Management of Upper Tract Urothelial Carcinoma in a Double Collecting System Kidney
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Study Selection and Data Extraction
- (1)
- Any case of UT malignancy out of urothelial origin was excluded (i.e., renal cell carcinoma).
- (2)
- Cases of urothelial malignancy of the UT in a kidney with anomalies other than DCS were excluded.
- (3)
- Any case of hemi-NU due to a nonfunctioning moiety was excluded.
- Articles were selected based on the following inclusion criteria:
- (1)
- Any original case report or series of UTUC in a DCS kidney was included.
- (2)
- Cases of urothelial neoplasia other than carcinoma in DCS or special UC variants (sarcomas) were included.
- (3)
- Cases of UTUC in DCS that were not surgically managed were included.
- (4)
- Abstracts and publications without full text were included.
- (5)
- A single case of a paper published with English abstract and Japanese content was translated via Google Translate for specific clarifications. Most of the information retrieved was in English; therefore, it has been included.
2.3. Data Analysis
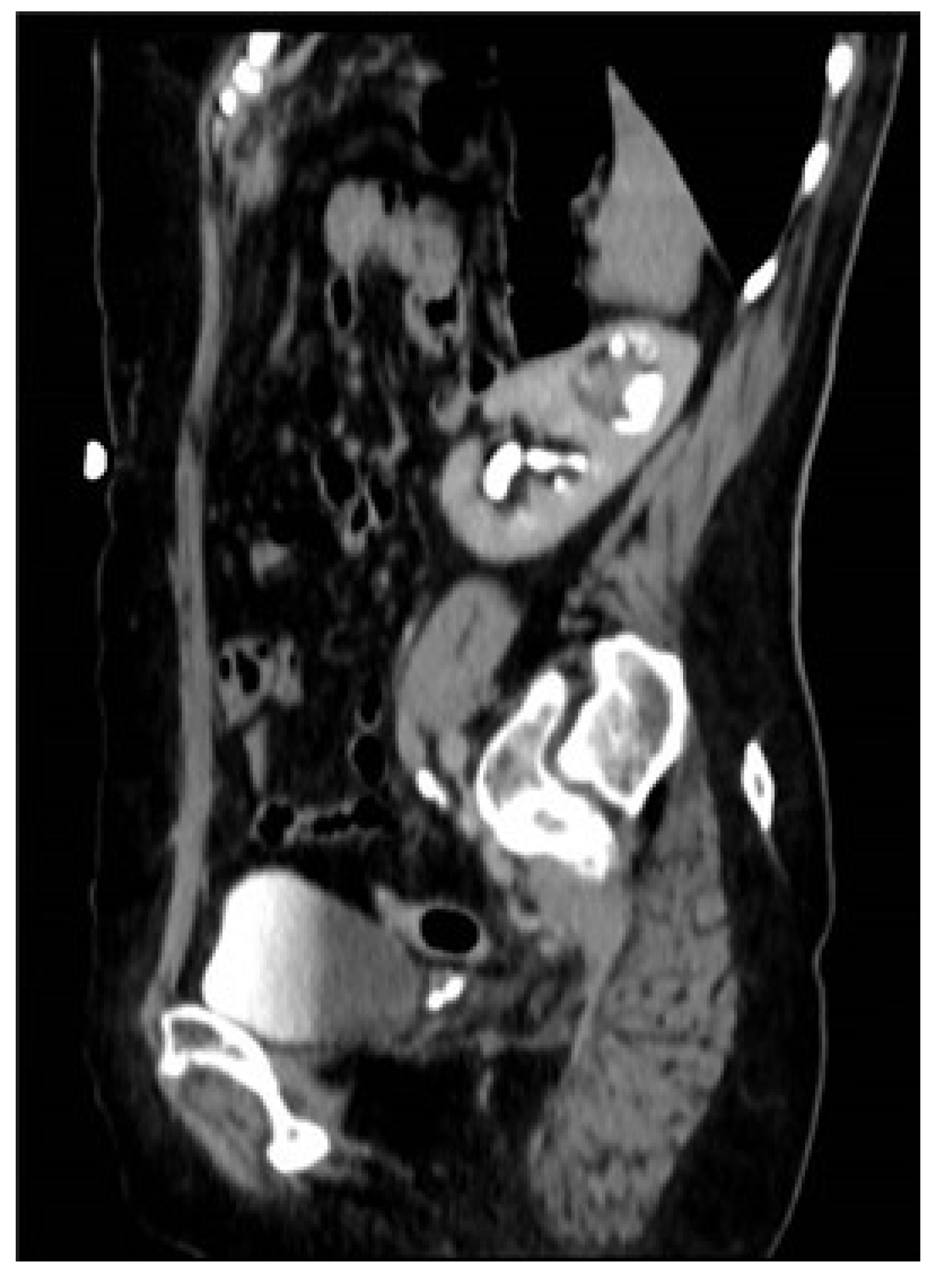
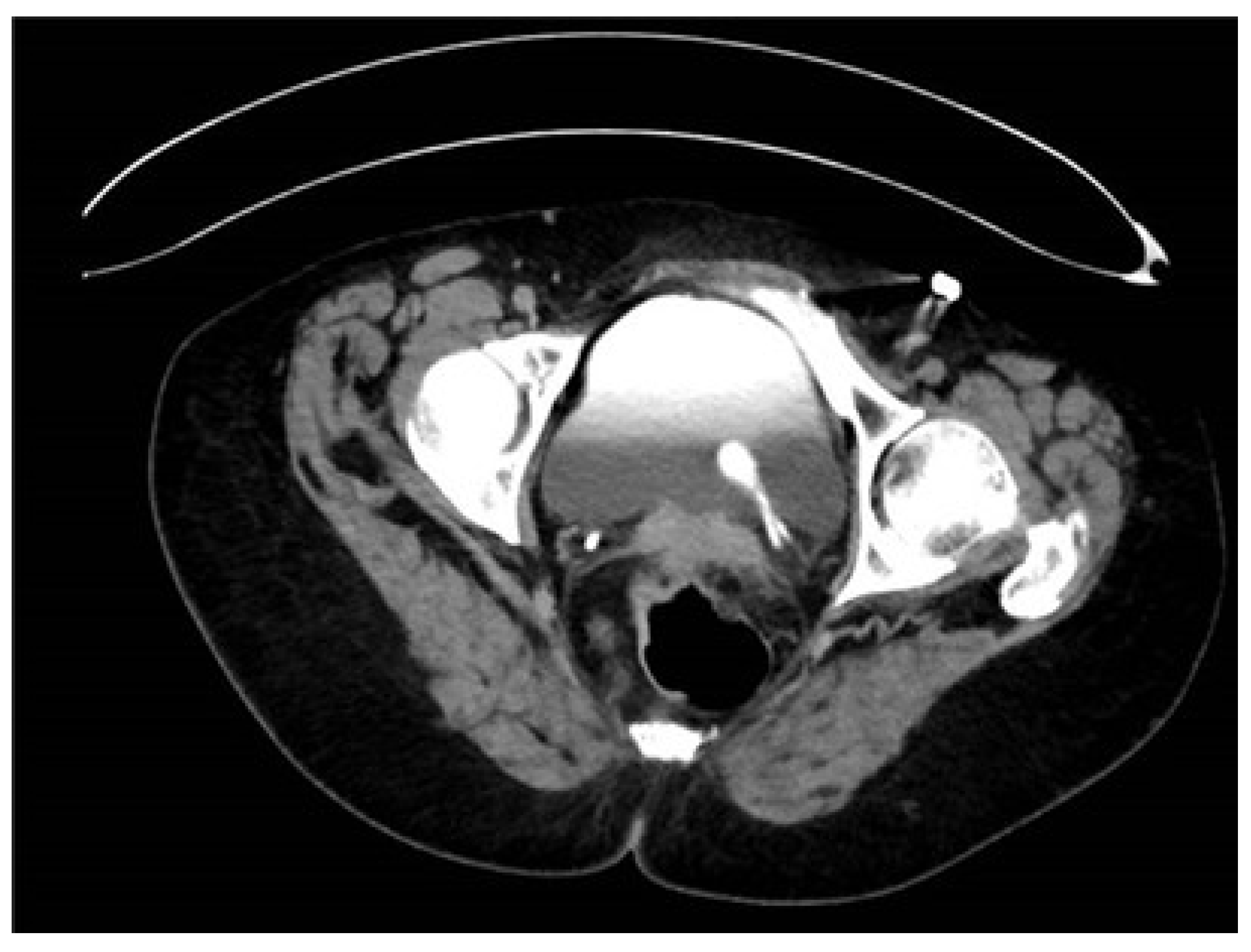
3. Case Presentation
3.1. Management
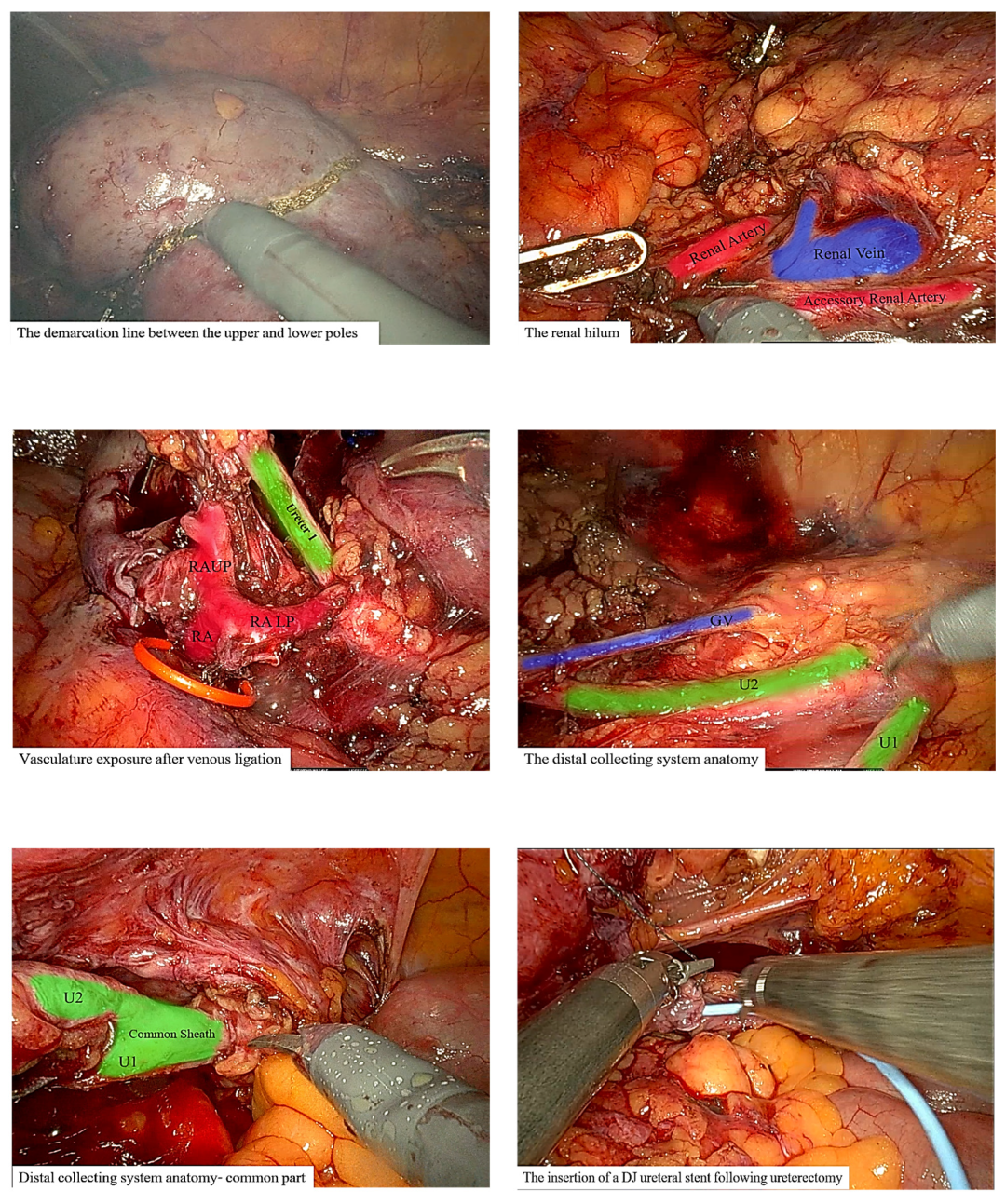
3.2. Surgical Intervention
3.3. Operative and Postoperative Course
3.4. Follow-Up
4. Results
5. Discussion
5.1. Hemi-Nephroureterectomy Considerations
5.2. Surgical Management of UTUC in Malformed Kidney
5.3. Pathophysiology of UTUC in Malformed Kidney
5.4. Oncological Considerations
5.5. Follow-Up Plan
5.6. Prognosis
5.7. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, J.; Chen, J.-F.; Al-Ahmadie, H. Urothelial Carcinoma. Surg. Pathol. Clin. 2022, 15, 641–659. [Google Scholar] [CrossRef]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bascietto, F.; Khalil, A.; Rizzo, G.; Makatsariya, A.; Buca, D.; Silvi, C.; Ucci, M.; Liberati, M.; Familiari, A.; D’Antonio, F. Prenatal imaging features and postnatal outcomes of isolated fetal duplex renal collecting system: A systematic review and meta-analysis. Prenat. Diagn. 2020, 40, 424–431. [Google Scholar] [CrossRef]
- Didier, R.A.; Chow, J.S.; Kwatra, N.S.; Retik, A.B.; Lebowitz, R.L. The duplicated collecting system of the urinary tract: Embryology, imaging appearances and clinical considerations. Pediatr. Radiol. 2017, 47, 1526–1538. [Google Scholar] [CrossRef]
- Houat, A.P.; Guimarães, C.T.S.; Takahashi, M.S.; Rodi, G.P.; Gasparetto, T.P.D.; Blasbalg, R.; Velloni, F.G. Congenital Anomalies of the Upper Urinary Tract: A Comprehensive Review. RadioGraphics 2021, 41, 462–486. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Margalit, R.; Efron, G.; Pleniceanu, O.; Tzur, D.; Stern-Zimmer, M.; Afek, A.; Erlich, T.; Derazne, E.; Kark, J.D.; Keinan-Boker, L.; et al. Congenital Anomalies of the Kidney and Urinary Tract and Adulthood risk of Urinary Tract Cancer. Kidney Int. Rep. 2021, 6, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-S.; Chuang, C.-K.; Wu, C.-H.; Liaw, C.-C.; Lee, N. Upper urinary tract tumor in a duplicated collecting system: Report of three cases and review of the literature. Chang Gung Med. J. 2003, 26, 377–382. [Google Scholar]
- Banya, Y.; Abe, T.; Sasaki, H.; Aoki, H.; Fujioka, T.; Akasaka, T.; Kubo, T.; Ohori, T. A case of primary ureteral carcinoma in the duplicated renal pelvis and ureter diagnosed by transurethral uretero-renoscopy. Hinyokika Kiyo 1986, 32, 454–461. [Google Scholar] [PubMed]
