Quality of Postoperative Recovery in Total Intravenous Anesthesia between Remimazolam and Propofol for Intraoperative Neurophysiological Monitoring: A Prospective Double-Blind Randomized Controlled Trial
Abstract
1. Introduction
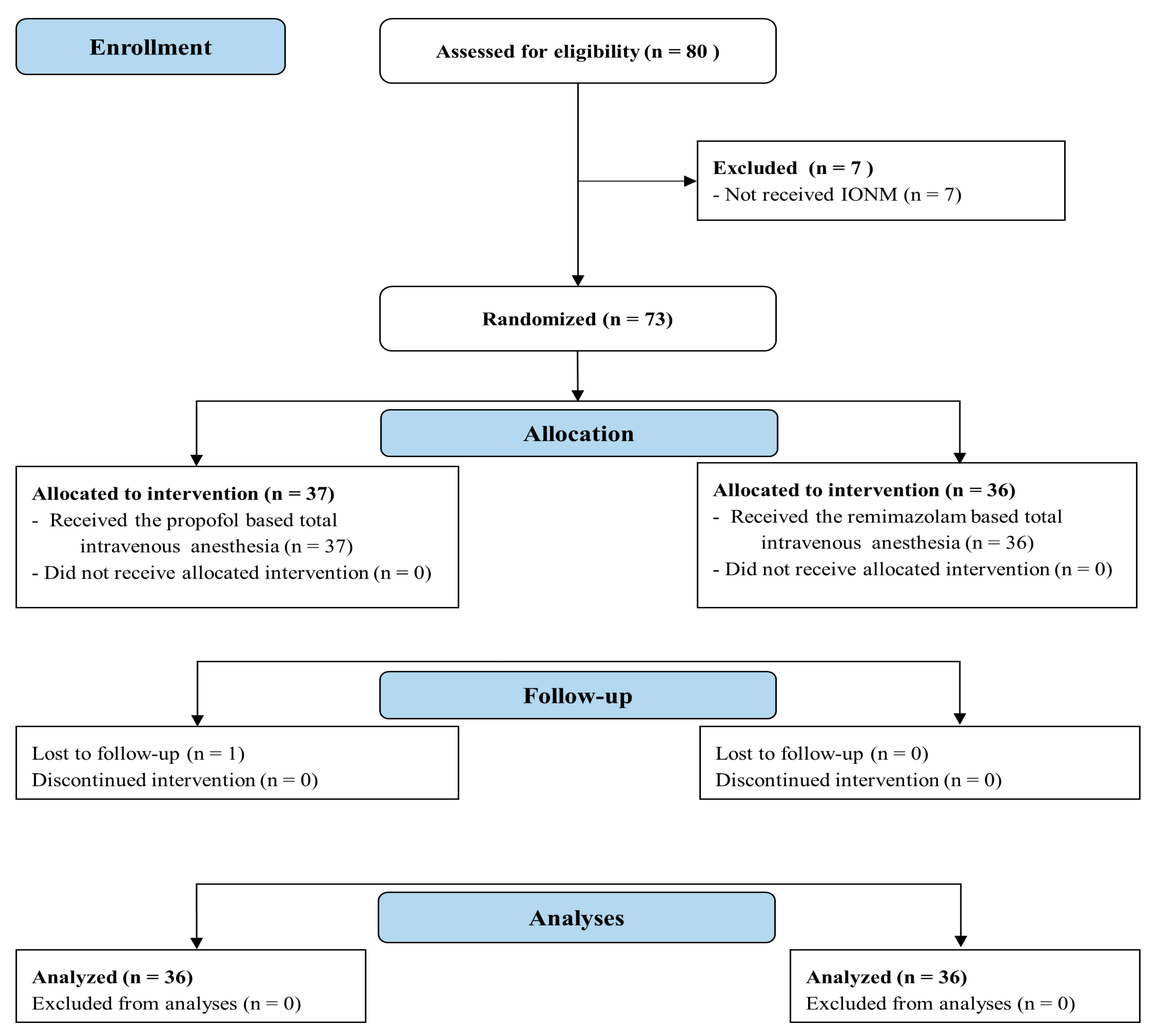
2. Materials and Methods
2.1. Participants Assignment
2.2. Intervention
2.3. Outcome Assessments
2.4. Statistical Analyses
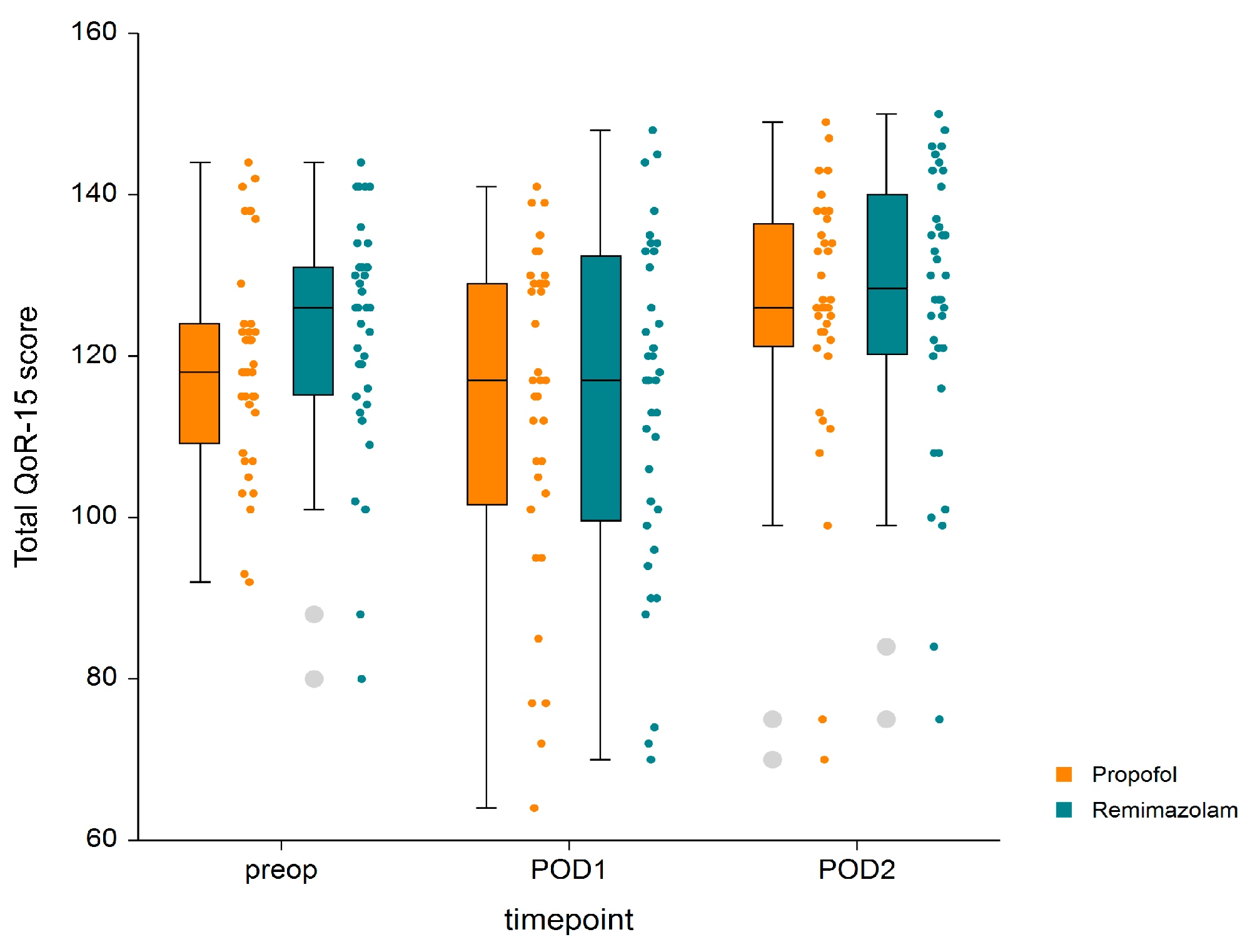
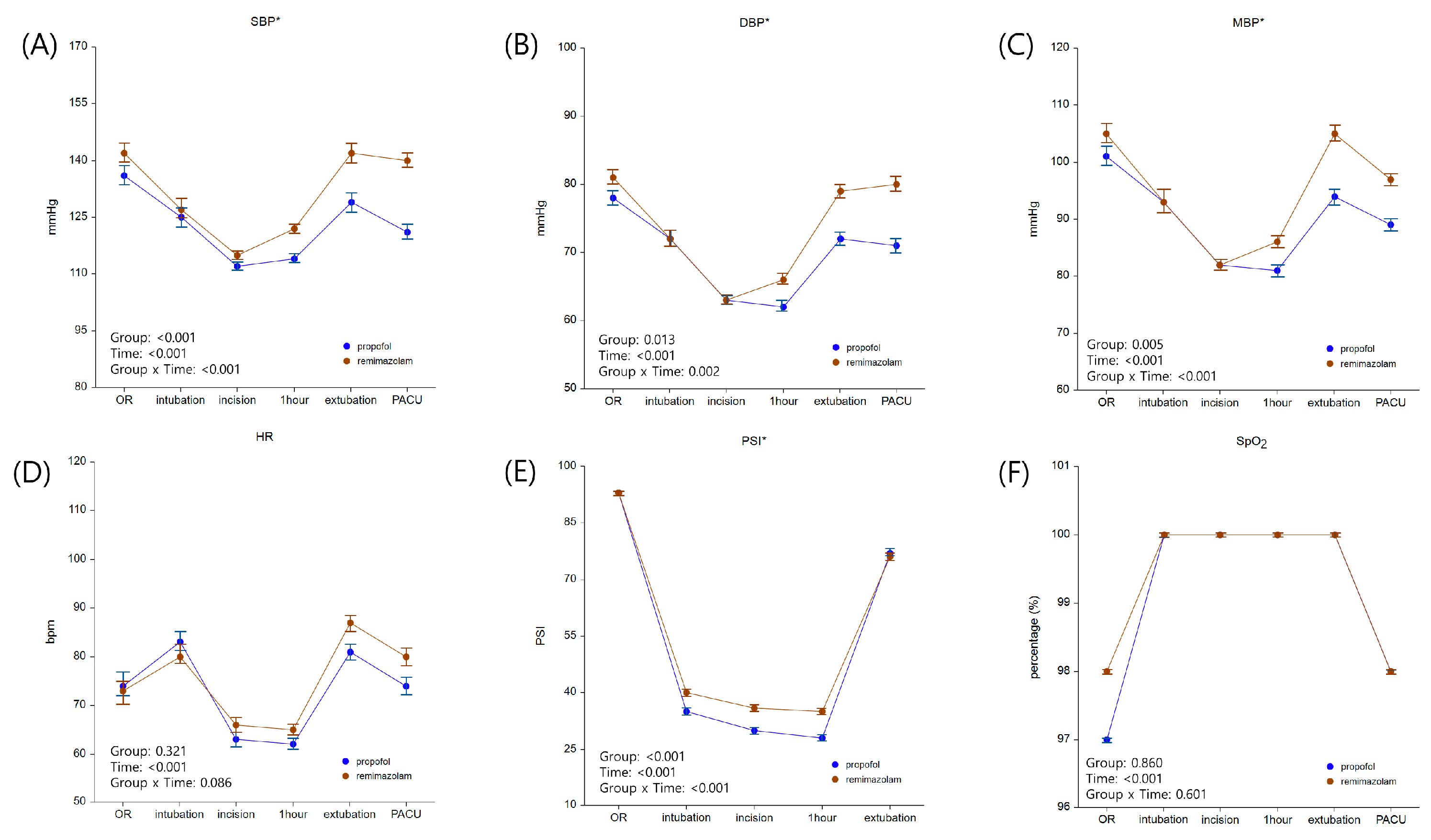
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain. Med. 2022, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, T.; Colin, P.J.; Ossig, J.; Pesic, M.; Borkett, K.; Winkle, P.; Struys, M.; Schippers, F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br. J. Anaesth. 2021, 127, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R.; Gambus, P.L.; Rigby-Jones, A.E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: A narrative review. Br. J. Anaesth. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ickx, B.; Cockshott, I.; Barvais, L.; Byttebier, G.; Pauw, L.; Vandesteene, A.; D’Hollander, A. Propofol infusion for induction and maintenance of anaesthesia in patients with end-stage renal disease. Br. J. Anaesth. 1998, 81, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Kim, M.A.; Eom, D.W.; Jung, J.W.; Chung, C.J.; Park, S.Y. Comparison of remimazolam-remifentanil and propofol-remifentanil during laparoscopic cholecystectomy. Anesth Pain Med. 2023, 18, 252–259. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.S.; Kim, J.Y.; Han, D.W.; Yang, J.Y.; Kim, M.J.; Song, Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J. Clin. Anesth. 2022, 82, 110955. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, A.; Royse, C. The importance of postoperative quality of recovery: Influences, assessment, and clinical and prognostic implications. Can. J. Anaesth. 2016, 63, 176–183. [Google Scholar] [CrossRef]
