A Genome-Wide Association Study of Oxypurinol Concentrations in Patients Treated with Allopurinol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Selection
2.2. Study Endpoints
2.3. Quantification of Oxypurinol, Allopurinol, and Allopurinol-Riboside Plasma Concentrations
2.4. Genotyping Quality Control and Imputation
2.5. Statistical Analyses
3. Results
3.1. Study Cohort
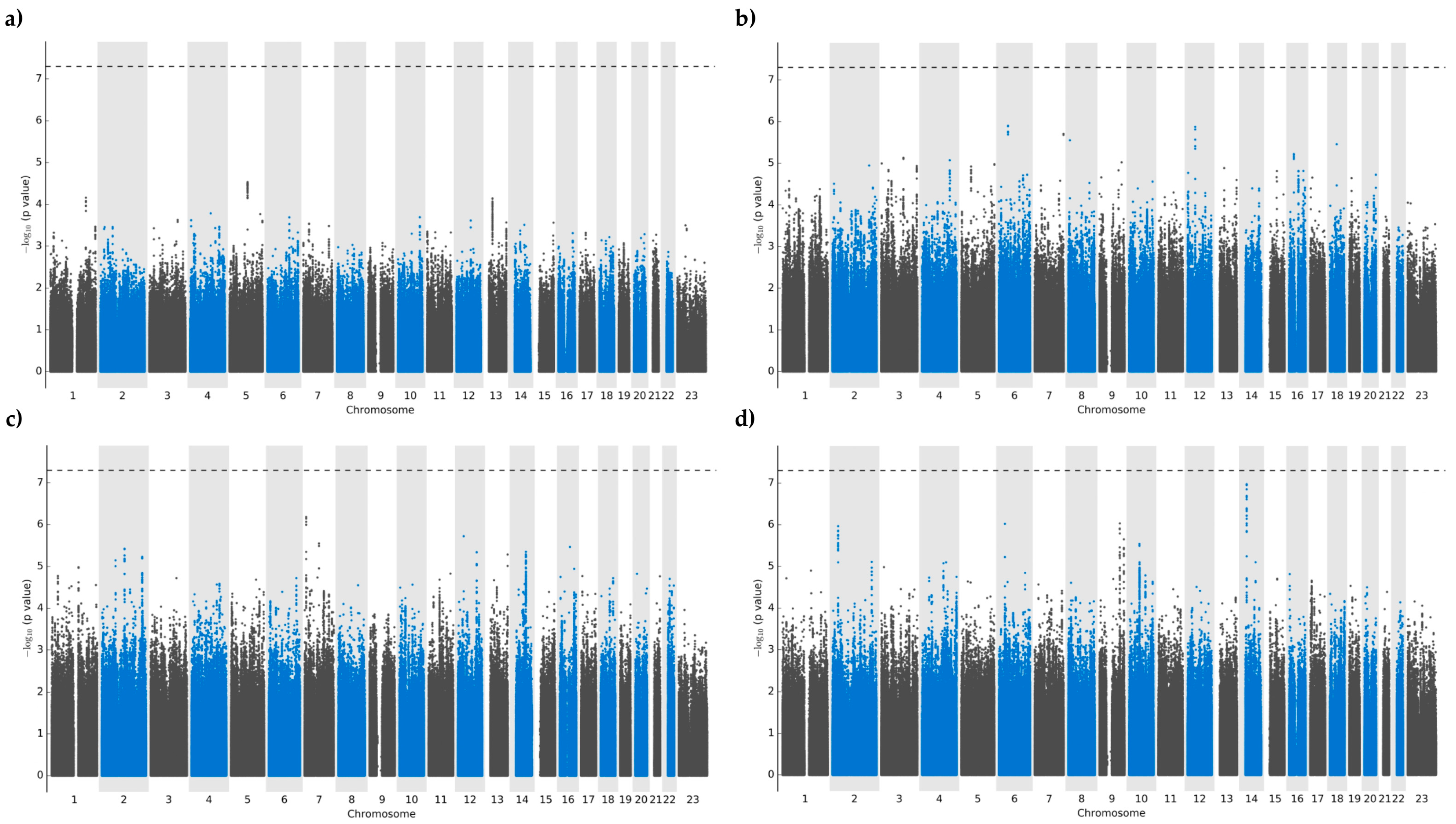
3.2. Genome-Wide Association Analyses: Allopurinol Metabolism and Dosing
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalbeth, N.; Choi, H.K.; Joosten, L.A.B.; Khanna, P.P.; Matsuo, H.; Perez-Ruiz, F.; Stamp, L.K. Gout. Nat. Rev. Dis. Primers 2019, 5, 69. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options. BMC Med. 2017, 15, 123. [Google Scholar] [CrossRef]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef]
- Kuo, C.F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Comorbidities in patients with gout prior to and following diagnosis: Case-control study. Ann. Rheum. Dis. 2016, 75, 210–217. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Joey, T.; Michael, R.K. Targeting uric acid levels in treating gout. Can. Fam. Physician 2020, 66, 671. [Google Scholar]
- Hui, M.; Carr, A.; Cameron, S.; Davenport, G.; Doherty, M.; Forrester, H.; Jenkins, W.; Jordan, K.M.; Mallen, C.D.; McDonald, T.M.; et al. The British Society for Rheumatology Guideline for the Management of Gout. Rheumatology 2017, 56, 1056–1059. [Google Scholar] [CrossRef]
- Kannangara, D.R.W.; Roberts, D.M.; Furlong, T.J.; Graham, G.G.; Williams, K.M.; Day, R.O. Oxypurinol, allopurinol and allopurinol-1-riboside in plasma following an acute overdose of allopurinol in a patient with advanced chronic kidney disease. Br. J. Clin. Pharmacol. 2012, 73, 828–829. [Google Scholar] [CrossRef]
- Day, R.O.; Graham, G.G.; Hicks, M.; McLachlan, A.J.; Stocker, S.L.; Williams, K.M. Clinical Pharmacokinetics and Pharmacodynamics of Allopurinol and Oxypurinol. Clin. Pharmacokinet. 2007, 46, 623–644. [Google Scholar] [CrossRef]
- Turnheim, K.; Krivanek, P.; Oberbauer, R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br. J. Clin. Pharmacol. 1999, 48, 501–509. [Google Scholar] [CrossRef]
- Simmonds, H.A. Urinary excretion of purines, pyrimidines and pyrazolopyrimidines in patients treated with allopurinol or oxipurinol. Clin. Chim. Acta 1969, 23, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, Y.; Yamamoto, T.; Tsutsumi, Z.; Takahashi, S.; Hada, T. Effects of angiotensin II infusion on renal excretion of purine bases and oxypurinol. Metabolism 2002, 51, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Richman, J.; Yang, S.; Bridges, S.L.; Saag, K. Allopurinol adherence and its predictors in gout: A national cohort study in US veterans. Lancet Rheumatol. 2020, 2, e281–e291. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.; Tomlinson, G.A.; Lipscombe, L.L.; Perkins, B.A.; Hawker, G.A. Allopurinol adherence, persistence and patterns of use in individuals with diabetes and gout: A retrospective, population-based cohort analysis. Semin. Arthritis Rheum. 