- Tudor, R.G.; Clear, J.D. Conservative surgery in the management of carcinoma in a duplex ureter. J. R. Coll. Surg. Edinb. 1986, 31, 323–324. [Google Scholar] [PubMed]
- Budd, J.S. Primary transitional cell carcinoma of the renal pelvis in a duplicated collecting system. Int. J. Clin. Pract. 1987, 41, 1063–1064. [Google Scholar] [CrossRef]
- Sreenevasan, G.; Por, P.K.Y.; Mukherjee, A.K.; Sabapathy, A.K. Carcinoma in One Limb of an Incompletely Duplicated Ureter. Br. J. Urol. 1987, 60, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Gepi-Attee, S.; Gingell, J.C. Ureteric Tumour in a Duplex System. Br. J. Urol. 1991, 68, 106. [Google Scholar] [CrossRef] [PubMed]
- Asase, D.; Frank, R.G.; Gerard, P.S.; Lindsay, K.; Wise, G.J. Transitional cell carcinoma of the renal pelvis in an incompletely duplicated collecting system. N. Y. State J. Med. 1993, 93, 59–61. [Google Scholar]
- Dudak, S.D.; Antun, R.A. Transitional cell carcinoma in a duplicated ectopic ureter. Urology 1995, 46, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-B.; Tserng, B.-R.; Huang, W.-H.; Tarn, C.-J. Synchronous Bilateral Carcinoma of the Ureter in Association with Unilateral Incomplete Duplication of the Ureter. Urol. Int. 1996, 56, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Tamada, H.; Tamura, T.; Kaneko, T.; Takata, K.; Sakuma, T.; Suzuki, Y. A case of renal pelvic carcinoma in a completely duplicated pelvis and ureter. Acta Urol. Jpn. 1998, 44, 733–735. [Google Scholar]
- Kawamura, H.; Sasaki, N. Transitional cell carcinoma in the blind-ending branch of the bifid ureter. BJU Int. 1998, 82, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Gohji, K.; Iwamoto, Y.; Masuda, H.; Segawa, N.; Kiura, H.; Ueda, H.; Katsuoka, Y. Ureter cancer of complete double renal pelvis and ureter: A case report. Acta Urol. Jpn. 2002, 48, 761–764. [Google Scholar]
- Hisataki, T.; Takahashi, A.; Taguchi, K.; Shimizu, T.; Suzuki, K.; Takatsuka, K.; Iwaki, H. Sarcomatoid transitional cell carcinoma originating from a duplicated renal pelvis. Int. J. Urol. 2001, 8, 704–706. [Google Scholar] [CrossRef]
- Unsal, A.; Cimentep, E.; Koc, A.; Akbulut, Z.; Balbay, M.D. A case of primary ureteral carcinoma in association with unilateral complete duplication of the ureter. Int. Urol. Nephrol. 2003, 35, 489–490. [Google Scholar] [CrossRef]
- Boris, R.S.; McIntire, L.; AlAlassi, O.; Peabody, J.O.; Savera, A. An unusual case of ureteral tumor in a duplex system. Int. Urol. Nephrol. 2007, 38, 473–474. [Google Scholar] [CrossRef]
- Chen, G.-M.; Chen, S.-W.; Xia, D.; Li, J.; Yan, S.; Jin, B.-Y. Sarcomatoid carcinoma of the renal pelvis in duplex kidney. Chin. Med. J. 2011, 124, 2074–2076. [Google Scholar]
- Kao, J.L.; Huang, C.H.; Chang, Y.M.; Tsai, S.C.; Yang, C.L.; Shiao, C.C. Ureteral cancer in a duplicated ureter. Qjm Int. J. Med. 2013, 106, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Chia-Hsiang Lin, V.; Wu, R.-H.; Yu, T.-J. Laparoscopic heminephrectomy and ureterectomy for Lower moiety urothelial carcinoma in a complete duplex right kidney. BJUI 2012, 20, A325. [Google Scholar] [CrossRef]
- Ogawa, M.; Morikawa, T.; Toyoshima, T.; Fukayama, M. Squamous cell carcinoma in a duplicated renal pelvis. Int. J. Clin. Exp. Pathol. 2014, 7, 7957–7961. [Google Scholar] [PubMed]
