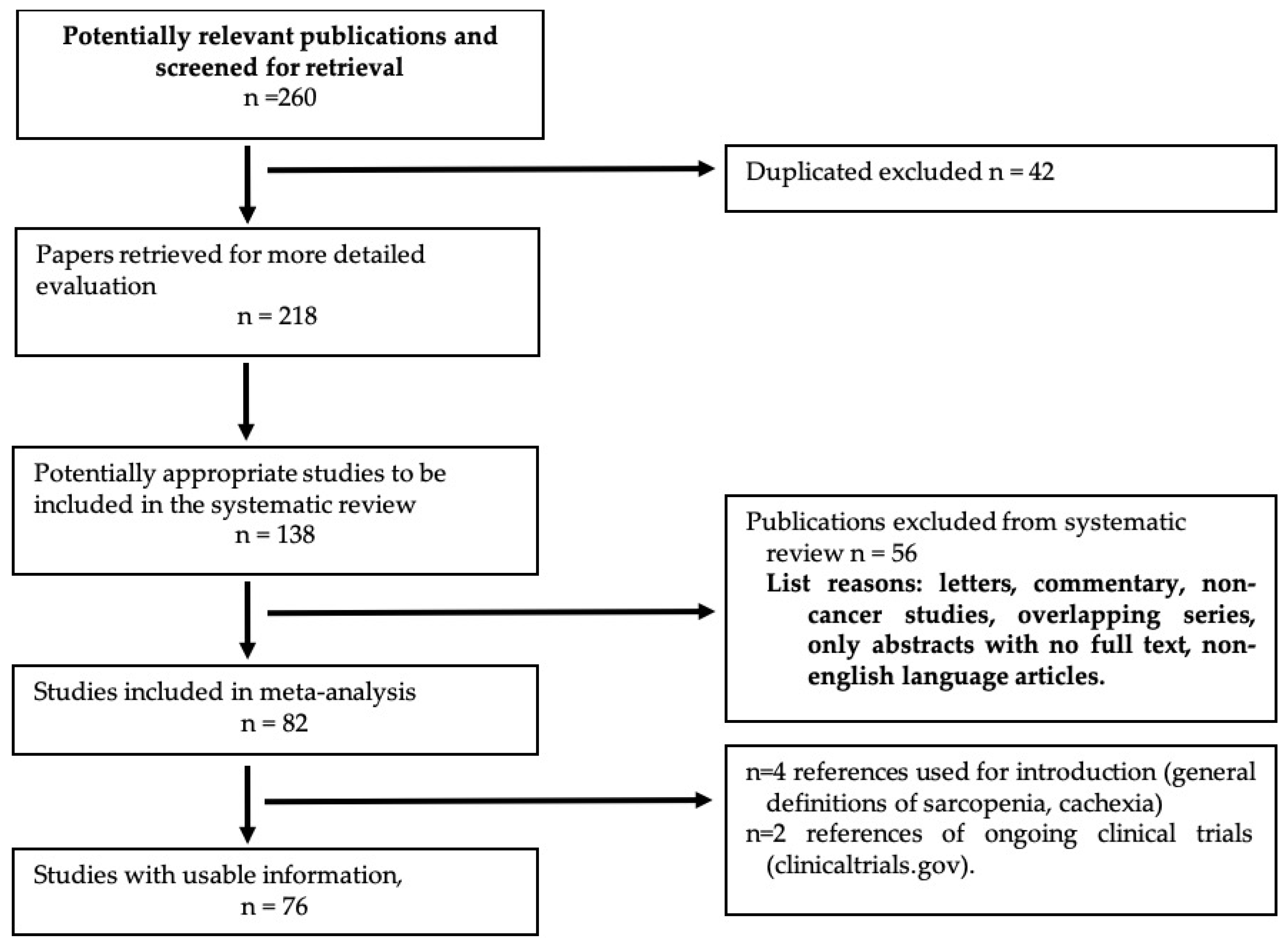
Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review
Abstract
1. Introduction
2. Traditional Body Composition Indicators
3. Quantitative Imaging Markers of Body Composition
3.1. Computed Tomography
3.2. Positron Emission Tomography (PET)
3.3. Dual-Energy X-ray Absorptiometry (DEXA)
3.4. Magnetic Resonance Imaging (MRI)
3.5. Ultrasound (US)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| BAT | brown adipose tissue |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| CT | computed tomography |
| DEXA | dual-energy X-ray absorptiometry |
| FDG-PET | fluoro-2-deoxy-D-glucose positron emission tomography |
| FFM: | fat-free mass |
| FM | fat mass |
| HU | Hounsfield units |
| MRI | magnetic resonance imaging |
| PhA | phase angle |
| SMM | skeletal muscle mass |
| US | ultrasound |
| VAT | visceral adipose tissue |
References
- Meza-Valderrama, D.; Marco, E.; Davalos-Yerovi, V.; Muns, M.D.; Tejero-Sanchez, M.; Duarte, E.; Sanchez-Rodriguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa, E.S.M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Voulgaridou, G.; Papadopoulou, S. Cancer, Phase Angle and Sarcopenia: The Role of Diet in Connection with Lung Cancer Prognosis. Lung 2022, 200, 347–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.J.; Bae, G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Stewart, G.D.; Skipworth, R.J.; Fearon, K.C. Cancer cachexia and fatigue. Clin. Med. 2006, 6, 140–143. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int. J. Mol. Sci. 2021, 22, 8491. [Google Scholar] [CrossRef]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Berriel Diaz, M. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(☆). ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.A.; Ballinger, T.J.; Bhandari, R.; Dieli-Cornwright, C.M.; Guertin, K.A.; Hibler, E.A.; Kalam, F.; Lohmann, A.E.; Ippolito, J.E. Imaging modalities for measuring body composition in patients with cancer: Opportunities and challenges. J. Natl. Cancer Inst. Monogr. 2023, 2023, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Abbass, T.; Dolan, R.D.; Laird, B.J.; McMillan, D.C. The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review. Cancers 2019, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, S.; Gianotti, L.; Wu, G. Evaluation and management of body composition changes in cancer patients. Nutrition 2023, 114, 112132. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kytta, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef]
- Abbass, T.; Dolan, R.D.; McMillan, D.C. Computed tomography-derived body composition analysis in patients with advanced cancer: Clinical utility and future research. Curr. Opin. Support. Palliat. Care 2020, 14, 309–315. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, M.; Gyarmathy, V.A.; Kaposi, A.; Kopa, Z. The potential role of central obesity in male infertility: Body mass index versus waist to hip ratio as they relate to selected semen parameters. BMC Public Health 2020, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.M.; Smith, S. Body composition and morphological assessment of nutritional status in adults: A review of anthropometric variables. J. Hum. Nutr. Diet. 2016, 29, 7–25. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (US); Office of Medical Applications of Research. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar] [CrossRef]
