The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- Patient with clinical stage II–III rectal neoplasm;
- -
- No distant metastases at the time of diagnosis;
- -
- ECOG score between 0 and 2;
- -
- Receiving neoadjuvant radiotherapy or radio-chemotherapy with total doses between 45 and 50.4 Gray;
- -
- Surgery with radical intent.
2.2. Treatment and Follow-Up
2.3. Statistical Analysis
3. Results
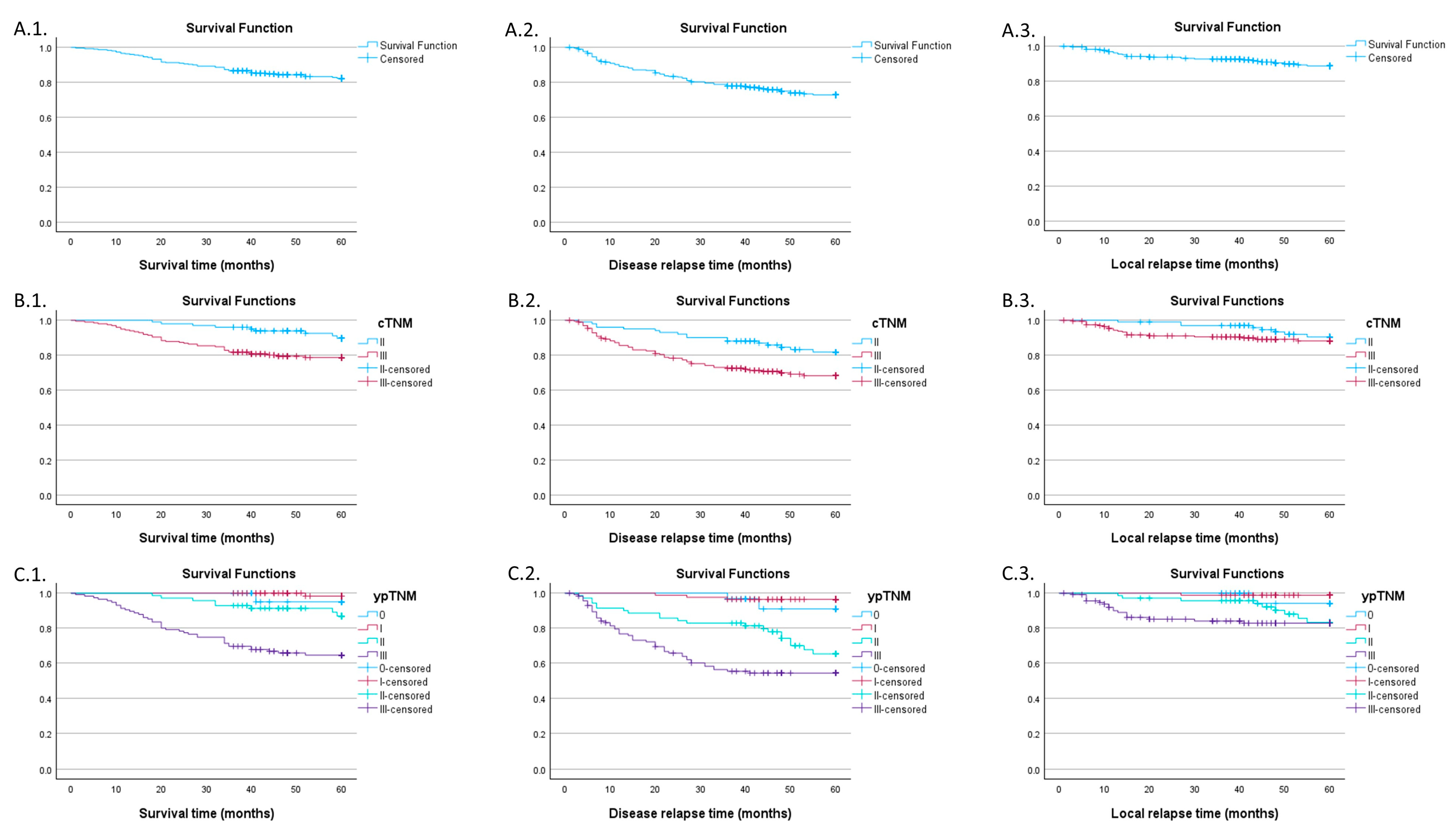
3.1. Global Survival Characteristics
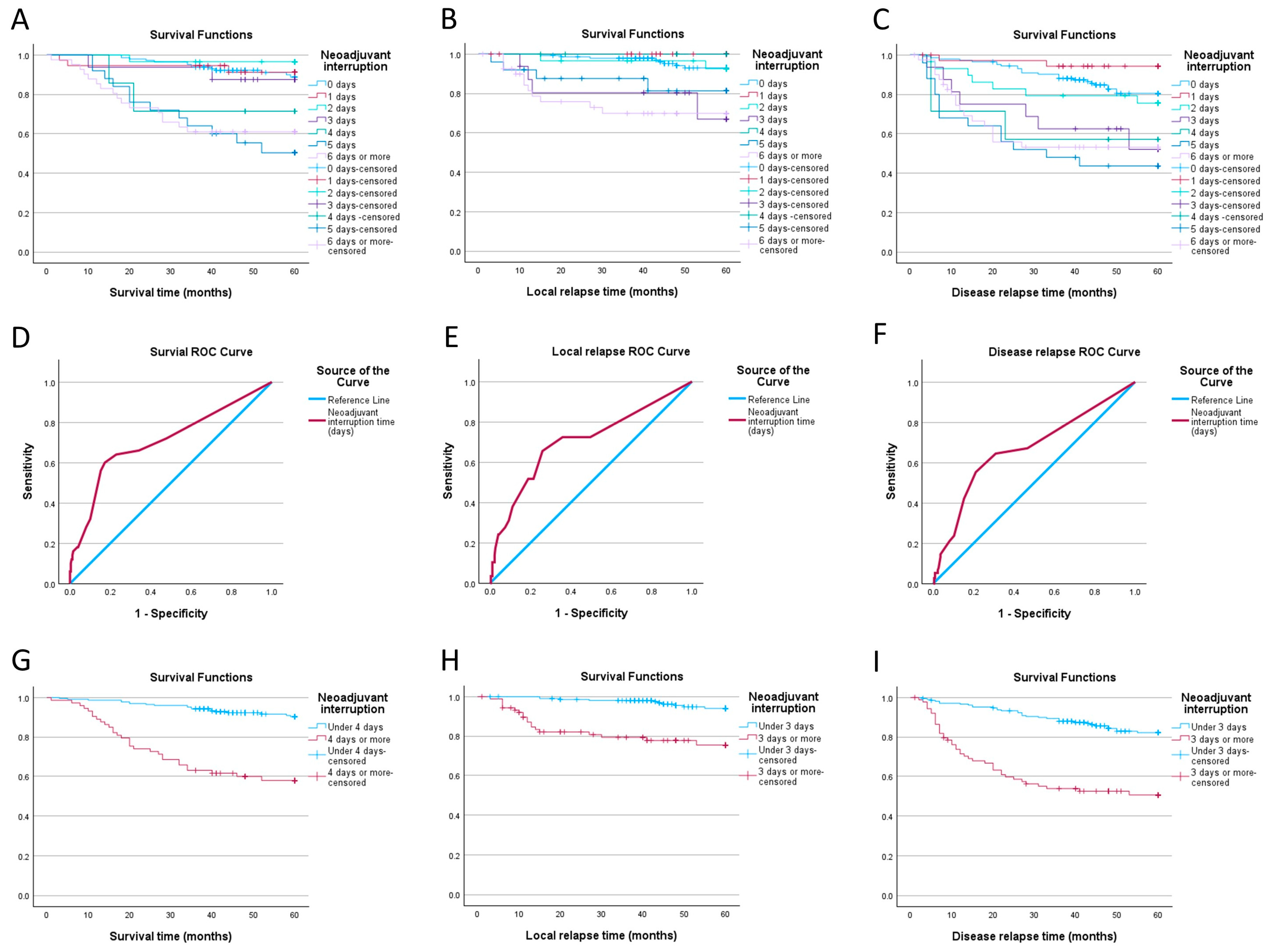
3.2. Impact of Neoadjuvant Therapy Interruption Days
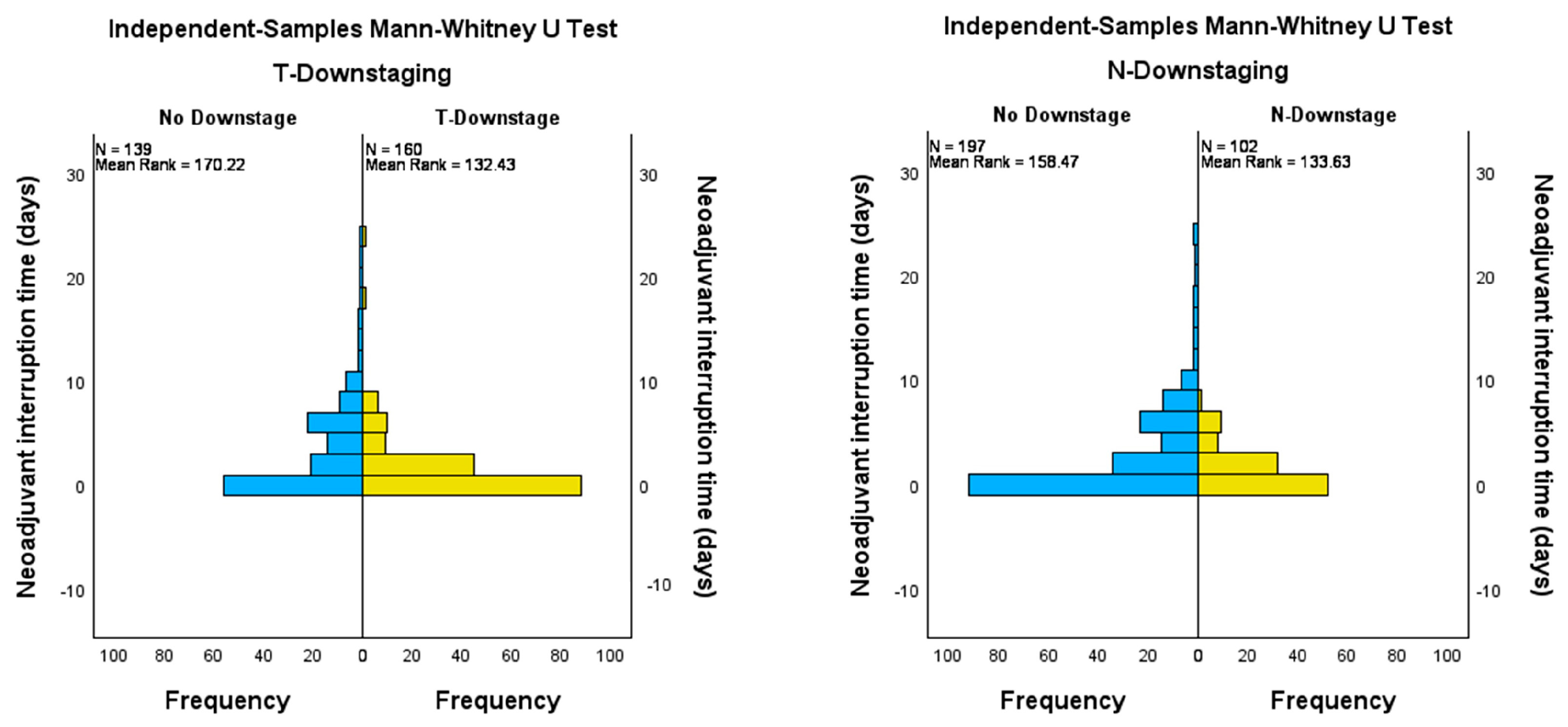
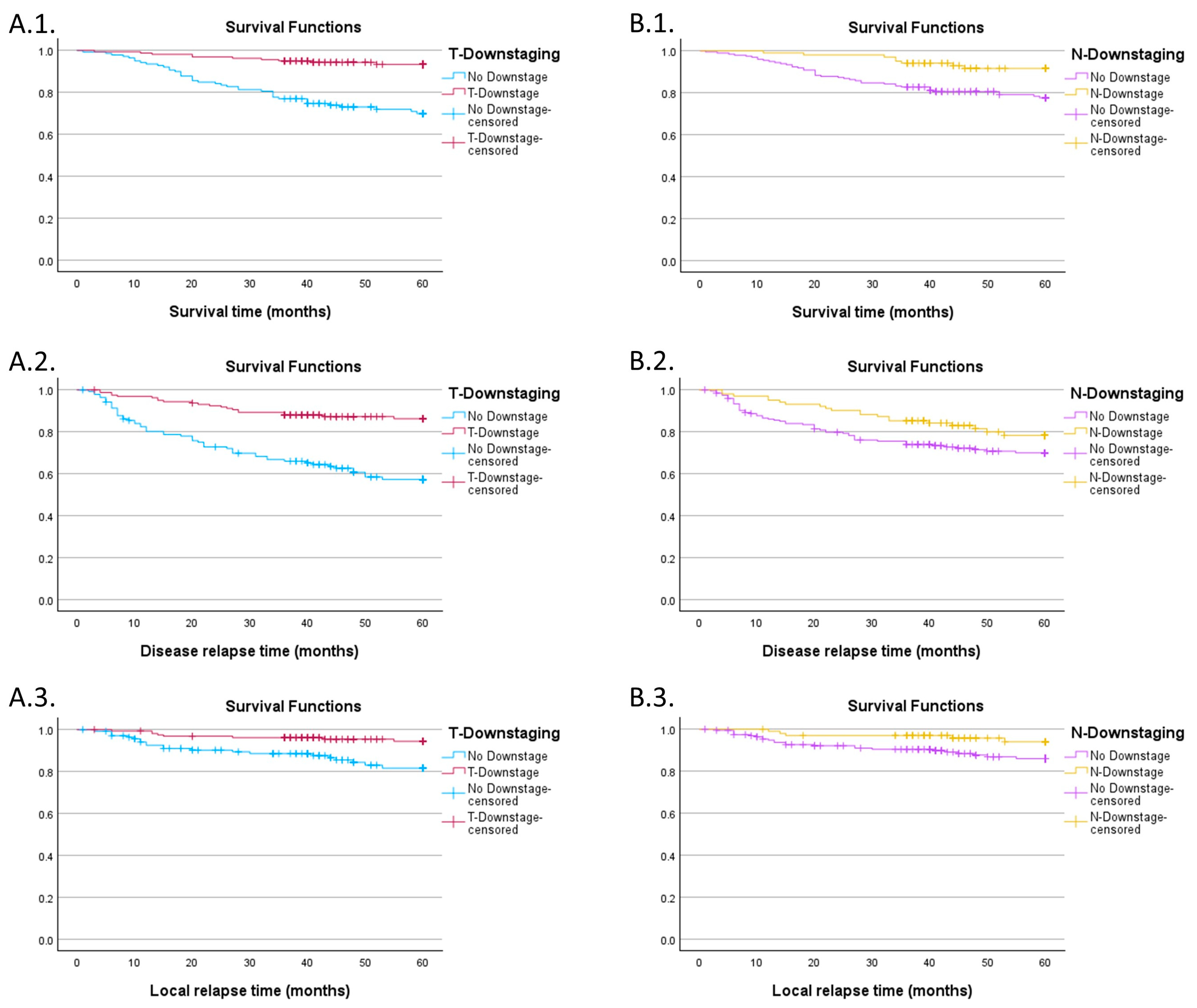
3.3. Impact of Downstaging on Survival Curves
4. Discussion
4.1. Radiobiology of Rectal Adenocarcinoma
4.2. Radiotherapy Dose Compensation