- Zhang, Y.; Yu, Q.; Zhang, Z.; Liu, R.; Xu, Y. Renal pelvis urothelial carcinoma of the upper moiety in complete right renal duplex: A case report. Int. J. Clin. Exp. Pathol. 2015, 8, 15422–15425. [Google Scholar] [PubMed]
- Karray, O.; Khouni, H.; Charfi, M.; Boulma, R.; Debaibi, M.; Makhlouf, R.; Bargaoui, K.; Nessej, O.; Seridi, A.; Fourti, S.; et al. Ureteral tumor in an ectopic duplex system: A case report. J. Med. Case Rep. 2019, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Haldar, B.; Pal, D.K. Transitional Cell Carcinoma in Pelvis of a Double Moiety Kidney- A Rare Presentation. J. Clin. Diagn. Res. 2020, 14, PD06–PD07. [Google Scholar] [CrossRef]
- Brnić, Z.; Schmidt, S.; Kovacević, D.; Bušić-Pavlek, I.; Krušlin, B.; Sjekavica, I. An Unusual Transitional Cell Carcinoma in Megaureter in the Patient with Duplicated Collecting System. Ann. Clin. Case Rep. 2020, 5, 1786. [Google Scholar]
- Storey, B.; Thirugnanasundralingam, V.; Raman, A. Crouching carcinoma, hidden ureter: A blind ending burnt out cancerous moiety. J. Clin. Urol. 2021, 17, 20514158211056440. [Google Scholar] [CrossRef]
- Nirei, T.; Tabei, T.; Ito, H.; Kobayashi, K. A Case of Renal Pelvic Cancer with a Complete Duplication of the Renal Pelvis and Ureter. Case Rep. Oncol. 2021, 14, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.; Memo, M. Upper tract urothelial carcinoma presenting at a bifurcation of a partially duplicated left ureter: A minimally invasive approach. Urol. Case Rep. 2022, 40, 101879. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.D.; Wittekind, C.T. TNM Classification of Malignant Tumors International Union Against Cancer, 8th ed.; Wiley: Oxford, UK, 2017. [Google Scholar]
- Barton, G.J.; Tan, W.P.; Inman, B.A. The nephroureterectomy: A review of technique and current controversies. Transl. Androl. Urol. 2020, 9, 3168–3190. [Google Scholar] [CrossRef] [PubMed]
- Masson-Lecomte, A.; Vaillant, V.; Roumiguié, M.; Lévy, S.; Pradère, B.; Peyromaure, M.; Duquesne, I.; De La Taille, A.; Lebâcle, C.; Panis, A.; et al. Oncological Outcomes of Distal Ureterectomy for High-Risk Urothelial Carcinoma: A Multicenter Study by The French Bladder Cancer Committee. Cancers 2022, 14, 5452. [Google Scholar] [CrossRef] [PubMed]
- Hora, M.; Albiges, L.; Bedke, J.; Campi, R.; Capitanio, U.; Giles, R.H.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; et al. European Association of Urology Guidelines Panel on Renal Cell Carcinoma Update on the New World Health Organization Classification of Kidney Tumours 2022: The Urologist’s Point of View. Eur. Urol. 2023, 83, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, M.T.; Ruiz, J.; Moldes, J.M.; Sentagne, A.; Tuchbaum, V.; Tessi, C.; Imizcoz, F.L.; Weller, S.; Vagni, R.; Ormaechea, M.N.; et al. Laparoscopic Upper-pole Heminephrectomy for the Management of Duplex Kidney: Outcomes of a Multicenter Cohort. Urology 2021, 156, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gözen, A.S.; Badawy, H.; Teber, D.; Assem, A.; Rassweiler, J. Outcome of laparoscopic upper pole heminephroureterectomy in children: A two-centre experience. Arab. J. Urol. 2016, 14, 287–291. [Google Scholar] [CrossRef]
- Seibold, J.; Schilling, D.; Nagele, U.; Anastasiadis, A.; Sievert, K.; Stenzl, A.; Corvin, S. Laparoscopic heminephroureterectomy for duplex kidney anomalies in the pediatric population. J. Pediatr. Urol. 2008, 4, 345–347. [Google Scholar] [CrossRef]