- Yoon, S.; Joo, H.; Oh, Y.M.; Lee, J.; Bahk, J.H.; Lee, H.J. Validation and clinical utility of the Korean version of the Quality of Recovery-15 with enhanced recovery after surgery: A prospective observational cohort study. Br. J. Anaesth. 2020, 125, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S. Measuring quality of recovery in perioperative clinical trials. Curr. Opin. Anaesthesiol. 2018, 31, 396–401. [Google Scholar] [CrossRef]
- Kleif, J.; Waage, J.; Christensen, K.B.; Gogenur, I. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br. J. Anaesth. 2018, 120, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.G.; Chung, C.K.E.; Ip, K.Y.; Wiles, M.D. Influence of propofol-based total intravenous anaesthesia on peri-operative outcome measures: A narrative review. Anaesthesia 2020, 75 (Suppl. S1), e90–e100. [Google Scholar] [CrossRef] [PubMed]
- Schraag, S.; Pradelli, L.; Alsaleh, A.J.O.; Bellone, M.; Ghetti, G.; Chung, T.L.; Westphal, M.; Rehberg, S. Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: A systematic review and meta-analysis. BMC Anesthesiol. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Bithal, P.K. Anaesthetic considerations for evoked potentials monitoring. J. Neuroanaesth Crit. Care 2014, 01, 002–012. [Google Scholar] [CrossRef]
- Ravussin, P.; de Tribolet, N.; Wilder-Smith, O.H. Total intravenous anesthesia is best for neurological surgery. J. Neurosurg. Anesthesiol. 1994, 6, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Sahinovic, M.M.; Struys, M.; Absalom, A.R. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.; Rasulo, F.; Candiani, A.; Latronico, N. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003, 29, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Onsiong, M.K. Pain on injection of propofol. Anaesthesia 1998, 53, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Schnider, T.W.; Minto, C.F.; Shafer, S.L.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Youngs, E.J. The influence of age on propofol pharmacodynamics. Anesthesiology 1999, 90, 1502–1516. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef]