4.3. Impact of Radiotherapy Treatment Interruptions
4.4. Reasons for Interruption
- 1.
- Acute toxicities
- 2.
- The impact of the COVID-19 pandemic
- 3.
- Machine failure
- 4.
- Days off
- 5.
- Other reasons
4.5. The Role of Total Neoadjuvant Treatment
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ypTNM Stage | Overall Survival | Disease-Free Survival | Local Control | |||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| 0 | 95% | 4.9% | 91% | 6.3% | 94.1% | 5.7% |
| I | 98.3% | 1.7% | 96.4% | 2% | 98.8% | 1.2% |
| II | 86.9% | 4.5% | 65.3% | 6.4% | 83.2% | 5.2% |
| III | 64.5% | 4.5% | 54.3% | 4.8% | 82.8% | 3.7% |
| Days of Interruption | Overall Survival | Disease-Free Survival | Local Control | |||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| 0 days | 88.8% | 2.9% | 80.5% | 3.6% | 93% | 2.4% |
| 1 day | 91.2% | 4.9% | 94.3% | 3.9% | na | na |
| 2 days | 96.6% | 3.4% | 75.5% | 8.1% | 92.5% | 5.1% |
| 3 days | 87.5% | 8.3% | 52.1% | 13.9% | 67% | 14.9% |
| 4 days | 71.4% | 17.1% | 57.1% | 18.7% | na | na |
| 5 days | 50.3% | 10.3% | 43.6% | 10% | 81.4% | 8.7% |
| 6+ days | 61% | 7.6% | 53.2% | 8.1% | 69.9% | 7.7% |
| cTNM | Total | ||
|---|---|---|---|
| II | III | ||
| Under 4 days | 81 | 145 | 226 |
| 4 days or more | 20 | 53 | 73 |
| Total | 101 | 198 | 299 |
References
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Büchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L. NCCN Guidelines Version 4.2022 Rectal Cancer Continue NCCN Guidelines Panel Disclosures. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 26 March 2023).
