Functional Status Correlates of Change and Stability in Appraisal after Spine Surgery: Earlier versus Later Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Design
2.2. Measures
2.3. Statistical Analysis
Software
3. Results
3.1. Change-Group Differences in ODI
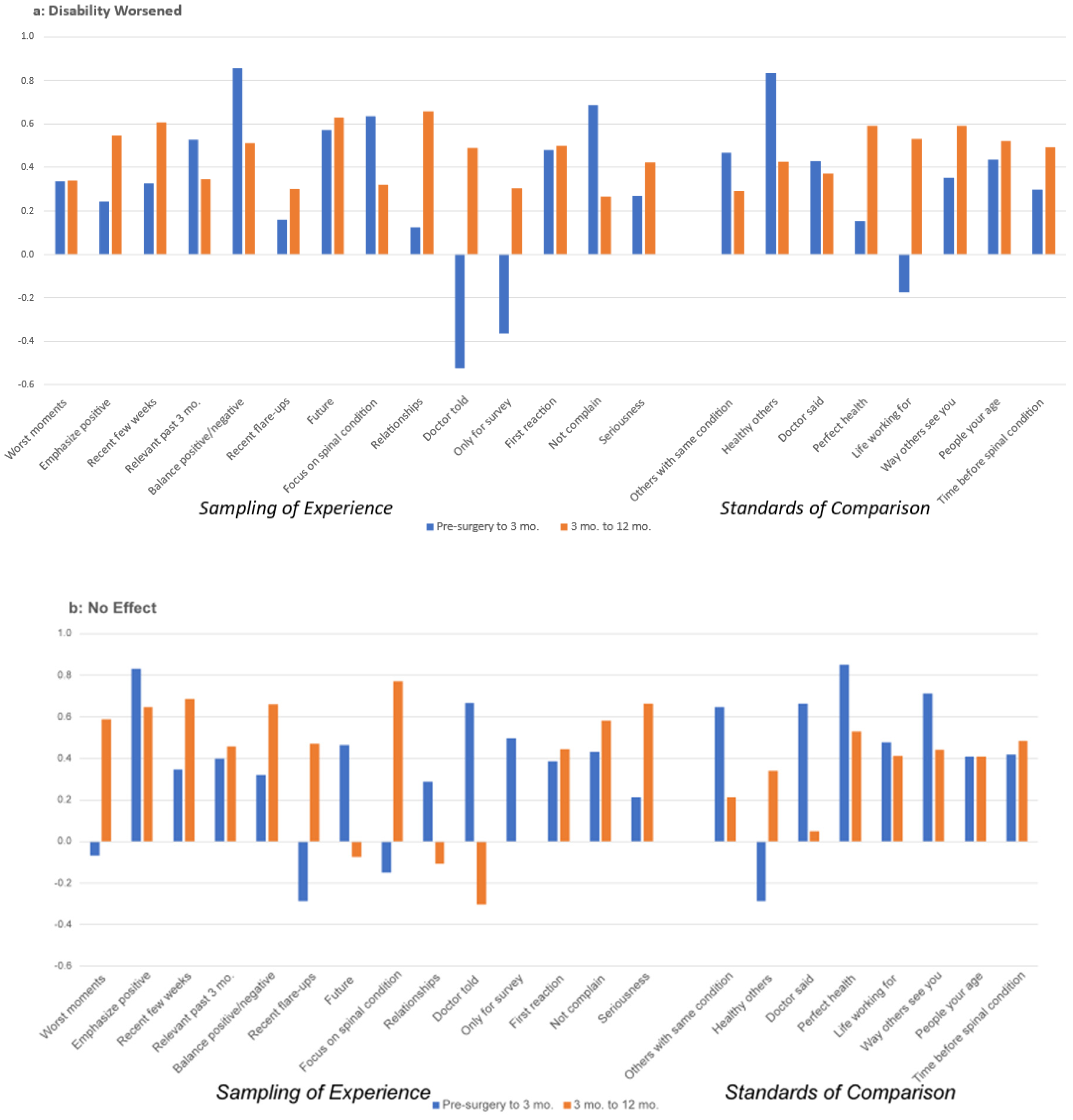
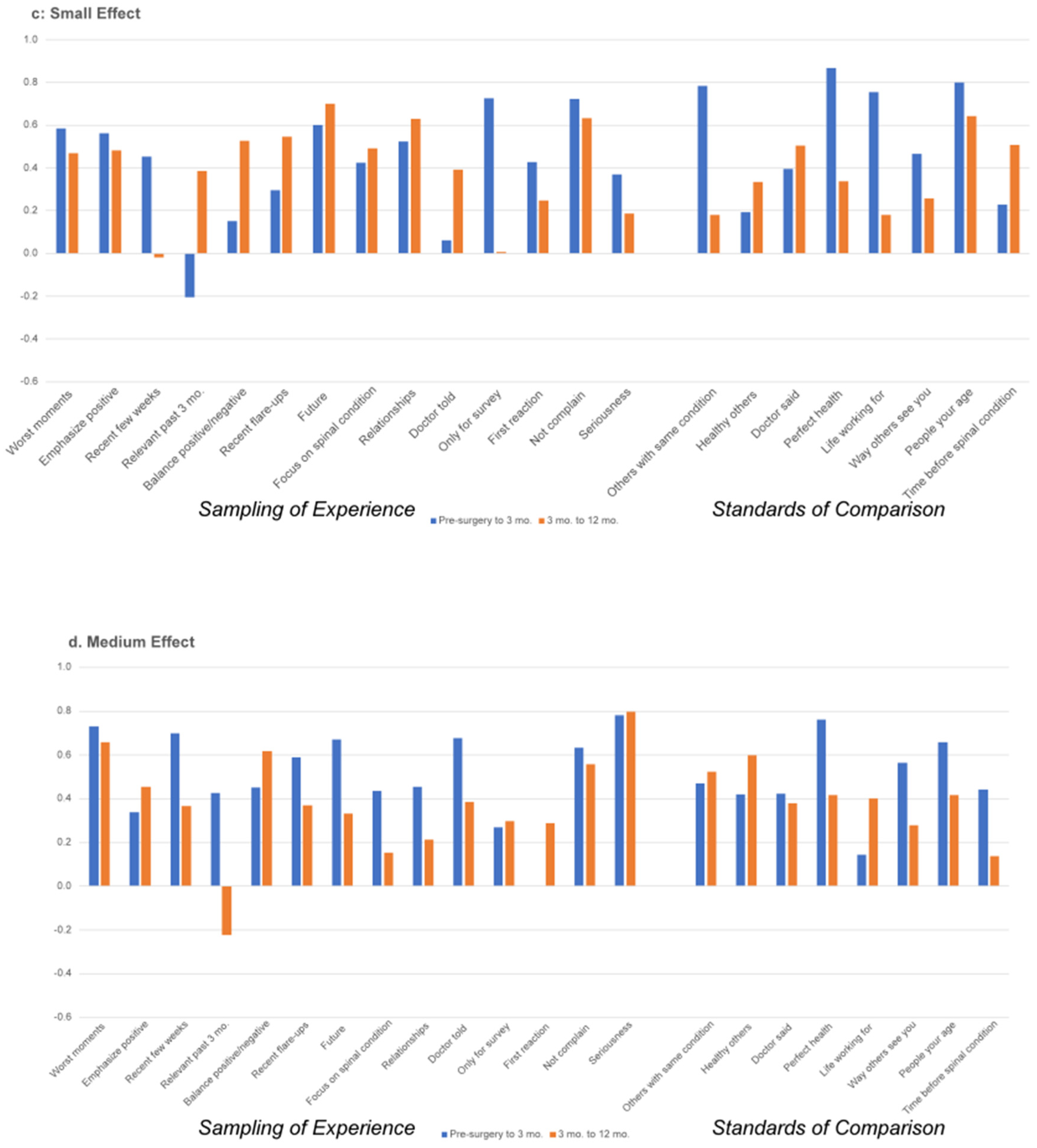
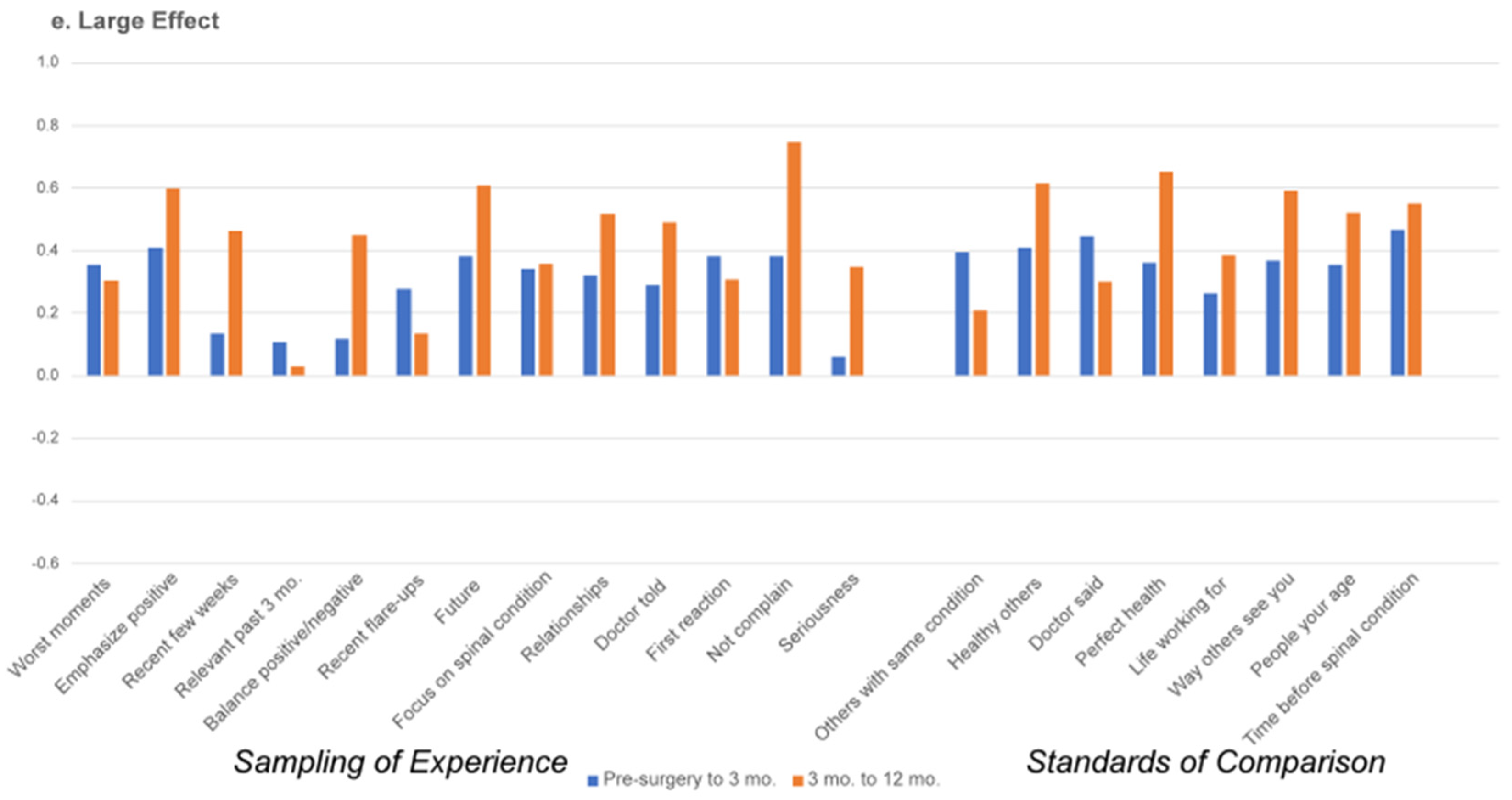
3.2. Appraisal Change over Time
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | Hedge’s g is interpreted as follows: 0.15 to 0.359 is a small effect, 0.36–0.649 is a medium effect, and >0.65 is a large effect. |
References
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Skinner, J.S.; Hanscom, B.; Tosteson, A.N.; Herkowitz, H.; Fischgrund, J.; Cammisa, F.P.; Albert, T.; et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA 2006, 296, 2451–2459. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Tosteson, A.N.; Blood, E.; Abdu, W.A.; Herkowitz, H.; Hilibrand, A.; Fischgrund, J. Surgical versus non operative treatment for lumbar disc herniation: Four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008, 33, 2789–2800. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Blood, E.; Hanscom, B.; Herkowitz, H.; Cammisa, F.; Albert, T.; Boden, S.D.; et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 794–810. [Google Scholar] [CrossRef]
- Khor, S.; Lavallee, D.C.; Cizik, A.M.; Bellabarba, C.; Dagal, A.; Hart, R.A.; Howe, C.R.; Martz, R.D.; Shonnard, N.; Flum, D.R. Hospital and surgeon variation in patient-reported functional outcomes after lumbar spine fusion: A statewide evaluation. Spine 2020, 45, 465–472. [Google Scholar] [CrossRef]
- Alvin, M.D.; Lubelski, D.; Alam, R.; Williams, S.K.; Obuchowski, N.A.; Steinmetz, M.P.; Wang, J.C.; Melillo, A.J.; Pahwa, A.; Benzel, E.C. Spine surgeon treatment variability: The impact on costs. Glob. Spine J. 2018, 8, 498–506. [Google Scholar] [CrossRef]