- Eleveld, D.J.; Colin, P.; Absalom, A.R.; Struys, M. Target-controlled-infusion models for remifentanil dosing consistent with approved recommendations. Br. J. Anaesth. 2020, 125, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.F.; Absalom, A.R.; Bagshaw, O.; Biswas, A.; Cook, T.M.; Costello, A.; Grimes, S.; Mulvey, D.; Shinde, S.; Whitehouse, T.; et al. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia 2019, 74, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Lohmer, L.L.; Schippers, F.; Petersen, K.U.; Stoehr, T.; Schmith, V.D. Time-to-Event Modeling for Remimazolam for the Indication of Induction and Maintenance of General Anesthesia. J. Clin. Pharmacol. 2020, 60, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Chew, C.; MacDonald, N.; Dennis, A. Minimal Clinically Important Difference for Three Quality of Recovery Scales. Anesthesiology 2016, 125, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Brodke, D.S.; Norvell, D.C.; Dettori, J.R. The evidence for intraoperative neurophysiological monitoring in spine surgery: Does it make a difference? Spine 2010, 35, S37–S46. [Google Scholar] [CrossRef] [PubMed]
- Banoub, M.; Tetzlaff, J.E.; Schubert, A. Pharmacologic and physiologic influences affecting sensory evoked potentials: Implications for perioperative monitoring. Anesthesiology 2003, 99, 716–737. [Google Scholar] [CrossRef] [PubMed]