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Liscu, H.-D.; Liscu, B.-R.; Mitre, R.; Anghel, I.-V.; Antone-Iordache, I.-L.; Balan, A.; Coniac, S.; Miron, A.-I.; Halcu, G. The Conditioning of Adjuvant Chemotherapy for Stage II and III Rectal Cancer Determined by Postoperative Pathological Characteristics in Romania. Medicina 2023, 59, 1224. [Google Scholar] [CrossRef] [PubMed]
- Enker, W.E. Total mesorectal excision—The new golden standard of surgery for rectal cancer. Ann. Med. 1997, 29, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Ryall, R.D.H. Recurrence and Survival after Total Mesorectal Excision for Rectal Cancer. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Kim, J.K.; Yuval, J.B.; Thompson, H.; Verheij, F.; Lee, M.; Saltz, L.B. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J. Clin. Oncol. 2020, 38 (Suppl. 15), 4008. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Jimenez-Rodriguez, R.M.; Quezada-Diaz, F.; Hameed, I.; Kalabin, A.; Patil, S.; Smith, J.J.; Garcia-Aguilar, J.M. Organ Preservation in Patients with Rectal Cancer Treated with Total Neoadjuvant Therapy. Dis. Colon Rectum 2021, 64, 1463–1470. [Google Scholar] [CrossRef]
- Bach, S.P.; Gilbert, A.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.G.; Khan, J.; et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): A randomised, open-label feasibility study. Lancet Gastroenterol. Hepatol. 2021, 6, 92–105. [Google Scholar] [CrossRef]
- Liang, J.-A.; Kuo, Y.-C.; Chao, K.C.; Chen, W.T.-L.; Ke, T.-W.; Chou, S.-H.; Li, C.-C.; Chien, C.-R. High vs. Standard Radiotherapy Dose in Locally Advanced Rectal Adenocarcinoma Patients Treated with Neoadjuvant Long Course Chemoradiotherapy: A Population-based Study. Anticancer Res. 2022, 42, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.; Pearson, K.; Fulton, R.; Hewitt, J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst. Rev. 2012, 12, CD008368. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Hollingshead, J. TNT and local recurrence in the RAPIDO trial—Untangling the puzzle. Nat. Rev. Clin. Oncol. 2023, 20, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.G.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Formenti, S.C. Cancer and COVID-19—Potentially deleterious effects of delaying radiotherapy. Nat. Rev. Clin. Oncol. 2020, 17, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Ciarleglio, F.A.; Rigoni, M.; Mereu, L.; Tommaso, C.; Carrara, A.; Malossini, G.; Tateo, S.; Tirone, G.; Johansen, T.E.B.; Benetollo, P.P.; et al. The negative effects of COVID-19 and national lockdown on emergency surgery morbidity due to delayed access. World J. Emerg. Surg. 2021, 16, 37. [Google Scholar] [CrossRef]
- DeMore, N.K.; Savage, S.J.; Lesher, A.P.; Abbott, A.; Giordano, A.; Zhang, B.; Mahvi, D.M.; Garcia, D.; Carneiro-Pla, D.; Camp, E.R.; et al. What is Elective Oncologic Surgery in the Time of COVID-19? A Literature Review of the Impact of Surgical Delays on Outcomes in Patients with Cancer. Clin. Oncol. Res. 2020, 3, 6. [Google Scholar] [CrossRef]
- RHunger, R.; König, V.; Stillger, R.; Mantke, R. Impact of the COVID-19 pandemic on delays in surgical procedures in Germany: A multi-center analysis of an administrative registry of 176,783 patients. Patient Saf. Surg. 2022, 16, 22. [Google Scholar] [CrossRef]
- Treder, M.; Vogelsang, R.P.; Janssen, S.; Schild, S.E.; Holländer, N.H.; Rades, D. Potential Prognostic Factors of Downstaging Following Preoperative Chemoradiation for High Rectal Cancer. In Vivo 2018, 32, 1481–1484. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Li, N.; Liu, W.; Jiang, J.; Jiang, L.; Chen, B.; Fang, H.; Ren, H.; Lu, N.; et al. Neoadjuvant Rectal Score And Downstaging Depth Score as Prognosis Predictors For Patients with Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e627–e628. [Google Scholar] [CrossRef]
- Valentini, V.; Coco, C.; Picciocchi, A.; Morganti, A.G.; Trodella, L.; Ciabattoni, A.; Cellini, F.; Barbaro, B.; Cogliandolo, S.; Nuzzo, G.; et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Sardari, D.; Verga, N. Calculation of externally applied electric field intensity for disruption of cancer cell proliferation. Electromagn. Biol. Med. 2010, 29, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Suwinski, R.; Wzietek, I.; Tarnawski, R.; Namysl-Kaletka, A.; Kryj, M.; Chmielarz, A.; Wydmanski, J. Moderately Low Alpha/Beta Ratio for Rectal Cancer May Best Explain the Outcome of Three Fractionation Schedules of Preoperative Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Iconaru, S.L.; Jiga, G.; Chapon, P.; Gaiaschi, S.; Verga, N.; Beuran, M.; Prodan, A.M.; Matei, M.; Marinescu, S.A.; et al. The Effect of the Ionizing Radiation on Hydroxyapatite–Polydimethylsiloxane Layers. Polym. Eng. Sci. 2019, 59, 2406–2412. [Google Scholar] [CrossRef]