- Block, A.R.; Gatchel, R.J.; Deardorff, W.W.; Guyer, R.D. The Psychology of Spine Surgery; American Psychological Association: Washington, DC, USA, 2003. [Google Scholar]
- Kaptain, G.J.; Shaffrey, C.I.; Alden, T.D.; Young, J.N.; Whitehill, R. The influence of secondary gain on surgical outcome: A comparison between cervical and lumbar discectomy. Neurosurg. Focus 1998, 5, e6. [Google Scholar] [CrossRef]
- Crombez, G.; Vlaeyen, J.W.; Heuts, P.H.; Lysens, R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain 1999, 80, 329–339. [Google Scholar] [CrossRef]
- Kanaan, S.F.; Melton, B.L.; Waitman, L.R.; Simpson, M.H.; Sharma, N.K. The effect of age and gender on acute postoperative pain and function following lumbar spine surgeries. Physiother. Res. Int. 2021, 26, e1888. [Google Scholar] [CrossRef]
- Finkelstein, J.A.; Stark, R.B.; Lee, J.; Schwartz, C.E. Patient factors that matter in predicting spine surgery outcomes: A machine learning approach. J. Neurosurg. Spine 2021, 35, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Herr, K.A.; Sohn, J.N.; Cha, B.K.; Yom, Y.H. Prediction of pain outcomes in Korean older adults: Use of a structural equation model. Pain Med. 2007, 8, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.F.; Reynolds, C.F., III; Butters, M.A.; Dew, M.A.; Mazumdar, S.; Begley, A.E.; Lenze, E.; Weiner, D.K. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006, 7, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kerns, R.D. Evidence that cognitive decline mediates the relationship between pain and disability in the elderly. Pain Med. 2006, 7, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Spinhoven, P.; Ter, K.M.; Kole-Snijders, A.M.; Hutten, M.M.; Den Ouden, D.J.; Vlaeyen, J.W. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur. J. Pain 2004, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Rapkin, B.D.; Borowiec, K.; Finkelstein, J.A. Cognitive processes during recovery: Moving toward personalized spine surgery outcomes. J. Pers. Med. 2022, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Folkman, S.; Lazarus, R.S. An analysis of coping in a middle-aged community sample. J. Health Soc. Behav. 1980, 21, 219–239. [Google Scholar] [CrossRef]