- Clapcich, A.J.; Emerson, R.G.; Roye, D.P., Jr.; Xie, H.; Gallo, E.J.; Dowling, K.C.; Ramnath, B.; Heyer, E.J. The effects of propofol, small-dose isoflurane, and nitrous oxide on cortical somatosensory evoked potential and bispectral index monitoring in adolescents undergoing spinal fusion. Anesth. Analg. 2004, 99, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Guo, J.; Yuan, J.; Zhao, E.; Yang, J. Quality of Recovery After General Anesthesia with Remimazolam in Patients’ Undergoing Urologic Surgery: A Randomized Controlled Trial Comparing Remimazolam with Propofol. Drug Des. Dev. Ther. 2022, 16, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.A.; Myles, P.S.; Burke, J.A. Development and psychometric evaluation of a postoperative quality of recovery score: The QoR-15. Anesthesiology 2013, 118, 1332–1340. [Google Scholar] [CrossRef]
- Masui, K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia! J. Anesth. 2020, 34, 479–482. [Google Scholar] [CrossRef]
- Lee, J.H.; Ki, M.; Choi, S.; Woo, C.J.; Kim, D.; Lim, H.; Kim, D.C. Validity and reliability of the Korean version of the Quality of Recovery-15 questionnaire. Korean J. Anesthesiol. 2021, 74, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Seo, D.; Son, J.S.; Kim, D.C. Validity and reliability of the Korean version of the Quality of Recovery-40 questionnaire. Korean J. Anesthesiol. 2018, 71, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Urabe, T.; Miyoshi, H.; Narasaki, S.; Yanase, Y.; Uchida, K.; Noguchi, S.; Hide, M.; Tsutsumi, Y.M.; Sakai, N. Characterization of intracellular calcium mobilization induced by remimazolam, a newly approved intravenous anesthetic. PLoS ONE 2022, 17, e0263395. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Gu, W.; Zhao, M.; Zhang, Y.; Wu, J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: A randomized trial. Front. Med. 2022, 9, 938940. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, M.H.; Kong, H.J.; Shin, H.J.; Yang, S.; Kim, N.Y.; Chae, D. Effects of Remimazolam vs. Sevoflurane Anesthesia on Intraoperative Hemodynamics in Patients with Gastric Cancer Undergoing Robotic Gastrectomy: A Propensity Score-Matched Analysis. J. Clin. Med. 2022, 11, 2643. [Google Scholar] [CrossRef]
