---|---|---|
| Conjunctivitis | 41 M | Chemosis, epiphora, photophobia, eye lid edema, watery discharge, follicular reaction of the palpebral conjunctiva | None | RT-PCR | Scalini S et al. [21] | |
| Anterior scleritis | 67 F | Redness, pain, and photophobia in both eyes | Fever, headache, myalgia, dry cough, and dyspnea. | RT-PCR | Ocular symptoms presented 1 week after systemic symptoms | Feizi S et al. [22] |
| 33 M | Red eye, foreign body sensation, epiphora, and photophobia | Fever, myalgia, anosmia, ageusia, dry cough. | RT-PCR | Ocular symptoms 2 weeks after systemic symptoms | Feizi S et al. [22] | |
| Episcleritis | 29 M | Redness and foreign body sensation. | Headache cough, and fever. | RT-PCR | Ocular symptoms presented 3 days before systemic symptoms | Otiaf W et al. [23] |
| Central retinal vein occlusion | 17 F | Diminished vision, optic disc examination—disc swelling, splinter hemorrhages, flame shaped and blot hemorrhages, OCT—neurosensory detachment and cystoid macular edema | Cough and fever. | RT-PCR | Ocular presentation occurred 21 days after the systemic presentation | Walinjkar et al. [24] |
| Bilateral central retinal vein occlusion | 40 M | Blurring of vision in both eyes | Fever, cough, and shortness of breath. | RT-PCR | Systemic symptoms began 3 days before the ocular presentation | Gaba WH et al. [25] |
| Vasculitis RVO | 52 M | Inferior hemiretinal vein occlusion with macular edema | Cough, fever, and malaise. | RT-PCR | Ocular symptoms presented 10 days after systemic ones | Sheth et al. [26] |
| Sno | Name | Vaccine Type | Route of Administration | Country of Development | Reported Efficacy against Mild COVID | Study |
|---|---|---|---|---|---|---|
| 1 | BNT163b2 Pfizer BioNTech | RNA (modified nucleoside in lipid nanoparticle) | Intra-muscular | Germany | 95% | [61] |
| 2 | mRNA-1273 Moderna: Spikewax | RNA (modified nucleoside in lipid nanoparticle) | Intra-muscular | Spain (Moderna Biotech) | 94.1% | [62] |
| 3 | Covovax (Novavax formulation) | Protein Subunit | Intra-muscular | India (Serum Institute of India) | 89.7% | [63] |
| 4 | Nuvaxovid (Novavax) | Protein Subunit | Intra-muscular | Czech Republic | 92.6% | [64] |
| 5 | Covishield (ChAdOx1 nCoV-19) | Non-replicating viral vector | Intra-muscular | India (Serum Institute of India) | 90% | [65] |
| 6 | Jcovden: Janssen (Johnson & Johnson) | Non-replicating viral vector | Intra-muscular | Belgium | 72% | [66] |
| 7 | Vaxzevria (Oxford AstraZeneca) | Non-replicating viral vector | Intra-muscular | Republic of Korea | 70.4% | [67] |
| 8 | Convidecia: CanSino (Ad5.CoV2-5) | Non-replicating viral vector | Intra-muscular | People’s Republic of China | - | [68] |
| 9 | Sinopharm: Covilo/BBIBP-CorV | Inactivated | Intra-muscular | China (BIBP) | - | [69] |
| 10 | Sinovac: Coronavac | Inactivated | Intra-muscular | China (Sinovac Biotech) | 67.7% | [70] |
| 11 | Covaxin | Inactivated (whole virion) | Intra-muscular | India (Bharat Biotech) | 93.4% | [71] |
| Ocular Side Effect | Age (years)/Age Range Study Type | Type of Vaccine | Symptoms | Time of Onset | Management | Study |
|---|---|---|---|---|---|---|