- Cullivan, O.; Byrnes, K.; D’arcy, F. Laparoscopic left hemi-nephroureterectomy for a patient with suspected urothelial carcinoma in a horseshoe kidney. J. Surg. Case Rep. 2022, 2022, rjac025. [Google Scholar] [CrossRef]
- Marshall, F.F.; Freedman, M.T. Crossed renal ectopia. J. Urol. 1978, 119, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Power, R.J.; MacDonald, L.; Johnston, P.; Organ, M. Nephroureterectomy of Right-to-Left Crossed Fused Renal Ectopia with Urothelial Carcinoma. Cureus 2020, 12, e8544. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Rausch, S.; Lotan, Y.; Youssef, R.F. Squamous cell carcinogenesis and squamous cell carcinoma of the urinary bladder: A contemporary review with focus on nonbilharzial squamous cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 32.e11–32.e16. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Robinson, B.D.; Green, D.A.; Cha, E.K.; Hansen, J.; Comploj, E.; Margulis, V.; Raman, J.D.; Ng, C.K.; Remzi, M.; et al. Impact of Histological Variants on Clinical Outcomes of Patients with Upper Urinary Tract Urothelial Carcinoma. J. Urol. 2012, 188, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wu, P.; Liu, S.; Seery, S.; Liu, J.; He, L.; Liu, M.; Zhang, Y.; Wang, J.-Y.; Xu, T. Presence of secondary bladder cancer following radical nephroureterectomy for upper tract urothelial carcinoma: Characteristics, risk factors, and predictive value. BMC Urol. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Colin, P.; Rouprêt, M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat. Rev. Urol. 2015, 12, 155–166. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Yang, C.; Cheng, C.; Yang, C.; Ou, Y.; Ho, H.; Lin, C.; Hung, S.; Chen, C.; et al. Significant predictors of contralateral upper tract recurrence after radical nephroureterectomy for upper tract urothelial carcinoma. Int. J. Urol. 2022, 29, 69–75. [Google Scholar] [CrossRef]
- Shigeta, K.; Matsumoto, K.; Tanaka, N.; Mikami, S.; Kosaka, T.; Yasumizu, Y.; Takeda, T.; Mizuno, R.; Kikuchi, E.; Oya, M. Profiling the Biological Characteristics and Transitions through Upper Tract Tumor Origin, Bladder Recurrence, and Muscle-Invasive Bladder Progression in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2022, 23, 5154. [Google Scholar] [CrossRef]
- Mullai, N.; Ruby, K.E.; Weiss, R. Importance of public awareness of the association of tobacco products to bladder cancer. J. Clin. Oncol. 2020, 38, e13564. [Google Scholar] [CrossRef]
- Tsumura, K.; Arai, E.; Tian, Y.; Shibuya, A.; Nishihara, H.; Yotani, T.; Yamada, Y.; Takahashi, Y.; Maeshima, A.M.; Fujimoto, H.; et al. Establishment of permutation for cancer risk estimation in the urothelium based on genome-wide DNA methylation analysis. Carcinog. 2019, 40, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Yu, H.; Ko, Y. Chronic arsenic exposure and its adverse health effects in Taiwan: A paradigm for management of a global environmental problem. Kaohsiung J. Med. Sci. 2011, 27, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Even, E.; Masuda, H.; Shibata, T.; Nojima, A.; Sakamoto, Y.; Murasaki, Y.; Chiba, H. Geochemical distribution and fate of arsenic in water and sediments of rivers from the Hokusetsu area, Japan. J. Hydrol. Reg. Stud. 2017, 9, 34–47. [Google Scholar] [CrossRef]