- Tourangeau, R.; Rips, L.J.; Rasinski, K. The Psychology of Survey Response; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Rapkin, B.D.; Schwartz, C.E. Toward a theoretical model of quality-of-life appraisal: Implications of findings from studies of response shift. Health Qual. Life Outcomes 2004, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.G.; Schwartz, C.E. Integrating response shift into health-related quality of life research: A theoretical model. Soc. Sci. Med. 1999, 48, 1507–1515. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Stark, R.B.; Rapkin, B.D. Creating idiometric short-form measures of cognitive appraisal: Balancing theory and pragmatics. J. Patient-Rep. Outcomes 2021, 5, 57. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Stark, R.B.; Rapkin, B.D. Capturing patient experience: Does quality-of-life appraisal entail a new class of measurement? J. Patient-Rep. Outcomes 2020, 4, 85. [Google Scholar] [CrossRef]
- Rapkin, B.D.; Schwartz, C.E. Advancing quality-of-life research by deepening our understanding of response shift: A unifying theory of appraisal. Qual. Life Res. 2019, 28, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Borowiec, K.; Rapkin, B.D. Depression trajectories during the COVID-19 pandemic: A secondary analysis of the impact of cognitive-appraisal processes. J. Patient-Rep. Outcomes 2023, 7, 67. [Google Scholar] [CrossRef]
- Alchemer. Boulder, CO, USA. Available online: https://www.alchemer.com (accessed on 19 March 2024).
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Sangha, O.; Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Care Res. 2003, 49, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Rosnow, R.L.; Rosenthal, R. Effect sizes for experimenting psychologists. Can. J. Exp. Psychol./Rev. Can. De Psychol. Expérimentale 2003, 57, 221–237. [Google Scholar] [CrossRef]