2021, 51, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Merriman, T.R.; Barclay, M.L.; Singh, J.A.; Roberts, R.L.; Wright, D.F.; Dalbeth, N. Impaired response or insufficient dosage? Examining the potential causes of “inadequate response” to allopurinol in the treatment of gout. Semin. Arthritis Rheum. 2014, 44, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Chapman, P.T.; Barclay, M.; Horne, A.; Frampton, C.; Merriman, T.R.; Wright, D.F.B.; Drake, J.; Dalbeth, N. Relationships Between Allopurinol Dose, Oxypurinol Concentration and Urate-Lowering Response-In Search of a Minimum Effective Oxypurinol Concentration. Clin. Transl. Sci 2020, 13, 110–115. [Google Scholar] [CrossRef]
- Stamp, L.K.; Chapman, P.T.; Barclay, M.; Horne, A.; Frampton, C.; Tan, P.; Drake, J.; Dalbeth, N. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: A post hoc analysis of a randomized controlled trial. Arthritis Res. Ther. 2017, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Chapman, P.T.; Barclay, M.L.; Horne, A.; Frampton, C.; Tan, P.; Drake, J.; Dalbeth, N. How much allopurinol does it take to get to target urate? Comparison of actual dose with creatinine clearance-based dose. Arthritis Res. Ther. 2018, 20, 255. [Google Scholar] [CrossRef]
- Graham, G.G.; Kannangara, D.R.W.; Stocker, S.L.; Portek, I.; Pile, K.D.; Indraratna, P.L.; Datta, I.; Williams, K.M.; Day, R.O. Understanding the dose–response relationship of allopurinol: Predicting the optimal dosage. Br. J. Clin. Pharmacol. 2013, 76, 932–938. [Google Scholar] [CrossRef]
- Tohkin, M.; Kaniwa, N.; Saito, Y.; Sugiyama, E.; Kurose, K.; Nishikawa, J.; Hasegawa, R.; Aihara, M.; Matsunaga, K.; Abe, M.; et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenom. J. 2013, 13, 60–69. [Google Scholar] [CrossRef]
- Jarjour, S.; Barrette, M.; Normand, V.; Rouleau, J.L.; Dubé, M.P.; de Denus, S. Genetic markers associated with cutaneous adverse drug reactions to allopurinol: A systematic review. Pharmacogenomics 2015, 16, 755–767. [Google Scholar] [CrossRef]
- Ko, T.M.; Tsai, C.Y.; Chen, S.Y.; Chen, K.S.; Yu, K.H.; Chu, C.S.; Huang, C.M.; Wang, C.R.; Weng, C.T.; Yu, C.L.; et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ 2015, 351, h4848. [Google Scholar] [CrossRef]
- Wang, C.-W.; Preclaro, I.A.C.; Lin, W.-H.; Chung, W.-H. An Updated Review of Genetic Associations With Severe Adverse Drug Reactions: Translation and Implementation of Pharmacogenomic Testing in Clinical Practice. Front. Pharmacol. 2022, 13, 886377. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Yamamoto, K.; Nakaoka, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Takahashi, A.; Nakamura, T.; Nakashima, H.; Takada, Y.; et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann. Rheum. Dis. 2016, 75, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, F.; Zhang, R.; Tang, S.S.; Chen, M.; Peng, D.F.; Yan, J.; Wang, T.; Wang, S.Y.; Bao, Y.Q.; et al. Serum uric acid levels are associated with polymorphisms in the SLC2A9, SF1, and GCKR genes in a Chinese population. Acta Pharmacol. Sin. 2014, 35, 1421–1427. [Google Scholar] [CrossRef]
- Köttgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggiero, D.; O'Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef]
- Kolz, M.; Johnson, T.; Sanna, S.; Teumer, A.; Vitart, V.; Perola, M.; Mangino, M.; Albrecht, E.; Wallace, C.; Farrall, M.; et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009, 5, e1000504. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, A.; Köttgen, A.; Yang, Q.; Hwang, S.J.; Kao, W.L.; Rivadeneira, F.; Boerwinkle, E.; Levy, D.; Hofman, A.; Astor, B.C.; et al. Association of three genetic loci with uric acid concentration and risk of gout: A genome-wide association study. Lancet 2008, 372, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.C.; Roberts, R.L.; Nanavati, P.; Miner, J.N.; Dalbeth, N.; Topless, R.; Merriman, T.R.; Stamp, L.K. Association between ABCG2 rs2231142 and poor response to allopurinol: Replication and meta-analysis. Rheumatology 2018, 57, 656–660. [Google Scholar] [CrossRef]
- Wen, C.; Yee, S.; Liang, X.; Hoffmann, T.; Kvale, M.; Banda, Y.; Jorgenson, E.; Schaefer, C.; Risch, N.; Giacomini, K. Genome-wide association study identifies ABCG2 (BCRP) as an allopurinol transporter and a determinant of drug response. Clin. Pharmacol. Ther. 2015, 97, 518–525. [Google Scholar] [CrossRef]
- Vora, B.; Brackman, D.J.; Zou, L.; Garcia-Cremades, M.; Sirota, M.; Savic, R.M.; Giacomini, K.M. Oxypurinol pharmacokinetics and pharmacodynamics in healthy volunteers: Influence of BCRP Q141K polymorphism and patient characteristics. Clin. Transl. Sci. 2021, 14, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Hollis-Moffatt, J.E.; Phipps-Green, A.J.; Chapman, B.; Jones, G.T.; van Rij, A.; Gow, P.J.; Harrison, A.A.; Highton, J.; Jones, P.B.; Montgomery, G.W.; et al. The renal urate transporter SLC17A1 locus: Confirmation of association with gout. Arthritis Res. Ther. 2012, 14, R92. [Google Scholar] [CrossRef] [PubMed]
- Brackman, D.J.; Yee, S.W.; Enogieru, O.J.; Shaffer, C.; Ranatunga, D.; Denny, J.C.; Wei, W.-Q.; Kamatani, Y.; Kubo, M.; Roden, D.M.; et al. Genome-Wide Association and Functional Studies Reveal Novel Pharmacological Mechanisms for Allopurinol. Clin. Pharmacol. Ther. 2019, 106, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Pardiñas, A.F.; Nalmpanti, M.; Pocklington, A.J.; Legge, S.E.; Medway, C.; King, A.; Jansen, J.; Helthuis, M.; Zammit, S.; MacCabe, J.; et al. Pharmacogenomic Variants and Drug Interactions Identified Through the Genetic Analysis of Clozapine Metabolism. Am. J. Psychiatry 2019, 176, 477–486. [Google Scholar] [CrossRef]