- Eaton-Evans, J. Nutritional assessment: Anthropometry. In Encyclopedia of Human Nutrition; Waltham, A.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 227–232. [Google Scholar]
- Butler, R.; McClinchy, J.; Morreale-Parker, C.; Marsh, W.; Rennie, K.L. BMI calculation in older people: The effect of using direct and surrogate measures of height in a community-based setting. Clin. Nutr. ESPEN 2017, 22, 112–115. [Google Scholar] [CrossRef]
- Misra, A. Ethnic-Specific Criteria for Classification of Body Mass Index: A Perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol. Ther. 2015, 17, 667–671. [Google Scholar] [CrossRef]
- Vatier, C.; Poitou, C.; Clement, K. Evaluation of visceral fat in massive obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, A.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–77. [Google Scholar]
- Moonen, H.; Van Zanten, A.R.H. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr. Opin. Crit. Care 2021, 27, 344–353. [Google Scholar] [CrossRef]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Sowers, M.R.; Tisch, J. Insulin resistance, body weight, obesity, body composition, and the menopausal transition. In Menopause, Biology and Pathobiology; Elsevier: Amsterdam, The Netherlands, 2000; pp. 245–260. [Google Scholar]
- Tylavsky, F.A.; Lohman, T.G.; Dockrell, M.; Lang, T.; Schoeller, D.A.; Wan, J.Y.; Fuerst, T.; Cauley, J.A.; Nevitt, M.; Harris, T.B. Comparison of the effectiveness of 2 dual-energy X-ray absorptiometers with that of total body water and computed tomography in assessing changes in body composition during weight change. Am. J. Clin. Nutr. 2003, 77, 356–363. [Google Scholar] [CrossRef]
- Goldman, L.W. Principles of CT and CT technology. J. Nucl. Med. Technol. 2007, 35, 115–128; quiz 129–130. [Google Scholar] [CrossRef]
- Kalender, W.A. X-ray computed tomography. Phys. Med. Biol. 2006, 51, R29. [Google Scholar] [CrossRef]
- Paris, M.T. Body Composition Analysis of Computed Tomography Scans in Clinical Populations: The Role of Deep Learning. Lifestyle Genom. 2020, 13, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Boutin, R.D.; Kaptuch, J.M.; Bateni, C.P.; Chalfant, J.S.; Yao, L. Influence of IV Contrast Administration on CT Measures of Muscle and Bone Attenuation: Implications for Sarcopenia and Osteoporosis Evaluation. AJR Am. J. Roentgenol. 2016, 207, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ban, M.J.; Park, J.H.; Lee, S.M. Visceral adipose tissue volume and CT-attenuation as prognostic factors in patients with head and neck cancer. Head. Neck 2019, 41, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Burden, S.T.; Strauss, B.J.; Todd, C.; Lal, S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: A systematic review. Eur. J. Clin. Nutr. 2015, 69, 1079–1086. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Witney, T.H.; Lewis, D.Y. Imaging Cancer Metabolism with Positron Emission Tomography (PET). Methods Mol. Biol. 2019, 1928, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Geus-Oei, L.F.; Oyen, W.J. Predictive and prognostic value of FDG-PET. Cancer Imaging 2008, 8, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Bos, S.A.; Gill, C.M.; Torriani, M.; Bredella, M.A. Brown adipose tissue and cancer progression. Skelet. Radiol. 2020, 49, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Sanders, V.; Pullarkat, P.; Teja, S.; Salter, A.; Watkins, M.P.; Atagu, N.; Ludwig, D.R.; Mhlanga, J.; Mellnick, V.M.; et al. Metabolic Biomarkers Assessed with PET/CT Predict Sex-Specific Longitudinal Outcomes in Patients with Diffuse Large B-Cell Lymphoma. Cancers 2022, 14, 2932. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Kitajima, K.; Toriihara, A.; Nakajo, M.; Hirata, K. Recent topics of the clinical utility of PET/MRI in oncology and neuroscience. Ann. Nucl. Med. 2022, 36, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kersting, D.; Rischpler, C.; Opitz, M.; Kirchner, J.; Pabst, K.M.; Mavroeidi, I.A.; Laschinsky, C.; Grueneisen, J.; Schaarschmidt, B.; et al. Clinical Use of PET/MR in Oncology: An Update. Semin. Nucl. Med. 2022, 52, 356–364. [Google Scholar] [CrossRef]
- Lundstrom, E.; Andersson, J.; Engstrom, M.; Lubberink, M.; Strand, R.; Ahlstrom, H.; Kullberg, J. PET/MRI of glucose metabolic rate, lipid content and perfusion in human brown adipose tissue. Sci. Rep. 2021, 11, 14955. [Google Scholar] [CrossRef]
- Sawicki, P.; Talalaj, M.; Zycinska, K.; Zgliczynski, W.S.; Wierzba, W. Current Applications and Selected Technical Details of Dual-Energy X-Ray Absorptiometry. Med. Sci. Monit. 2021, 27, e930839. [Google Scholar] [CrossRef]
- Slart, R.; Tsoumpas, C.; Glaudemans, A.; Noordzij, W.; Willemsen, A.T.M.; Borra, R.J.H.; Dierckx, R.; Lammertsma, A.A. Long axial field of view PET scanners: A road map to implementation and new possibilities. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4236–4245. [Google Scholar] [CrossRef]
- Smoot, B.J.; Mastick, J.; Shepherd, J.; Paul, S.M.; Kober, K.M.; Cooper, B.A.; Conley, Y.P.; Dixit, N.; Hammer, M.J.; Fu, M.R.; et al. Use of Dual-Energy X-Ray Absorptiometry to Assess Soft Tissue Composition in Breast Cancer Survivors with and without Lymphedema. Lymphat. Res. Biol. 2022, 20, 391–397. [Google Scholar] [CrossRef]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle dysfunction in cancer patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef]
- Kroll, L.; Mathew, A.; Baldini, G.; Hosch, R.; Koitka, S.; Kleesiek, J.; Rischpler, C.; Haubold, J.; Fuhrer, D.; Nensa, F.; et al. CT-derived body composition analysis could possibly replace DXA and BIA to monitor NET-patients. Sci. Rep. 2022, 12, 13419. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022, 47, 3205–3216. [Google Scholar] [CrossRef]
- Han, J.; Harrison, L.; Patzelt, L.; Wu, M.; Junker, D.; Herzig, S.; Berriel Diaz, M.; Karampinos, D.C. Imaging modalities for diagnosis and monitoring of cancer cachexia. EJNMMI Res. 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Boutin, R.D.; Yao, L.; Canter, R.J.; Lenchik, L. Sarcopenia: Current Concepts and Imaging Implications. AJR Am. J. Roentgenol. 2015, 205, W255–W266. [Google Scholar] [CrossRef] [PubMed]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Shangguan, J.; Pan, L.; Zhou, X.; Yaghmai, V.; Velichko, Y.; Hu, C.; Yang, J.; Zhang, Z. MRI Assessment of Associations between Brown Adipose Tissue and Cachexia in Murine Pancreatic Ductal Adenocarcinoma. Intern. Med. Open Access 2019, 9, 301. [Google Scholar] [CrossRef]