- Masters, B.; Pascoe, A.; Walker, G.; Sundar, S. Radiobiology of Acute Rectal Toxicity. Clin. Oncol. 2018, 30, 594–595. [Google Scholar] [CrossRef]
- Lemanska, A.; Dearnaley, D.; Jena, R.; Sydes, M.; Faithfull, S. Older Age, Early Symptoms and Physical Function are Associated with the Severity of Late Symptom Clusters for Men Undergoing Radiotherapy for Prostate Cancer. Clin. Oncol. 2018, 30, 334–345. [Google Scholar] [CrossRef]
- Brand, D.H.; Brüningk, S.C.; Wilkins, A.; Fernandez, K.; Naismith, O.; Gao, A.; Syndikus, I.; Dearnaley, D.P.; Tree, A.C.; van As, N.; et al. Estimates of Alpha/Beta (α/β) Ratios for Individual Late Rectal Toxicity Endpoints: An Analysis of the CHHiP Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 596–608. [Google Scholar] [CrossRef]
- Marzi, S.; Saracino, B.; Petrongari, M.G.; Arcangeli, S.; Gomellini, S.; Arcangeli, G.; Benassi, M.; Landoni, V. Modeling of αβ for late rectal toxicity from a randomized phase II study: Conventional versus hypofractionated scheme for localized prostate cancer. J. Exp. Clin. Cancer Res. 2009, 28, 117. [Google Scholar] [CrossRef]
- Brenner, D.J. Fractionation and late rectal toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1013–1015. [Google Scholar] [CrossRef]
- Georgescu, M.-I.; Ionescu, R.T.; Miron, A.-I.; Savencu, O.; Ristea, N.-C.; Verga, N.; Khan, F.S. Multimodal Multi-Head Convolutional Attention with Various Kernel Sizes for Medical Image Super-Resolution. In Proceedings of the 2023 IEEE Winter Conference on Applications of Computer Vision, WACV 2023, Waikoloa, HI, USA, 2–7 January 2023; pp. 2194–2204. [Google Scholar] [CrossRef]
- Elkind, M.M.; Sutton, H. Radiation response of mammalian cells grown in culture. 1. Repair of X-ray damage in surviving Chinese hamster cells. Radiat. Res. 1960, 13, 556–593. [Google Scholar] [CrossRef]
- Kawahara, D.; Nakano, H.; Saito, A.; Ozawa, S.; Nagata, Y. Dose compensation based on biological effectiveness due to interruption time for photon radiation therapy. Br. J. Radiol. 2020, 93, 20200125. [Google Scholar] [CrossRef] [PubMed]
- Putora, P.M.; Schmuecking, M.; Aebersold, D.; Plasswilm, L. Compensability index for compensation radiotherapy after treatment interruptions. Radiat. Oncol. 2012, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Abolfath, R.; Khalili, M.; Senejani, A.G.; Kodery, B.; Ivker, R. The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy. Onco 2022, 2, 264–281. [Google Scholar] [CrossRef]
- Atkočius, V.; Burneckis, A.; Atkočiené, E. Clinical radiobiology of HDR Cf-252 brachytherapy for rectal cancer. Radiother. Oncol. 1995, 37, S13. [Google Scholar] [CrossRef]
- Buckley, H.; Wilson, C.; Ajithkumar, T. High-Dose-Rate Brachytherapy in the Management of Operable Rectal Cancer: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Diefenhardt, M.; Trommel, M.; Scherf, C.; Ramm, U.; Chatzikonstantinou, G.; Fokas, E.; Rödel, C.; Tselis, N. Image-guided high-dose-rate brachytherapy for rectal cancer: Technical note and first clinical experience on an organ-preserving approach. Strahlenther. Onkol. 2022, 198, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.A.; Oates, J.P.; Dale, R.G. BED-time charts and their application to the problems of interruptions in external beam radiotherapy treatments. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 381–389. [Google Scholar] [CrossRef]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of Prolongation of Overall Treatment Time Due To Unplanned Interruptions During Radiotherapy of Different Tumor Sites and Practical Methods for Compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef]
- Giuglea, C.; Marin, A.; Gavrila, I.; Paunescu, A.; Dobrete, N.A.; Marinescu, S.A. Basal Cell Carcinoma—A Retrospective Descriptive Study Integrated in Current Literature. Life 2023, 13, 832. [Google Scholar] [CrossRef]
- The Royal College of Radiologists. The Timely Delivery of Radical Radiotherapy: Guidelines for the Management of Unscheduled Treatment Interruptions, 4th ed.; The Royal College of Radiologists: London, UK, 2019; Available online: www.rcr.ac.uk (accessed on 31 January 2024).