| Endothelial Graft Rejection | Median age 68 (27–83) IQR Systematic Review | BNT162b2 (mRNA) vaccine 8 patients | Hyperemia, diffuse conjunctival edema, flare, keratoprecipitates | 17 days 7 days 3 days 14 days 9 days 13 days 3 weeks 4 days | Oral methyl prednisone, dexamethasone eye drops 0.2% hourly and hypertonic saline | Fujio K et al. [76] |
| mRNA-1273 8 patients | 1 week 1 week 2 weeks 1 week 15 days 3 days 1 week 1 week | |||||
| ChAdOx1 4 patients | 5 days 10 days 2 days 6 weeks | |||||
| CoronaVac | 1 day | |||||
| 73 yrs 56 yrs Case report | BNT162b2 (mRNA) vaccine | Discomfort in the eye, ciliary injection, corneal edema, Descemet folds and keratoprecipitates | 2 weeks | Dexamethasone 0.1% eye drops hourly and oral prednisone 60 mg daily | Wesser LM et al. [77] | |
| 94 yrs Case report | BNT162b2 (mRNA) vaccine | Painless worsening of vision | 2 weeks | Topical dexamethasone and tobramycin | Forshaw, T et al. [78] | |
| 71 yrs Case report | BNT162b2 (mRNA) vaccine | Conjunctival injection, sudden decrease in vision | 1 week | Topical dexamethasone sodium 1 mg/mL/2 hourly | Crnej, A et al. [79] | |
| 68 yrs Case report | BNT162b2 (mRNA) vaccine | Discomfort in the eye, conjunctival hyperemia, and epithelial rejection line. | 1 day | Dexamethasone 0.1% eye drops hourly and acyclovir 400 mg 5 times a day for a week | Rallis et al. [80] | |
| Case series | BNT162b2 (mRNA) vaccine 3 cases | Decreased visual acuity, ocular pain, photophobia | 16.86 ± 6.96 days (mean) | Topical steroids | Molero-Senosiain, et al. [81] | |
| AZD1222 2 cases | 17 ± 11.89 days | |||||
| 83 yrs 66 yrs Case report | BNT162b2 (mRNA) vaccine | Discomfort, painless decrease in vision hyperemia | 7 days 3 weeks | Topical steroids | Phylactou et al. [82] | |
| Acute Anterior Uveitis | 23 yrs Case report | BNT162b2 (mRNA) vaccine | Pain, photophobia and conjunctival hyperemia | 14 days | Topical steroids and cycloplegic eye drops for 10 days | Renisi et al. [83] |
| 46 yrs Case report | BNT162b2 (mRNA) vaccine | Photophobia, pain, blurring of vision | 3 weeks | Azathioprine 50 mg once daily Topical triamcinolone drops | Al-Allaf, et al. [84] | |
| 92 yrs 85 yrs Case Report | BNT162b2 (mRNA) vaccine | Hyperemia, ocular pain, headache | 3 days 3 days | Cycloplegic eye drops every 8 h and moxifloxacin every 4 h | Ortiz Egea et al. [85] | |
| 79 yrs 55 yrs Case report | BNT162b2 (mRNA) vaccine | Keratoprecipitates, vitreous opacity, inflammatory cells in the anterior chamber | 3 days 2 days | Topical steroids | Choi et al. [86] | |
| 62 yrs Case report | AZD1222 | 1 day | ||||
| Panuveitis | 43 F | BNT162b2 (mRNA) vaccine | On examination—vitreous inflammation and thickening of the choroid | 2 weeks | Oral and topical steroids | Mudie et al. [87] |
| Herpes Zoster Ophthalmicus | 29 yrs 34 yrs Case report | BNT162b2 (mRNA) vaccine | Painful grouped vesicles in the S3 dermatome, painful inguinal lymph nodes | 15 days 13 days | Valacyclovir 1 g 3×/day for 10 days | Van Dam, C. S et al. [88] |
| 58 yrs 47 yrs 39 yrs 56 yrs 41 yrs Case series | BNT162b2 (mRNA) vaccine | Umbilicated vesicles, rash in dermatomal pattern, lymphadenopathy | 1 day 5 days 3 days 2 days 16 days | Valacyclovir 1 g 3×/day for 7 days | Rodriguez-Jimenez P et al. [89] | |
| 68 yrs Case report | Inactivated COVID-19 vaccine | Erythematous rash with vesicular lesions, stinging sensation and pain | 5 days | Valacyclovir 1 g 3×/day for 7 days | Aksu, S.B et al. [90] | |