- Lee, C.; Lim, J.; Hong, H.; Yu, H.; Lee, H. Effect of Remimazolam on Pain Perception and Opioid-Induced Hyperalgesia in Patients Undergoing Laparoscopic Urologic Surgery—A Prospective, Randomized, Controlled Study. Medicina 2024, 9, 123. [Google Scholar] [CrossRef]
| Group P (n = 36) | Group R (n = 36) | Standardized Difference | |
|---|---|---|---|
| Sex (male/female) | 23/13 | 19/17 | 0.227 |
| Age (years) | 50.3 [33–66] | 54.2 [27–66] | 0.447 |
| Height (cm) | 168.0 (6.2) | 165.8 (9.5) | 0.272 |
| Weight (kg) | 65.5 (11.6) | 65.0 (9.4) | 0.050 |
| Diagnosis | |||
| Stenosis | 17 (47.2%) | 14 (38.9%) | 0.169 |
| Disc herniation | 8 (22.2%) | 9 (25.0%) | 0.065 |
| Cervical myelopathy | 3 (8.3%) | 7 (19.4) | 0.326 |
| OPLL | 3 (8.3%) | 3 (8.3%) | 0 |
| Others | 5 (13.9%) | 3 (8.3%) | 0.178 |
| Past medical history | |||
| Smoking | 15 (41.7%) | 9 (25.0%) | 0.359 |
| Steroid use | 3 (8.3%) | 2 (5.6%) | 0.109 |
| Psychiatric medication | 3 (8.3%) | 4 (11.1%) | 0.094 |
| Comorbidities | |||
| Hypertension | 9 (25.0%) | 6 (16.7%) | 0.206 |
| DM | 4 (11.1%) | 4 (11.1%) | 0 |
| Cardiac | 0 | 3 (8.3%) | 0.426 |
| Respiratory | 4 (11.1%) | 9 (13.9%) | 0.084 |
| Others | 6 (16.7%) | 4 (11.1%) | 0.161 |
| Group P (n = 36) | Group R (n = 36) | p-Value | |
|---|---|---|---|
| Time to loss of consciousness (s) | 78.1 (30.5) | 77.1 (14.5) | 0.868 |
| Time to PSI < 50 (s) | 146.1 (68.3) | 156.8 (44.2) | 0.433 |
| Duration of anesthesia (min) | 174.1 (43.4) | 176.5 (48.6) | 0.831 |
| Duration of operation (min) | 117.6 (36.0) | 118.2 (41.4) | 0.949 |
| Total input (mL) | 1350.7 (543.9) | 1330.6 (475.1) | 0.809 |
| Total output (mL) | 440.0 (75.0, 1075.0) | 400.0 (100.0, 600.0) | 0.368 |
| Total consumption of remifentanil (μg) * | 1425.4 (410.2) | 2066.8 (680.8) | <0.001 |