- Ritz, A.; Froeba-Pohl, A.; Kolorz, J.; Vigodski, V.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Haberle, B.; Schmid, I.; Kappler, R.; et al. Total Psoas Muscle Area as a Marker for Sarcopenia Is Related to Outcome in Children With Neuroblastoma. Front. Surg. 2021, 8, 718184. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.S.; Ormiston, W.; Heron, R.; Pontrè, B.; MacLeod, R.; Doyle, A. Body composition skeletal muscle analysis in cancer cachexia studies: Is there a place for 3T MRI analysis? JCSM Clin. Rep. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Gray, C.; MacGillivray, T.J.; Eeley, C.; Stephens, N.A.; Beggs, I.; Fearon, K.C.; Greig, C.A. Magnetic resonance imaging with k-means clustering objectively measures whole muscle volume compartments in sarcopenia/cancer cachexia. Clin. Nutr. 2011, 30, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Bastijns, S.; Sanchez-Rodriguez, D.; Piotrowicz, K.; De Cock, A.M.; Full SARCUS Working Group. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update: Reply to the letter to the editor: SARCUS working group on behalf of the Sarcopenia Special Interest Group of the European Geriatric Medicine Society. Eur. Geriatr. Med. 2021, 12, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.L.N.; Borges, T.C.; Pichard, C.; Pimentel, G.D. Correlation between SARC-F Score and Ultrasound-Measured Thigh Muscle Thickness in Older Hospitalized Cancer Patients. J. Nutr. Health Aging 2020, 24, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Escriche-Escuder, A.; Trinidad-Fernandez, M.; Pajares, B.; Iglesias-Campos, M.; Alba, E.; Cuesta-Vargas, A.I.; Roldan-Jimenez, C. Ultrasound use in metastatic breast cancer to measure body composition changes following an exercise intervention. Sci. Rep. 2021, 11, 8858. [Google Scholar] [CrossRef]
- Galli, A.; Colombo, M.; Carrara, G.; Lira Luce, F.; Paesano, P.L.; Giordano, L.; Bondi, S.; Tulli, M.; Mirabile, A.; De Cobelli, F.; et al. Low skeletal muscle mass as predictor of postoperative complications and decreased overall survival in locally advanced head and neck squamous cell carcinoma: The role of ultrasound of rectus femoris muscle. Eur. Arch. Otorhinolaryngol. 2020, 277, 3489–3502. [Google Scholar] [CrossRef]
- Lortie, J.; Rush, B.; Osterbauer, K.; Colgan, T.J.; Tamada, D.; Garlapati, S.; Campbell, T.C.; Traynor, A.; Leal, T.; Patel, V.; et al. Myosteatosis as a Shared Biomarker for Sarcopenia and Cachexia Using MRI and Ultrasound. Front. Rehabil. Sci. 2022, 3, 896114. [Google Scholar] [CrossRef]
- Weber, M.A.; Krakowski-Roosen, H.; Schroder, L.; Kinscherf, R.; Krix, M.; Kopp-Schneider, A.; Essig, M.; Bachert, P.; Kauczor, H.U.; Hildebrandt, W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009, 48, 116–124. [Google Scholar] [CrossRef]
- Jatoi, A.; Kaasa, S.; Strijbos, M. Esmo Handbook of Nutrition and Cancer, 2nd ed.; Education Library: Oatlands, Australia, 2023. [Google Scholar]
- Neacsu, F.; Varban, A.S.; Simion, G.; Surghie, R.; Patrascu, O.M.; Sajin, M.; Dumitru, M.; Vrinceanu, D. Lung cancer mimickers—A case series of seven patients and review of the literature. Rom. J. Morphol. Embryol. 2021, 62, 697–704. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29-Identifier NCT06007794, Correlation Between Ultrasound-Assessed Quadriceps Muscle Mass and Baseline Whole-Body Densitometry Muscle Index in the Post-Cancer Population (JUMP Research II) (JUMPresearchII). 2023. Available online: https://classic.clinicaltrials.gov/ct2/history/NCT06007794?V_1=View (accessed on 10 February 2024).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29-Identifier NCT03867357, Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia. 2023. Available online: https://clinicaltrials.gov/study/NCT03867357?term=NCT03867357&rank=1 (accessed on 10 February 2024).