- Brydges, C.R. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov. Aging 2019, 3, igz036. [Google Scholar] [CrossRef]
- Lovakov, A.; Agadullina, E.R. Empirically derived guidelines for effect size interpretation in social psychology. Eur. J. Soc. Psychol. 2021, 51, 485–504. [Google Scholar] [CrossRef]
- IBM. IBM SPSS Statistics for Windows, version 26; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Copay, A.G.; Glassman, S.D.; Subach, B.R.; Berven, S.; Schuler, T.C.; Carreon, L.Y. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008, 8, 968–974. [Google Scholar] [CrossRef]
- Nakarai, H.; Kato, S.; Kawamura, N.; Higashikawa, A.; Takeshita, Y.; Fukushima, M.; Ono, T.; Hara, N.; Azuma, S.; Tanaka, S. Minimal clinically important difference in patients who underwent decompression alone for lumbar degenerative disease. Spine J. 2022, 22, 549–560. [Google Scholar] [CrossRef]
- Copay, A.G.; Chung, A.S.; Eyberg, B.; Olmscheid, N.; Chutkan, N.; Spangehl, M.J. Minimum clinically important difference: Current trends in the orthopaedic literature, part I: Upper extremity: A systematic review. JBJS Rev. 2018, 6, e1. [Google Scholar] [CrossRef]
- Copay, A.G.; Eyberg, B.; Chung, A.S.; Zurcher, K.S.; Chutkan, N.; Spangehl, M.J. Minimum clinically important difference: Current trends in the orthopaedic literature, part II: Lower extremity: A systematic review. JBJS Rev. 2018, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.; Morley, S. Cognitive-behavioral treatments for chronic pain: What works for whom? Clin.J.Pain 2005, 21, 1–8. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Zhang, J.; Rapkin, B.D.; Finkelstein, J.A. Reconsidering the minimally important difference: Evidence of instability over time and across groups. Spine J. 2019, 19, 726–734. [Google Scholar] [CrossRef]
- Sniderman, J.; Stark, R.B.; Schwartz, C.E.; Imam, H.; Finkelstein, J.A.; Nousiainen, M.T. Patient Factors That Matter in Predicting Hip Arthroplasty Outcomes: A Machine-Learning Approach. J. Arthroplast. 2021, 36, 2024–2032. [Google Scholar] [CrossRef]