- Oetting, W.S.; Wu, B.; Schladt, D.P.; Guan, W.; van Setten, J.; Keating, B.J.; Iklé, D.; Remmel, R.P.; Dorr, C.R.; Mannon, R.B.; et al. Genetic Variants Associated With Immunosuppressant Pharmacokinetics and Adverse Effects in the DeKAF Genomics Genome-wide Association Studies. Transplantation 2019, 103, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Chami, N.; Chen, M.H.; Slater, A.J.; Eicher, J.D.; Evangelou, E.; Tajuddin, S.M.; Love-Gregory, L.; Kacprowski, T.; Schick, U.M.; Nomura, A.; et al. Exome Genotyping Identifies Pleiotropic Variants Associated with Red Blood Cell Traits. Am. J. Hum. Genet. 2016, 99, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Turcot, V.; Brunet, J.; Daneault, C.; Tardif, J.C.; Des Rosiers, C.; Lettre, G. Validation of fatty acid intakes estimated by a food frequency questionnaire using erythrocyte fatty acid profiling in the Montreal Heart Institute Biobank. J. Hum. Nutr. Diet. 2015, 28, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Pilon, M.-O.; Leclair, G.; Oussaïd, E.; St-Jean, I.; Jutras, M.; Gaulin, M.-J.; Mongrain, I.; Busseuil, D.; Rouleau, J.L.; Tardif, J.-C.; et al. An association study of ABCG2 rs2231142 on the concentrations of allopurinol and its metabolites. Clin. Transl. Sci. 2022, 15, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nieuwenhuis, L.M.; Keating, B.J.; Festen, E.A.M.; de Meijer, V.E. The Impact of Donor and Recipient Genetic Variation on Outcomes After Solid Organ Transplantation: A Scoping Review and Future Perspectives. Transplantation 2022, 106, 1548–1557. [Google Scholar] [CrossRef]
- Monek, O.; Paintaud, G.; Bechtel, Y.; Miguet, J.P.; Mantion, G.; Bechtel, P.R. Influence of donor and recipient genotypes on CYP2D6 phenotype after liver transplantation: A study of mutations CYP2D6*3 and CYP2D6*4. Eur. J. Clin. Pharmacol. 1998, 54, 47–52. [Google Scholar] [CrossRef]
- Laverdière, J.; Meloche, M.; Provost, S.; Leclair, G.; Oussaïd, E.; Jutras, M.; Perreault, L.L.; Valois, D.; Mongrain, I.; Busseuil, D.; et al. Pharmacogenomic markers of metoprolol and α-OH-metoprolol concentrations: A genome-wide association study. Pharmacogenomics 2023, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lemieux Perreault, L.P.; Provost, S.; Legault, M.A.; Barhdadi, A.; Dubé, M.P. pyGenClean: Efficient tool for genetic data clean up before association testing. Bioinformatics 2013, 29, 1704–1705. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Loh, P.R.; Danecek, P.; Palamara, P.F.; Fuchsberger, C.; A Reshef, Y.; K Finucane, H.; Schoenherr, S.; Forer, L.; McCarthy, S.; Abecasis, G.R.; et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016, 48, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Nielsen, J.B.; Fritsche, L.G.; Dey, R.; Gabrielsen, M.E.; Wolford, B.N.; LeFaive, J.; VandeHaar, P.; Gagliano, S.A.; Gifford, A.; et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 2018, 50, 1335–1341. [Google Scholar] [CrossRef]
- Becker, M.A.; Schumacher, H.R.; Espinoza, L.R.; Wells, A.F.; MacDonald, P.; Lloyd, E.; Lademacher, C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: The CONFIRMS trial. Arthritis Res. Ther. 2010, 12, R63. [Google Scholar] [CrossRef]