- Chen, C.-H.; Dickman, K.G.; Moriya, M.; Zavadil, J.; Sidorenko, V.S.; Edwards, K.L.; Gnatenko, D.V.; Wu, L.; Turesky, R.J.; Wu, X.-R.; et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Daza, J.; Poltiyelova, E.; Gul, Z.; Heard, J.R.; Ferket, B.S.; Waingankar, N.; Galsky, M.D.; Sfakianos, J.P. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int. 2019, 124, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Yip, W.; Wong, N.C.; Sjoberg, D.D.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Cha, E.K.; Donahue, T.F.; et al. Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients with High-Grade Upper Tract Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Martini, A.; Raggi, D.; Cucchiara, V.; Colecchia, M.; Lucianò, R.; Villa, L.; Mazzone, E.; Basile, G.; Scuderi, S.; et al. A feasibility study of preoperative pembrolizumab before radical nephroureterectomy in patients with high-risk, upper tract urothelial carcinoma: PURE-02. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 10.e1–10.e6. [Google Scholar] [CrossRef]
- Veccia, A.; Carbonara, U.; Djaladat, H.; Mehrazin, R.; Eun, D.D.; Reese, A.C.; Meng, X.; Uzzo, R.; Srivastava, A.; Porter, J.R.; et al. Robotic vs. Laparoscopic Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Multicenter Propensity-Score Matched Pair “tetrafecta” Analysis (ROBUUST Collaborative Group). J. Endourol. 2022, 36, 752–759. [Google Scholar] [CrossRef]
- Zhang, X.; Bu, R.; Liu, Z.; Wu, B.; Bai, S. Development and Validation of a Model for Predicting Intravesical Recurrence in Organ-confined Upper Urinary Tract Urothelial Carcinoma Patients after Radical Nephroureterectomy: A Retrospective Study in One Center with Long-term Follow-up. Pathol. Oncol. Res. 2020, 26, 1741–1748. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Chang, C.-H.; Huang, C.-P.; Yu, C.-C.; Lo, C.-W.; Chung, S.-D.; Wu, W.-C.; Chen, I.-H.A.; Lin, J.-T.; Jiang, Y.-H.; et al. Is Lymph Node Dissection Necessary During Radical Nephroureterectomy for Clinically Node-Negative Upper Tract Urothelial Carcinoma? A Multi-Institutional Study. Front. Oncol. 2022, 12, 791620. [Google Scholar] [CrossRef]
- Qiwei, C.; Jiajun, S.; Cheng, L.; Shengbo, H.; Yue, K.; Shujing, W.; Liu, W.; Xinqing, Z.; Hongyu, W.; Deyong, Y. Comparison between renal pelvic and ureteral tumors in muscle-invasive upper tract urothelial carcinoma. Cancer Sci. 2023, 114, 984–994. [Google Scholar] [CrossRef]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Matsubara, N.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; Gunduz, S.; Loriot, Y.; Rodriguez-Vida, A.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; E Gschwend, J.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Treatment of muscle-invasive urothelial cancer with nivolumab (CheckMate 274 study): A plain language summary. Futur. Oncol. 2023, 19, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Tomita, Y.; Nasroulah, F.; Li, J.; Collette, S.; Valderrama, B.P.; Grimm, M.O.; et al. Disease-free Survival Analysis for Patients with High-risk Muscle-invasive Urothelial Carcinoma from the Randomized CheckMate 274 Trial by PD-L1 Combined Positive Score and Tumor Cell Score. Eur Urol. 2023, 83, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Katims, A.B.; Kuo, F.; Reisz, P.; Tracey, A.; Thomas, J.; Yip, W.; Merghoub, T.; Bochner, B.H.; Pietzak, E.J.; Solit, D.B.; et al. Characterizing the immune phenotype of FGFR3 mutated upper tract urothelial carcinoma (UTUC) using single-cell (sc)RNA-sequencing (seq). J. Clin. Oncol. 2023, 41, 558. [Google Scholar] [CrossRef]