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Popescu-Vâlceanu, H.-C.; Stoicea, M.C.; Enache, V.; Bratu, R.M.; Mustăţea, P.; Drăguţ, R.M.; Rusu, E.; Ionescu-Tîrgovişte, C.; Radulian, G. Bcl-2 and p53 immunophenotypes in colorectal adenocarcinoma in type 2 diabetes mellitus versus non-diabetic patients. Rom. J. Morphol. Embryol. 2022, 63, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.F.W.; Strous, M.T.A.; Bongers, B.C.; Vogelaar, F.J.; Janssen-Heijnen, M.L.G. The Association Between Treatment Interval and Survival in Patients with Colon or Rectal Cancer: A Systematic Review. World J. Surg. 2021, 45, 2924–2937. [Google Scholar] [CrossRef] [PubMed]
- CROCODILE Study Group. Catastrophic expenditure and treatment attrition in patients seeking comprehensive colorectal cancer treatment in India: A prospective multicentre study. Lancet Reg. Health-Southeast Asia 2022, 6, 100058. [Google Scholar] [CrossRef]
- Overgaard, J.; Hjelm-Hansen, M.; Johansen, L.V.; Andersen, A.P. Comparison of conventional and split-course radiotherapy as primary treatment in carcinoma of the larynx. Acta Oncol. 1988, 27, 147–152. [Google Scholar] [CrossRef]
- Barton, M.B.; Keane, T.J.; Gadalla, T.; Maki, E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother. Oncol. 1992, 23, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, B.; Preuss-Bayer, G.; Trott, K.-R. The influence of the number of fractions and of overall treatment time on local control and late complication rate in squamous cell carcinoma of the larynx. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 321–328. [Google Scholar] [CrossRef]
- Skladowski, K.; Law, M.G.; Maciejewski, B.; Steel, G.G. Planned and unplanned gaps in radiotherapy: The importance of gap position and gap duration. Radiother. Oncol. 1994, 30, 109–120. [Google Scholar] [CrossRef]
- Cox, J.D.; Pajak, T.F.; Asbell, S.; Russell, A.H.; Pederson, J.; Byhardt, R.W.; Emami, B.; Roach, M. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: Analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 493–498. [Google Scholar] [CrossRef]
- Machtay, M.; Hsu, C.; Komaki, R.; Sause, W.T.; Swann, R.S.; Langer, C.J.; Byhardt, R.W.; Curran, W.J. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: Analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 667–671. [Google Scholar] [CrossRef]
- Weber, D.C.; Kurtz, J.M.; Allal, A.S. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Lanciano, R.M.; Pajak, T.F.; Martz, K.; Hanks, G.E. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care study. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Samuelian, J.M.; Callister, M.D.; Ashman, J.B.; Young-Fadok, T.M.; Borad, M.J.; Gunderson, L.L. Reduced Acute Bowel Toxicity in Patients Treated with Intensity-Modulated Radiotherapy for Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1981–1987. [Google Scholar] [CrossRef]
- Ng, S.Y.; Colborn, K.L.; Cambridge, L.; Hajj, C.; Yang, T.J.; Wu, A.J.; Goodman, K.A. Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer. Radiother. Oncol. 2016, 121, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Liscu, H.-D.; Miron, A.-I.; Rusea, A.-R.; Oprea, A.-M.N.; Mitre, R.; Herdea, A.; Negreanu, R. Short-Course Radiotherapy versus Long-Course Radio-Chemotherapy as Neoadjuvant Treatment for Locally Advanced Rectal Cancer: Meta-Analysis from a Toxicity Perspective. Maedica 2021, 16, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.W.; Kang, H.-C.; Wu, H.-G.; Chie, E.K.; Choi, N.; Park, J.M.; Kim, J.-I.; Huang, C.-M.; Wang, J.-Y.; Ng, S.Y.; et al. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: A meta-analysis and pooled-analysis of acute toxicity. Jpn. J. Clin. Oncol. 2018, 48, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Coniac, S.; Outas, M.C.C.; Pirvu, E.-E.; Patru, R.-I.; Gainariu, E.; Aldea, C.; Iorga, P.G.; Ambroci, M.; Liscu, H.-D.; Miron, A.-I.; et al. Challenges and Limitations of Endocrine Toxicity Evaluation in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy-Retrospective Study from a Tertiary-Level Hospital in Romania. Diagnostics 2023, 13, 1788. [Google Scholar] [CrossRef]
- Grigorean, V.T.; Erchid, A.; Coman, I.S.; Liţescu, M. Colorectal Cancer—The ‘Parent’ of Low Bowel Obstruction. Medicina 2023, 59, 875. [Google Scholar] [CrossRef]
- Bernabucci, L.; Cornacchione, P.; Boldrini, L.; Pasini, D.; Dinapoli, L.; Smiljanic, L.; Valentini, V.; Dinapoli, N. Radiotherapy during the COVID-19: A review about management and treatment strategies. Rep. Pract. Oncol. Radiother. 2022, 27, 291–302. [Google Scholar] [CrossRef]
- Peiris, G.S.; Pawiro, S.A.; Kasim, M.F.; Sheehy, S.L. Failure modes and downtime of radiotherapy LINACs and multileaf collimators in Indonesia. J. Appl. Clin. Med. Phys. 2023, 24, e13756. [Google Scholar] [CrossRef]
- Wroe, L.; Ige, T.; Asogwa, O.; Aruah, S.; Grover, S.; Makufa, R.; Fitz-Gibbon, M.; Sheehy, S. Comparative Analysis of Radiotherapy Linear Accelerator Downtime and Failure Modes in the UK, Nigeria and Botswana. Clin. Oncol. 2020, 32, e111–e118. [Google Scholar] [CrossRef] [PubMed]
- Countries with the Most Public Holidays. WorldAtlas. Available online: https://www.worldatlas.com/articles/countries-with-the-most-public-holidays.html (accessed on 31 January 2024).