| Total amount of phenylephrine (μg) * | 1200.0 (340.0, 2420.0) | 185.0 (0.0, 360.0) | <0.001 |
| Sugammadex | 0 | 1 (2.8%) | >0.999 |
| Flumazenil | 0 | 2 (5.6%) | >0.999 |
| Group P (n = 36) | Group R (n = 36) | p-Value | |
|---|---|---|---|
| After anesthesia completion | |||
| Time to obey command (s) * | 714.0 (254.4) | 594.7 (197.9) | 0.030 |
| Time to PSI > 50 (s) * | 609.9 (243.2) | 366.1 (206.9) | <0.001 |
| Time to extubation (s) | 785.9 (268.6) | 702.1 (239.2) | 0.167 |
| Adverse events related extubation | 0 | 2 (5.6%) | 0.493 |
| In PACU | |||
| NRS of pain intensity (resting) * | 3.0 (0.0) | 2.8 (0.5) | 0.009 |
| NRS of pain intensity (acting) | 5.2 (0.7) | 4.8 (0.9) | 0.086 |
| Requested rescue analgesics | 9 (25%) | 7 (19.4%) | 0.571 |
| Morphine equivalent dose of analgesics (mg) | 1(2.2) | 1.3(2.2) | 0.688 |
| Requested antiemetics | 0 (0%) | 0 (0%) | >0.999 |
| Duration of PACU stay (min) | 43.1 (21.5) | 43.3 (17.9) | 0.953 |
| Postoperative 1–6 h | |||
| NRS of pain intensity (at rest) | 3.4 (0.5) | 3.3 (0.7) | 0.703 |
| NRS of pain intensity (during movement) | 5.3 (0.7) | 5.1 (0.7) | 0.130 |
| Requested rescue analgesics | 2 (5.6%) | 5 (13.9%) | 0.429 |
| Morphine equivalent dose of analgesics (mg) | 0.7(1.8) | 0.3(1.2) | 0.239 |
| Requested antiemetics | 1 (2.8%) | 0 (0%) | 0.239 |
| Postoperative 6–24 h | |||
| NRS of pain intensity (at rest) | 1.9 (0.7) | 1.9 (0.7) | 0.748 |
| NRS of pain intensity (during movement) | 3.8 (1.4) | 3.7 (1.0) | 0.848 |
| Requested rescue analgesics | 3 (8.3%) | 7 (19.4%) | 0.173 |
| Morphine equivalent dose of analgesics (mg) | 1(2) | 0.4(1.4) | 0.178 |
| Requested antiemetics | 3 (8.3%) | 2 (5.6%) | >0.999 |
| Postoperative 24–48 h | |||
| NRS of pain intensity (at rest) | 1.5 (0.6) | 1.3 (0.6) | 0.169 |
| NRS of pain intensity (during movement) | 3.4 (0.9) | 3.1 (0.6) | 0.105 |