| 51 yrs 56 yrs 89 yrs 86 yrs 90 yrs 91 yrs 94 yrs Case Series | BNT162b2 (mRNA) vaccine | Dermatomal rash, vesicles, pain, headache | 9 days 2 weeks 8 days 1 week 9 days 1 week 10 days | Acyclovir 5×/day, prednisone phosphate 0.5%, doxycycline 50 mg orally once a day | Psichogiou M, et al. [91] | |
| Acute macular neuro retinopathy | 27 yrs 22 yrs 28 yrs Case report | AZD1222 | Paracentral scotoma with a macular lesion nasal to the fovea | 2 days 2 days 2 days | Self-limiting | Mambretti, et al. [92] |
| 21 yrs | AZD1222 | Bilateral paracentral scotoma, OCT with outer plexiform layer thickening | 3 days | Self-limiting | Book, et al. [93] | |
| 27 yrs Case report | AstraZeneca | Visual disturbances, on examination—paracentral scotoma, tear shaped macular lesion | - | Bøhler et al. [94] | ||
| Retinal detachment | 22 yrs Case report | mRNA-1273 (Moderna) | Progressive painless loss of vision | Vitrectomy | Subramony R et al. [26] | |
| Optic Neuritis | 19 yrs Case report | Ad26.COV2.S | Eye pain, pain with movement of the eye | Oral steroids | Garcia-Estarada C et al. [95] | |
| Retinal vein occlusion | 50 yrs 56 yrs Case report | BNT162b2 (mRNA) vaccine | Sudden onset vision loss, reduced best corrected visual acuity (BCVA) on examination | 3 days 3 days | Intravitreal ranibizumab | Tanaka H, et al. [96] |
| 58 yrs 73 yrs | AstraZeneca | Pianless decrease in vision | 3 days 3 days | Intravitreal ranibizumab | Peters MC, et al. [97] | |
| 47 yrs 36 yrs Case series | BNT162b2 (mRNA) vaccine | 5 days 1–3 days | ||||
| 50 yrs 43 yrs Case report | AstraZeneca | Sudden onset blurry vision | 4 days 3 days | Intravitreal ranibizumab Close follow up | Sonawane NJ, et al. [98] | |
| 66 yrs 51 yrs 66 yrs 54 yrs Case series | AstraZeneca | Painless vision loss, cotton wool spots on examination | 16 days 6 days 4 days 10 days | Intravitreal steroids | Da Silva LSC, et al. [99] | |
| 71 yrs 72 yrs | BNT162b2 (mRNA) vaccine | Blurry vision, decreased BCVA | 1 day 1 day | Intravitreal ranibizumab | Tanaka H, et al. [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, U.P.S.; Surico, P.L.; Singh, R.B.; Musa, M.; Scarabosio, A.; Surico, G.; Maniaci, A.; Lavalle, S.; D’Esposito, F.; Longo, A.; et al. Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events. J. Pers. Med. 2024, 14, 780. https://doi.org/10.3390/jpm14080780
Parmar UPS, Surico PL, Singh RB, Musa M, Scarabosio A, Surico G, Maniaci A, Lavalle S, D’Esposito F, Longo A, et al. Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events. Journal of Personalized Medicine. 2024; 14(8):780. https://doi.org/10.3390/jpm14080780
Chicago/Turabian StyleParmar, Uday Pratap Singh, Pier Luigi Surico, Rohan Bir Singh, Mutali Musa, Anna Scarabosio, Giorgio Surico, Antonino Maniaci, Salvatore Lavalle, Fabiana D’Esposito, Antonio Longo, and et al. 2024. "Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events" Journal of Personalized Medicine 14, no. 8: 780. https://doi.org/10.3390/jpm14080780
APA StyleParmar, U. P. S., Surico, P. L., Singh, R. B., Musa, M., Scarabosio, A., Surico, G., Maniaci, A., Lavalle, S., D’Esposito, F., Longo, A., Russo, A., Gagliano, C., & Zeppieri, M. (2024). Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events. Journal of Personalized Medicine, 14(8), 780. https://doi.org/10.3390/jpm14080780