| Overall (N = 156) | ||
|---|---|---|
| Variable | Mean | SD |
| Age (at time of surgery) | 60.9 | 14.8 |
| Range | 20–87 | |
| Missing | 18 | 12% |
| No. of Comorbidities *^ | 1.8 | 1.5 |
| Range | 0–6 | |
| Missing | 3 | 2% |
| Follow-up Time in Days | ||
| Follow-up 1 (~3 months) | 107.9 | 99.5 |
| Range | 29–1009 | |
| Follow-up 2 (~12 months) | 395.6 | 217.8 |
| Range | 64–1477 | |
| Baseline Oswestry Disability Index score | 46.7 | 16.0 |
| Range | 11.1–95.6 | |
| Missing | 0 | 0% |
| No. | % of total sample | |
| Gender | ||
| Male | 65 | 42% |
| Female | 72 | 46% |
| Other | 1 | 1% |
| Missing | 18 | 12% |
| Smoking Status | ||
| Never smoked/used tobacco | 76 | 49% |
| Used to smoke/use tobacco | 61 | 39% |
| Currently smoke/use tobacco | 16 | 10% |
| Decline to answer | 1 | 1% |
| Missing | 2 | 1% |
| Level of Education | ||
| Less than high school | 7 | 4% |
| Graduated from high school or GED | 18 | 12% |
| Some college or technical school | 28 | 18% |
| Completed technical school (college) | 6 | 4% |
| Graduated from college | 40 | 26% |
| Postgraduate school or degree | 42 | 27% |
| Decline to answer | 1 | 1% |
| Missing | 14 | 9% |
| Diagnoses ^ | ||
| Disc Herniation | ||
| Yes | 45 | 29% |
| No | 102 | 65% |
| Missing | 9 | 6% |
| Radiculopathy/sciatica | ||
| Yes | 13 | 8% |
| No | 134 | 86% |
| Missing | 9 | 6% |
| Spinal stenosis with neurogenic claudication | ||
| Yes | 100 | 64% |
| No | 47 | 30% |
| Missing | 9 | 6% |
| Spondylolisthesis (Lytic) | ||
| Yes | 5 | 3% |
| No | 142 | 91% |
| Missing | 9 | 6% |
| Spondylolisthesis (degenerative) | ||
| Yes | 34 | 22% |
| No | 113 | 72% |
| Missing | 9 | 6% |
| Primary Procedure | ||
| Lami/disc | 98 | 63% |
| Instr/fusion alone | 20 | 13% |
| Instr/fusion w lami disc | 31 | 20% |
| Missing | 7 | 4% |
| Number of Vertebrae Fused if Fusion Surgery | ||
| Instr/fusion alone | ||
| 2 | 12 | 8% |
| 3 | 4 | 3% |
| 4 | 3 | 2% |