- Sidani, S.; Fox, M.; Streiner, D.L.; Miranda, J.; Fredericks, S.; Epstein, D.R. Examining the influence of treatment preferences on attrition, adherence and outcomes: A protocol for a two-stage partially randomized trial. BMC Nurs. 2015, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Leykin, Y.; DeRubeis, R.J.; Gallop, R.; Amsterdam, J.D.; Shelton, R.C.; Hollon, S.D. The relation of patients’ treatment preferences to outcome in a randomized clinical trial. Behav. Ther. 2007, 38, 209–217. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.-D.; Ghadimi, M.; Minsky, B.D.; Rödel, C.; Fokas, E. Association of Treatment Adherence with Oncologic Outcomes for Patients with Rectal Cancer: A Post Hoc Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.U.; Kim, H.O.; Kim, H.; Lee, D.; Cheong, C. Unveiling the profound advantages of total neoadjuvant therapy in rectal cancer: A trailblazing exploration. Ann. Surg. Treat. Res. 2023, 105, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.-I.; Atasiei, D.-I.; Ionescu, R.-T.; Ultimescu, F.; Barnonschi, A.-A.; Anghel, A.-V.; Anghel, C.-A.; Antone-Iordache, I.-L.; Mitre, R.; Bobolocu, A.M.; et al. Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients-A Review Using AI Tools. Cancers 2024, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Gebert, P.; Schindel, D.; Frick, J.; Schenk, L.; Grittner, U. Characteristics and patient-reported outcomes associated with dropout in severely affected oncological patients: An exploratory study. BMC Med. Res. Methodol. 2021, 21, 77. [Google Scholar] [CrossRef]
- Mehedintu, C.; Frincu, F.; Brinduse, L.A.; Carp-Veliscu, A.; Bratila, E.; Hennetier, C.; Roman, H. Postoperative Assessment of the Quality of Life in Patients with Colorectal Endometriosis. J. Clin. Med. 2021, 10, 5211. [Google Scholar] [CrossRef]
- Stalmeier, P.F.; van Tol-Geerdink, J.J.; van Lin, E.N.; Schimmel, E.; Huizenga, H.; van Daal, W.A.; Leer, J.-W. Doctors’ and patients’ preferences for participation and treatment in curative prostate cancer radiotherapy. J. Clin. Oncol. 2007, 25, 3096–3100. [Google Scholar] [CrossRef]
- Miron, A.-I.; Anghel, A.-V.; Barnonschi, A.-A.; Mitre, R.; Liscu, H.-D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics 2023, 13, 1938. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.-M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023, 41, LBA3504. [Google Scholar] [CrossRef]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2021, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Foulon, V.; Schöffski, P.; Wolter, P. Patient adherence to oral anticancer drugs: An emerging issue in modern oncology. Acta Clin. Belg. 2011, 66, 85–96. [Google Scholar]
- Bosset, J.; Calais, G.; Daban, A.; Berger, C.; Radosevic-Jelic, L.; Maingon, P.; Bardet, E.; Pierart, M.; Briffaux, A. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: Assessment of acute toxicity and treatment compliance: Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur. J. Cancer 2004, 40, 219–224. [Google Scholar] [CrossRef]