- Yang, C.M.; Chang, H.S.; Chen, H.C.; You, J.J.; Liou, H.H.; Ting, S.C.; Ger, L.P.; Li, S.C.; Tsai, K.W. Low C6orf141 Expression is Significantly Associated with a Poor Prognosis in Patients with Oral Cancer. Sci. Rep. 2019, 9, 4520. [Google Scholar] [CrossRef]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Güneş, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef]
- Boocock, J.; Leask, M.; Okada, Y.; Matsuo, H.; Kawamura, Y.; Shi, Y.; Li, C.; Mount, D.B.; Mandal, A.K.; Wang, W.; et al. Genomic dissection of 43 serum urate-associated loci provides multiple insights into molecular mechanisms of urate control. Hum. Mol. Genet. 2020, 29, 923–943. [Google Scholar] [CrossRef]
- Cho, C.; Kim, B.; Kim, D.S.; Hwang, M.Y.; Shim, I.; Song, M.; Lee, Y.C.; Jung, S.-H.; Cho, S.K.; Park, W.-Y.; et al. Large-scale cross-ancestry genome-wide meta-analysis of serum urate. Nat. Commun. 2024, 15, 3441. [Google Scholar] [CrossRef]
- Takei, R.; Cadzow, M.; Markie, D.; Bixley, M.; Phipps-Green, A.; Major, T.J.; Li, C.; Choi, H.K.; Li, Z.; Hu, H.; et al. Trans-ancestral dissection of urate- and gout-associated major loci SLC2A9 and ABCG2 reveals primate-specific regulatory effects. J. Hum. Genet. 2021, 66, 161–169. [Google Scholar] [CrossRef]
- Okada, Y.; Sim, X.; Go, M.J.; Wu, J.Y.; Gu, D.; Takeuchi, F.; Takahashi, A.; Maeda, S.; Tsunoda, T.; Chen, P.; et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 2012, 44, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Wallace, M.C.; Phipps-Green, A.J.; Topless, R.; Drake, J.M.; Tan, P.; Dalbeth, N.; Merriman, T.R.; Stamp, L.K. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenom. J 2017, 17, 201–203. [Google Scholar] [CrossRef]
- Hishe, H.Z.; Stocker, S.L.; Stamp, L.K.; Dalbeth, N.; Merriman, T.R.; Phipps-Green, A.; Wright, D.F.B. The impact of genetic variability in urate transporters on oxypurinol pharmacokinetics. Clin. Transl. Sci. 2023, 16, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.E.; Albrecht, E.; Teumer, A.; Mangino, M.; Kapur, K.; Johnson, T.; Kutalik, Z.; Pirastu, N.; Pistis, G.; Lopez, L.M.; et al. Modulation of Genetic Associations with Serum Urate Levels by Body-Mass-Index in Humans. PLoS ONE 2015, 10, e0119752. [Google Scholar] [CrossRef]
- Cleophas, M.C.; Joosten, L.A.; Stamp, L.K.; Dalbeth, N.; Woodward, O.M.; Merriman, T.R. ABCG2 polymorphisms in gout: Insights into disease susceptibility and treatment approaches. Pharmgenom. Pers. Med. 2017, 10, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jarmuzewska, E.A. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: A critical appraisal of the evidence. Br. J. Clin. Pharmacol. 2019, 85, 20–36. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Kappel, D.B.; Roberts, M.; Tipple, F.; Shitomi-Jones, L.M.; King, A.; Jansen, J.; Helthuis, M.; Owen, M.J.; O'Donovan, M.C.; et al. Pharmacokinetics and pharmacogenomics of clozapine in an ancestrally diverse sample: A longitudinal analysis and genome-wide association study using UK clinical monitoring data. Lancet Psychiatry 2023, 10, 209–219. [Google Scholar] [CrossRef]
| Characteristics | n = 439 (100%) 1 |