| >4 | 1 | 1% |
| Instr/fusion w lami disc | ||
| 2 | 17 | 11% |
| 3 | 8 | 5% |
| 4 | 2 | 1% |
| >4 | 4 | 3% |
| Pain Medicine Frequency | ||
| Not at all | 23 | 15% |
| Once a week | 2 | 1% |
| Once every couple days | 13 | 8% |
| Once or twice a day | 54 | 35% |
| 3 or more times a day | 62 | 40% |
| Decline to answer | 1 | 1% |
| Missing | 1 | 1% |
| Specific Comorbidities ^ (back-pain excluded) | ||
| Anemia or other blood disorder | 5 | 3% |
| Arthritis (rheumatoid or unspecified type) | 26 | 17% |
| Asthma | 4 | 3% |
| Cancer | 7 | 4% |
| Depression | 13 | 8% |
| Diabetes | 20 | 13% |
| Heart disease | 15 | 10% |
| High blood pressure | 60 | 38% |
| Insomnia | 5 | 3% |
| Kidney disease | 1 | 1% |
| Liver disease | 0 | 0% |
| Lung disease | 8 | 5% |
| Osteoarthritis, degenerative arthritis | 49 | 31% |
| Stroke | 1 | 1% |
| Ulcer or stomach disease | 6 | 4% |
| One or more additional comorbidities | 45 | 29% |
| Missing | 3 | 2% |
| Groups Classifying ODI Change 2nd Interval (3 mo. to 12 mo.) | |||||||
|---|---|---|---|---|---|---|---|
| Disability Worsened | No Effect | Small Effect | Medium Effect | Large Effect | Total | ||
| Groups classifying ODI change 1st Interval (pre-surgery to 3 mo.) | Disability worsened | 1 | 0 | 2 | 0 | 6 | 9 |
| No effect | 6 | 1 | 1 | 2 | 0 | 10 | |
| Small effect | 4 | 4 | 1 | 1 | 4 | 14 | |
| Medium effect | 6 | 1 | 2 | 3 | 5 | 17 | |
| Large effect | 47 | 17 | 18 | 9 | 15 | 106 | |
| Total | 64 | 23 | 24 | 15 | 30 | 156 | |
| ODI Change Group Baseline to 3 Months (1st Interval) | ODI Change Group 3 Months to 12 Months (2nd Interval) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appraisal Item | Disability Worsened | No Effect | Small Effect | Medium Effect | Large Effect | Disability Worsened | No Effect | Small Effect | Medium Effect | Large Effect | ||
| n = 9 | n = 10 | n = 14 | n = 17 | n = 106 | n = 64 | n = 23 | n = 24 | n = 15 | n = 30 | |||
| Sampling of Experience | Worst moments | 0.544 | 0.000 | −0.491 | −0.243 | −0.646 | Worst moments | 0.163 | 0.418 | −0.038 | −0.232 | −0.183 |