- Iwata, T.; Kimura, S.; Abufaraj, M.; Janisch, F.; Karakiewicz, P.I.; Seebacher, V.; Rouprêt, M.; Nasu, Y.; Shariat, S.F. The role of adjuvant radiotherapy after surgery for upper and lower urinary tract urothelial carcinoma: A systematic review. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Giri, S.; Pathak, R.; Bhatt, V.R.; Martin, M.G. Effect of adjuvant radiotherapy on survival in patients with locoregional urothelial malignancies of the upper urinary tract. Anticancer Res. 2016, 36, 4051–4055. [Google Scholar] [PubMed]
- Tian, C.; Liu, J.; An, L.; Hong, Y.; Xu, Q. Prognostic nomogram for overall survival in upper urinary tract urothelial carcinoma (UTUC) patients treated with chemotherapy: A SEER-based retrospective cohort study. BMC Urol. 2023, 23, 2. [Google Scholar]
- Wu, J.; Chen, S.; Wu, X.; Mao, W.; Wang, Y.; Xu, B.; Zheng, D.; Chen, M. Trends of incidence and prognosis of upper tract urothelial carcinoma. Bosn. J. Basic Med. Sci. 2021, 21, 607–619. [Google Scholar] [CrossRef]
| Reference | Year | Region | Age | Gender | Presentation | Exposure | Cr | Diagnosis | Anatomy | Side of Duplicated System | Location of Tumor | Management | Approach | Pathology | Follow-Up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kumon et al. [8] | 1981 | Japan | 52 | M | N/A | N/A | N/A | N/A | Incomplete | Right | At the junction site of the incomplete duplicated ureters | N/A | N/A | N/A | N/A |
| 2 | 67 | M | Incomplete | Left | ||||||||||||
| 3 | 70 | F | Incomplete | Right | ||||||||||||
| 4 | 67 | F | Incomplete | Left | ||||||||||||
| 5 | 62 | F | Incomplete | Right | Near the junction site | |||||||||||
| 6 | Banya et al. [9] | 1986 | Japan | 78 | M | Hematuria | N/A | N/A | IVU, RPG | Incomplete | Right | Polyp-like filling defect in the lower segment of duplicated ureter at about 4 cm from the fusion of the ureters | Radical NU + partial cystectomy | N/A | Prior to NU, patient underwent diagnostic URS with tumor resection, primary pathology confirmed T1 LG disease. Histopathology after NU was negative for malignancy | N/A |
| 7 | Tudor et al. [10] | 1986 | Japan | 56 | F | Hematuria | N/A | N/A | IVU, US | Complete | Right | Right lower moiety ureter near the orifice | Local excision with ureteroureterostomy | Laparotomy | N/A | N/A |
| 8 | Budd et al. [11] | 1987 | United Kingdom | 40 | M | Hematuria | N/A | N/A | IVU | Complete | Right | Right upper moiety pelvis | Hemi-NU | Laparotomy | N/A | N/A |
| 9 | G. SREENEVASAN et al. [12] | 1987 | Malaysia | 67 | M | hematuria | N/A | N/A | IVU | Incomplete | Right | Lower moiety proximal ureter 2 cm above fusion | Radical NU + bladder cuff | Laparotomy | Papillary TCC | 2 years follow-up NED |
| 10 | Gepi-attee [13] | 1991 | Japan | 74 | M | Hematuria | Smoking | N/A | US, RPG | Incomplete | Left | Left upper moiety | Hemi-NU | Laparotomy | G2pT2 invasive papillary UC resection margins free of tumor | N/A |
| 11 | Asase et al. [14] | 1992 | US | 66 | M | Hematuria, weakness | N/A | N/A | US, RPG | Incomplete | Left | Left renal pelvis of lower moiety | Radical NU with bladder cuff | Laparotomy | N/A | N/A |