|---|---|
| Age (years) | 69.4 (8.0) |
| Females, n (%) | 64 (14.6) |
| Smoking status, n (%) | |
| Never-smoker | 117 (26.7) |
| Past-smoker | 301 (68.6) |
| Current-smoker | 21 (4.8) |
| Weight (kg) | 90.1 (18.3) |
| BMI | 31.4 (5.5) |
| Hypertension, n (%) | 379 (86.3) |
| Diabetes mellitus, n (%) | |
| Type 1 | 1 (0.2) |
| Type 2 | 182 (41.5) |
| Dyslipidemia, n (%) | 383 (87.6) |
| Myocardial infarction, n (%) | 173 (39.7) |
| Chronic heart failure, n (%) | 113 (25.9) |
| Chronic renal failure, n (%) | 115 (26.2) |
| Analyte concentrations | |
| Mean daily allopurinol dose (mg) | 194.5 (77.1) |
| Mean quantifiable oxypurinol plasma concentrations (ng/mL) | 13,374.4 (8,656.6) |
| Mean allopurinol plasma concentrations (ng/mL) | 277.6 (358.1) |
| Mean allopurinol-riboside plasma concentrations (ng/mL) | 228.3 (206.3) |
| Concomitant medications, n (%) | |
| Aspirin | 307 (70.1) |
| Other antiplatelet agents | 62 (14.2) |
| ACE inhibitors | 159 (36.2) |
| Angiotensin II receptor blockers | 172 (39.2) |
| Beta-blockers | 315 (71.8) |
| Calcium channel blockers | 149 (33.9) |
| Amiodarone | 20 (4.6) |
| Warfarin | 116 (26.4) |
| Novel oral anticoagulants | 19 (4.3) |
| Digoxin | 54 (12.3) |
| Diuretics | 263 (59.9) |
| Statins | 356 (81.1) |
| Fibrates | 16 (3.6) |
| Other hypolipidemic agents | 53 (12.1) |
| Oral hypoglycemic agents | 159 (36.3) |
| Insulin | 34 (7.7) |
| Serum creatinine (n = 391, 89.1%) | |
| Concentrations (µmol/L) | 118.7 (54.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloche, M.; Pilon, M.-O.; Provost, S.; Leclair, G.; Oussaïd, E.; St-Jean, I.; Jutras, M.; Gaulin, M.-J.; Lemieux Perreault, L.-P.; Valois, D.; et al. A Genome-Wide Association Study of Oxypurinol Concentrations in Patients Treated with Allopurinol. J. Pers. Med. 2024, 14, 649. https://doi.org/10.3390/jpm14060649
Meloche M, Pilon M-O, Provost S, Leclair G, Oussaïd E, St-Jean I, Jutras M, Gaulin M-J, Lemieux Perreault L-P, Valois D, et al. A Genome-Wide Association Study of Oxypurinol Concentrations in Patients Treated with Allopurinol. Journal of Personalized Medicine. 2024; 14(6):649. https://doi.org/10.3390/jpm14060649
Chicago/Turabian StyleMeloche, Maxime, Marc-Olivier Pilon, Sylvie Provost, Grégoire Leclair, Essaïd Oussaïd, Isabelle St-Jean, Martin Jutras, Marie-Josée Gaulin, Louis-Philippe Lemieux Perreault, Diane Valois, and et al. 2024. "A Genome-Wide Association Study of Oxypurinol Concentrations in Patients Treated with Allopurinol" Journal of Personalized Medicine 14, no. 6: 649. https://doi.org/10.3390/jpm14060649
APA StyleMeloche, M., Pilon, M.-O., Provost, S., Leclair, G., Oussaïd, E., St-Jean, I., Jutras, M., Gaulin, M.-J., Lemieux Perreault, L.-P., Valois, D., Mongrain, I., Busseuil, D., Rouleau, J.-L., Tardif, J.-C., Dubé, M.-P., & de Denus, S. (2024). A Genome-Wide Association Study of Oxypurinol Concentrations in Patients Treated with Allopurinol. Journal of Personalized Medicine, 14(6), 649. https://doi.org/10.3390/jpm14060649