| Emphasize positive | −0.504 | −0.707 | 0.000 | 0.346 | 0.343 | Emphasize positive | −0.158 | −0.348 | −0.086 | −0.065 | 0.321 | |
| Recent few weeks | 0.267 | −0.316 | 0.109 | 0.000 | −0.196 | Recent few weeks | −0.093 | −0.493 | −0.158 | 0.054 | −0.420 | |
| Relevant past 3 mo. | 0.000 | 0.000 | 0.058 | 0.433 | −0.234 | Relevant past 3 mo. | −0.329 | 0.084 | −0.346 | 0.128 | −0.077 | |
| Balance positive/negative | −0.185 | −0.194 | 0.539 | 0.211 | 0.226 | Balance positive/negative | −0.281 | −0.254 | 0.216 | 0.567 | 0.194 | |
| Recent flare-ups | 0.095 | −0.224 | 0.185 | −0.200 | −0.457 | Recent flare-ups | 0.012 | −0.327 | −0.042 | −0.229 | −0.025 | |
| Future | 0.185 | −1.037 | −0.584 | 0.323 | 0.030 | Future | −0.245 | −0.228 | −0.246 | −0.165 | −0.216 | |
| Focus on spinal condition | −0.120 | −0.523 | −0.467 | −0.676 | −0.805 | Focus on spinal condition | 0.232 | −0.160 | −0.357 | −0.204 | −0.463 | |
| Relationships | −0.142 | −0.572 | 0.185 | 0.122 | −0.180 | Relationships | −0.086 | −0.240 | 0.412 | 0.393 | −0.143 | |
| Doctor told | 0.312 | −0.254 | −0.214 | −0.081 | −0.102 | Doctor told | −0.255 | −0.184 | 0.171 | 0.106 | −0.204 | |
| First reaction | 0.203 | 0.349 | 0.070 | −0.069 | 0.207 | First reaction | −0.223 | −0.134 | −0.379 | 0.422 | 0.069 | |
| Not complain | 0.000 | −0.372 | 0.000 | 0.323 | 0.106 | Not complain | −0.065 | −0.234 | −0.240 | −0.056 | 0.000 | |
| Seriousness | 0.438 | −0.316 | −0.087 | 0.174 | −0.481 | Seriousness | 0.144 | 0.041 | −0.216 | −0.098 | −0.052 | |
| Others with same condition | 0.360 | −0.101 | 0.185 | 0.000 | 0.000 | Others with same condition | −0.035 | −0.121 | −0.057 | −0.483 | −0.135 | |
| Standards of Comparison | Healthy others | 0.000 | −0.396 | 0.111 | −0.368 | −0.079 | Healthy others | 0.024 | −0.300 | −0.209 | −0.166 | −0.224 |
| Doctor said | 0.000 | 0.000 | 0.098 | −0.056 | −0.147 | Doctor said | −0.281 | −0.177 | −0.171 | −0.338 | −0.046 | |