| 12 | DUDAK et al. [15] | 1995 | US | 81 | M | Hematuria | No per anamnesis | 1.2 | IVU, CT, RPG | Complete | Left | Left upper moiety ectopic ureter distal part adjacent to orifice | Radical NU following nuclear renal scan (R 32% L 68% Cr level 1.2 mg/dL) | Laparotomy | pTa G I-II/III multifocal involvement in upper moiety pelvis | 7 months NED |
| 13 | Tan et al. [16] | 1996 | Taiwan | 62 | M | Hematuria, dysuria, suprapubic pain, cachexia | Lives in Taiwan (Blackfoot disease endemic area, long-term exposure to inorganic arsenic) | 10.7 mg/dL | US, CT, RPG | Incomplete | Left | Right distal ureter Left middle ureter area, after the point of ureteral fusion | Right—radical NU Left—segmental ureterectomy with bladder cuff and ileal ureter | Exploratory laparotomy | Right—pT1 G1 TCC Left-pT1G2 TCC | Follow-up RPG 6 months later- no recurrence, Postoperative Cr 4.9 mg/dL 2 years follow-up—NED |
| 14 | Tamada et al. [17] | 1998 | Japan | 72 | M | Hematuria | N/A | N/A | CTU, RPG | Complete | Left | Left upper moiety | Radical NU | N/A | TCC | N/A |
| 15 | KAWAMURA et al. [18] | 1998 | Japan | 67 | F | Hematuria | History of surgical and irradiation therapy for breast cancer | N/A | IVU, CT | Blind-ending bifid ureter | Right | The bifid ureter was filled with serous dark fluid with a narrow lumen | Radical NU | Laparotomy | pT3N0M0UC | Patient received adjuvant chemotherapy. 6 months follow-up NED |
| 16 | Chen et al. [8] | 2002 | Japan | 66 | M | Hematuria | No per anamnesis | 1 | IVU + RPG | Incomplete | Right | Right lower moiety | Radical NU | Laparoscopic | TCC in pelvis, ureters were free of tumor | 6 months NED |
| 17 | Chen et al. [8] | 2002 | Japan | 58 | M | Hematuria | No per anamnesis | 1.9 | IVU, CT | Complete | Right | Renal pelvis of upper moiety | Bilateral radical NU + bladder cuff | Laparotomy | TCC | After 1 year follow-up, patient developed bladder tumor, he died of sepsis 2 years after NU |
| Incomplete | Left | Mass in left lower moiety | ||||||||||||||
| 18 | Chen et al. [8] | 2002 | Japan | 65 | F | Intermittent hematuria for 2 months followed by an acute presentation with syncope and N and V | No per anamnesis | 16.6 mg/dL | CT (under hemodialysis), APG + RPG | Incomplete | Left | Distal end of the left lower moiety ureter immediately above the junction site | Radical NU + bladder cuff | Laparotomy | TCC | Maintenance hemodialysis 6-month follow-up cystoscopy revealed bladder recurrence. At 2-year follow-up, patient received intravesical chemotherapy |
| 19 | Takagi [19] | 2002 | Japan | 66 | M | Left flank pain | Lives in Japan | N/A | IVG, CT, MRI | Complete | Left | Papillary tumor from the left ureteral orifice of the lower renal moiety | Diagnostic URS confirmed pTaG2 UC followed by radical NU with partial cystectomy | Not mentioned | pT3G3N1MX UC | Patient received adjuvant chemotherapy (M-VAC) that was initially discontinued due to severe side effects, however, re-administrated 22 months later because of recurrence detected in retroperitoneal lymph nodes |
| 20 | Hisataki et al. [20] | 2002 | Japan | 43 | F | Hematuria and left flank pain | N/A | N/A | IVP, CTU | Incomplete | Left | Upper pole renal pelvis | Radical NU | N/A | Sarcomatoid TCC | Recurrence within 10 months Overall survival after recurrence 4 months |