| Features | Cachexia | Sarcopenia |
|---|---|---|
| Definition | Disease-related malnutrition based on the GLIM definition and the presence of systemic inflammation [12]. | Decline in skeletal muscle mass and function, increased risk of falls, physical disability, poor quality of life, and mortality [3,4,9]. |
| Causes | Negative energy balance (increased energy expenditure and decrease in energy intake) and metabolic alterations generated by a pro-inflammatory environment [8,12,13]. | Inflammation, reduced physical activity, malnutrition, and direct effects of cancer therapies [4]. |
| Clinical features | Weight loss, decreased skeletal muscle mass, anorexia, and metabolic abnormalities [2,10,11]. | Low muscle strength, low muscle quantity/quality, and low physical performance [4,5,6]. |
| Source | Pathology | Diagnostic Method | Results |
|---|---|---|---|
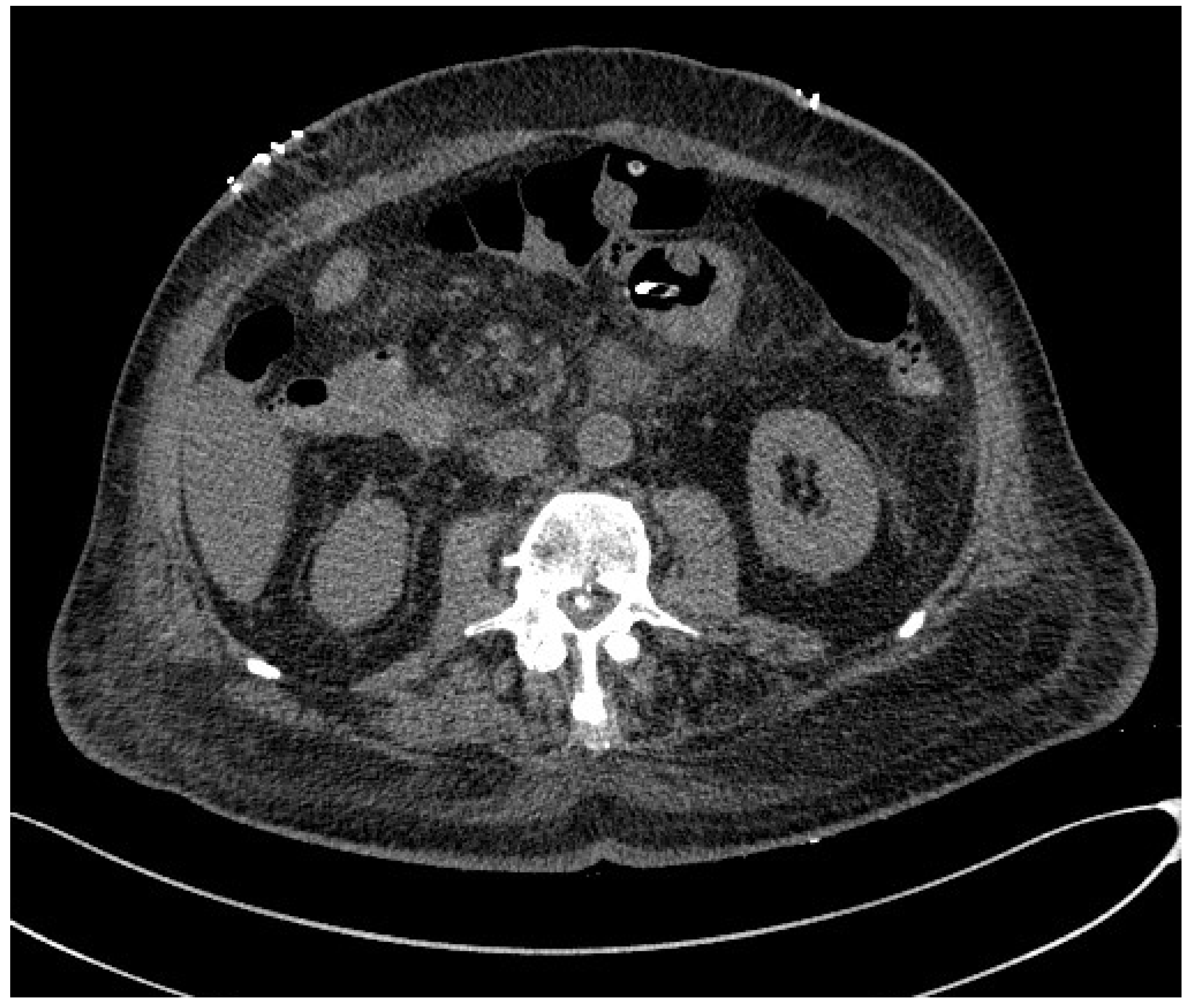
| Aleixo GFP. et al. [3] | Sarcopenia | CT |
|
| Lee JW. et al. [41] | Cachexia |
| |
| Witney TH. et al. [45] Chu K. et al. [48] Jaswal S. et al. [49] Seifert R. et al. [51] | Sarcopenia | FDG-PET |
|
| Chu K. et al. [48] | Cachexia |
| |
| Chianca V. et al. [59] | Sarcopenia Cachexia | MRI |
|
| Tagliafico AS et al. [61] Han J et al. [60] | Sarcopenia Cachexia |
| |
| Boutin RD et al. [62] | Sarcopenia |
| |
| Zhang Y et al. [64] | Sarcopenia | MRI |
|
| Ritz A et al. [65] | Sarcopenia |
| |
| Rogers ES et al. [66] | Cachexia |
| |
| Gray C et al. [67] | Sarcopenia |
| |
| Casey P et al. [68] | Sarcopenia | US |
|
| Perkisas S et al. [69] | Sarcopenia |
| |
| Gomes TLN et al. [70] | Sarcopenia |