| Perfect health | 0.356 | −0.176 | −0.215 | 0.283 | −0.136 | Perfect health | 0.081 | −0.342 | 0.250 | −0.128 | −0.315 | |
| Life working for | 0.558 | −0.163 | 0.086 | 0.093 | −0.239 | Life working for | 0.013 | −0.443 | −0.254 | −0.126 | −0.085 | |
| Way others see you | −0.280 | −0.218 | 0.145 | −0.112 | −0.030 | Way others see you | −0.028 | −0.223 | −0.232 | −0.308 | −0.218 | |
| People your age | 0.000 | 0.194 | 0.086 | −0.320 | −0.227 | People your age | 0.071 | −0.192 | 0.037 | 0.050 | 0.112 | |
| Time before spinal condition | 0.272 | 0.296 | −0.145 | 0.045 | −0.357 | Time before spinal condition | 0.000 | −0.188 | −0.367 | −0.137 | −0.200 | |
| Conditional formatting according to published interpretation standards for Cohen’s d: 0.20 to 0.49 is a small effect, 0.50−0.79 is a medium effect, and > 0.80 is a large effect. | ||||||||||||
 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, C.E.; Borowiec, K.; Rapkin, B.D.; Finkelstein, J.A. Functional Status Correlates of Change and Stability in Appraisal after Spine Surgery: Earlier versus Later Effects. J. Pers. Med. 2024, 14, 329. https://doi.org/10.3390/jpm14030329
Schwartz CE, Borowiec K, Rapkin BD, Finkelstein JA. Functional Status Correlates of Change and Stability in Appraisal after Spine Surgery: Earlier versus Later Effects. Journal of Personalized Medicine. 2024; 14(3):329. https://doi.org/10.3390/jpm14030329
Chicago/Turabian StyleSchwartz, Carolyn E., Katrina Borowiec, Bruce D. Rapkin, and Joel A. Finkelstein. 2024. "Functional Status Correlates of Change and Stability in Appraisal after Spine Surgery: Earlier versus Later Effects" Journal of Personalized Medicine 14, no. 3: 329. https://doi.org/10.3390/jpm14030329
APA StyleSchwartz, C. E., Borowiec, K., Rapkin, B. D., & Finkelstein, J. A. (2024). Functional Status Correlates of Change and Stability in Appraisal after Spine Surgery: Earlier versus Later Effects. Journal of Personalized Medicine, 14(3), 329. https://doi.org/10.3390/jpm14030329