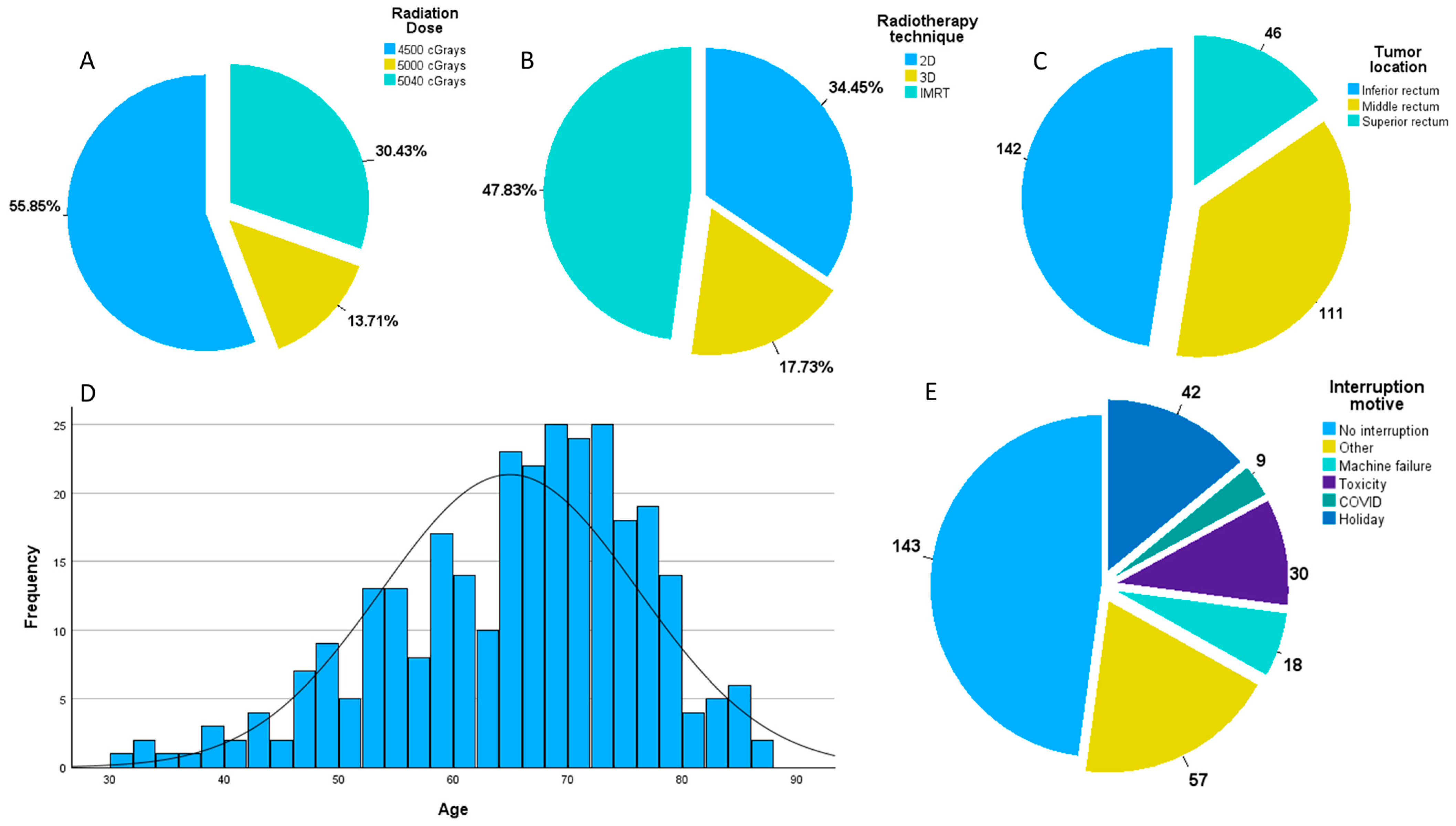
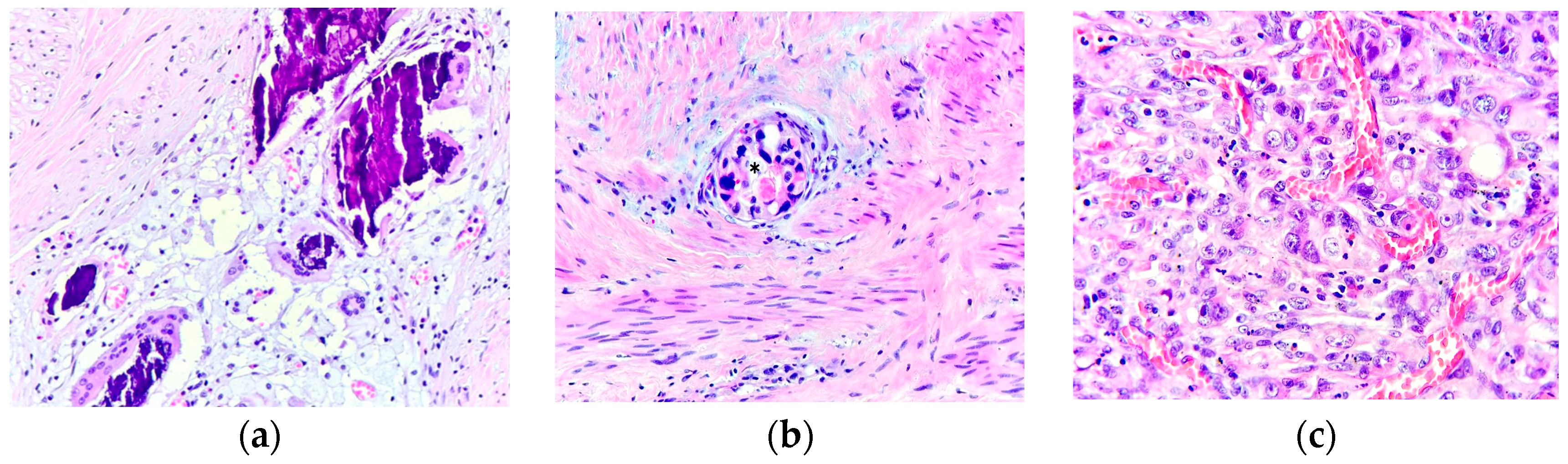
| Variable | Number | Frequency (%) | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 172 | 57.53 | ||
| Female | 127 | 42.47 | ||
| cTNM | ||||
| II | 101 | 33.78 | ||
| III | 198 | 66.22 | ||
| ypTNM | ||||
| 0 | 30 | 10.03 | ||
| I | 84 | 28.10 | ||
| II | 70 | 23.41 | ||
| III | 115 | 38.46 | ||
| Neoadjuvant therapy | ||||
| RT | 84 | 28.09 | ||
| RT + CHT | 215 | 71.90 | ||
| Tumor location | ||||
| Low rectum | 142 | 47.49 | ||
| Mid rectum | 111 | 37.12 | ||
| Upper rectum | 46 | 15.39 | ||
| Radiotherapy technique | ||||
| 2D | 103 | 34.45 | ||
| 3D | 53 | 17.73 | ||
| IMRT | 143 | 47.82 | ||
| Radiation dose | ||||
| 4500 cGrays | 167 | 55.85 | ||
| 5000 cGrays | 41 | 13.71 | ||
| 5040 cGrays | 91 | 30.44 | ||
| Tumor downstaging | ||||
| Yes | 139 | 46.49 | ||
| No | 160 | 53.51 | ||
| Nodal downstaging | ||||
| Yes | 197 | 65.89 | ||
| No | 102 | 34.11 | ||
| Neoadjuvant interruption | ||||
| 0 days | 144 | 48.16 | ||
| 1 day | 37 | 12.38 | ||
| 2 days | 29 | 9.70 | ||
| 3 days | 16 | 5.35 | ||
| 4 days | 7 | 2.34 | ||
| 5 days | 25 | 8.36 | ||
| 6+ days | 41 | 13.71 | ||
| Reason for interruption | Mean | SD | ||
| Holiday | 42 | 2 days | 1.3 | 26.92 |
| Toxicity | 30 | 5.1 days | 1.9 | 19.23 |
| Machine failure | 18 | 2.4 days | 1.5 | 11.54 |
| COVID | 9 | 15.9 days | 4.8 | 5.77 |
| Other reasons | 57 | 5.3 days | 4.6 | 36.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lișcu, H.-D.; Antone-Iordache, I.-L.; Atasiei, D.-I.; Anghel, I.V.; Ilie, A.-T.; Emamgholivand, T.; Ionescu, A.-I.; Șandru, F.; Pavel, C.; Ultimescu, F. The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients. J. Pers. Med. 2024, 14, 266. https://doi.org/10.3390/jpm14030266
Lișcu H-D, Antone-Iordache I-L, Atasiei D-I, Anghel IV, Ilie A-T, Emamgholivand T, Ionescu A-I, Șandru F, Pavel C, Ultimescu F. The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients. Journal of Personalized Medicine. 2024; 14(3):266. https://doi.org/10.3390/jpm14030266
Chicago/Turabian StyleLișcu, Horia-Dan, Ionut-Lucian Antone-Iordache, Dimitrie-Ionuț Atasiei, Ioana Valentina Anghel, Andreea-Teodora Ilie, Taraneh Emamgholivand, Andreea-Iuliana Ionescu, Florica Șandru, Christopher Pavel, and Flavia Ultimescu. 2024. "The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients" Journal of Personalized Medicine 14, no. 3: 266. https://doi.org/10.3390/jpm14030266
APA StyleLișcu, H.-D., Antone-Iordache, I.-L., Atasiei, D.-I., Anghel, I. V., Ilie, A.-T., Emamgholivand, T., Ionescu, A.-I., Șandru, F., Pavel, C., & Ultimescu, F. (2024). The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients. Journal of Personalized Medicine, 14(3), 266. https://doi.org/10.3390/jpm14030266