| |
| Escriche-Escuder A et al. [71] | Sarcopenia |
| |
| Galli A et al. [72] | Cachexia | US |
|
| Lortie J et al. [73] | Sarcopenia Cachexia |
| |
| Weber MA et al. [74] | Cachexia |
|
| Availability | Costs | Transportability | Examiner Dependency | |
|---|---|---|---|---|
| CT | High | Medium | Not transportable | Independent |
| FDG-PET | Medium | High | Not transportable | Independent |
| MRI | Medium | High | Not transportable | Independent |
| US | High | Low | Transportable | Dependent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortellaro, S.; Triggiani, S.; Mascaretti, F.; Galloni, M.; Garrone, O.; Carrafiello, G.; Ghidini, M. Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review. J. Pers. Med. 2024, 14, 243. https://doi.org/10.3390/jpm14030243
Mortellaro S, Triggiani S, Mascaretti F, Galloni M, Garrone O, Carrafiello G, Ghidini M. Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review. Journal of Personalized Medicine. 2024; 14(3):243. https://doi.org/10.3390/jpm14030243
Chicago/Turabian StyleMortellaro, Sveva, Sonia Triggiani, Federica Mascaretti, Micol Galloni, Ornella Garrone, Gianpaolo Carrafiello, and Michele Ghidini. 2024. "Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review" Journal of Personalized Medicine 14, no. 3: 243. https://doi.org/10.3390/jpm14030243
APA StyleMortellaro, S., Triggiani, S., Mascaretti, F., Galloni, M., Garrone, O., Carrafiello, G., & Ghidini, M. (2024). Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review. Journal of Personalized Medicine, 14(3), 243. https://doi.org/10.3390/jpm14030243