| Requested rescue analgesics | 3 (8.3%) | 4 (11.1%) | >0.999 |
| Morphine equivalent dose of analgesics (mg) | 0.6(1.6) | 0.4(1.4) | 0.696 |
| Requested antiemetics | 0 (0%) | 0 (0%) | >0.999 |
| PCA withdrawal due to PONV | 12 (33.3%) | 13 (36.1%) | 0.805 |
| Postoperative adverse events | 1 (2.8%) | 0 | >0.999 |
| Postoperative hospital stay duration | 4.4 (2.4) | 4.2 (1.9) | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Han, D.W.; Song, Y.; Lee, J.; Jeon, S.; Kim, M.H. Quality of Postoperative Recovery in Total Intravenous Anesthesia between Remimazolam and Propofol for Intraoperative Neurophysiological Monitoring: A Prospective Double-Blind Randomized Controlled Trial. J. Pers. Med. 2024, 14, 382. https://doi.org/10.3390/jpm14040382
Lee J, Han DW, Song Y, Lee J, Jeon S, Kim MH. Quality of Postoperative Recovery in Total Intravenous Anesthesia between Remimazolam and Propofol for Intraoperative Neurophysiological Monitoring: A Prospective Double-Blind Randomized Controlled Trial. Journal of Personalized Medicine. 2024; 14(4):382. https://doi.org/10.3390/jpm14040382
Chicago/Turabian StyleLee, Jiwon, Dong Woo Han, Young Song, Jongyun Lee, Soyoung Jeon, and Myoung Hwa Kim. 2024. "Quality of Postoperative Recovery in Total Intravenous Anesthesia between Remimazolam and Propofol for Intraoperative Neurophysiological Monitoring: A Prospective Double-Blind Randomized Controlled Trial" Journal of Personalized Medicine 14, no. 4: 382. https://doi.org/10.3390/jpm14040382
APA StyleLee, J., Han, D. W., Song, Y., Lee, J., Jeon, S., & Kim, M. H. (2024). Quality of Postoperative Recovery in Total Intravenous Anesthesia between Remimazolam and Propofol for Intraoperative Neurophysiological Monitoring: A Prospective Double-Blind Randomized Controlled Trial. Journal of Personalized Medicine, 14(4), 382. https://doi.org/10.3390/jpm14040382