| 21 | Unsal et al. [21] | 2003 | Turkey | 68 | F | Hematuria and right flank pain | S/P TAH + BSO due to endometrial carcinoma | Within normal limits | IVU, RPG, cystography (confirmed right side reflux) MRI | Complete | Right | Upper 1/3 of right upper moiety ureter | Hemi-NU+ bladder cuff | Laparotomy | TCC | N/A |
| 22 | Boris et al. [22] | 2006 | US | 51 | F | Hematuria | N/A | N/A | CTU, RPG | Incomplete | Right | Distal right ureter progress proximally into bifurcation | Radical NU | Laparotomy | T1 LG papillary UC | 2 years NED |
| 23 | G. M. Chen et al. [23] | 2011 | Japan | 77 | M | LUQ abdominal pain | No per anamnesis | N/A | Dipstick, no evidence of microhematuria CT, MRI | Complete | left | Upper pole moiety 16 cm mass | Radical NU + bladder cuff | N/A | Invasive sarcomatoid TCC | Full recovery on follow-up (adjuvant chemotherapy and radiotherapy were offered however patient refused) |
| 24 | Kao et al. [24] | 2012 | Taiwan | 87 | F | Hematuria, abdominal fullness | No per anamnesis | Within normal limits | CTU | Unclear | Left | duplicated left kidney with the normal excretory upper moiety along with the hydronephrosis caused by tumor infiltration involving upper ureter of the lower moiety | HG UC was confirmed via urine cytology, considering her age; patients received palliative radiotherapy | - | - | 6 months overall survival from diagnosis |
| 25 | LIN et al. [25] | 2012 | Taiwan | 82 | F | Hematuria | N/A | N/A | RPG | Complete | right | Lower moiety renal pelvis | Hemi-NU+ bladder cuff | Laparoscopic+ open for bladder cuff (Gibson’s incision) | pT1 HG UC | Two years follow-up NED |
| 26 | Ogawa et al. [26] | 2014 | Japan | 71 | F | Hematuria | N/A | N/A | CT, RPG | Incomplete | left | Left upper renal pelvis with invasion to renal parenchyma | Radical NU | N/A | pT4N0Mx squamous cell carcinoma | N/A |
| 27 | Zhang et al. [27] | 2015 | China | 65 | M | Hematuria and right flank pain | Smoking and drinking history Lives in Japan | N/A | US, CTU, MRU | Bilateral complete duplex collecting system on cystoscopy: right ectopic ureter insertion to posterior urethra | Right | Upper moiety renal pelvis | Radical NU | Laparotomy | Poorly differentiated UC showing invasive growth | N/A |
| 28 | Karray et al. [28] | 2019 | Tunisia | 52 | M | Hematuria | Smoking | Within normal limits | CTU | Complete + ectopic ureter | Right | Right upper pole ureter at the L2-4 level | Hemi-NU | Laparotomy | pT2G2 TCC | 2 years follow-up NED |
| 29 | Sarkar et al. [29] | 2020 | India | 46 | M | Hematuria | No per anamnesis | N/A | CTU | incomplete | Right | Renal pelvis of lower moiety | Radical NU | Laparoscopic | Infiltrating TCC | N/A |
| 30 | Brnić, Zoran et al. [30] | 2020 | Croatia | 63 | M | Hematuria | N/A | Within normal limits | CTU | Complete | Left | Distal part of upper moiety ureter was tortuous and largely dilated (megaureter), crossing lower moiety ureter at the level of iliac crest. Two centimeters caudally from that point, within megaureter, a slightly hyperdense 3 cm × 5 cm irregularly shaped tumor was identified | Radical NU | Laparotomy | HG invasive UC T1NxMxG3 | 2 years follow-up NED |
| 31 | Storey et al. [31] | 2021 | Australia | 76 | M | Hematuria | N/A | N/A | CTU, RPG | Complete with ectopic ureter of upper pole moiety which inserts into bladder neck | Right | Upper pole moiety distal ureter mass | N/A | N/A | N/A | N/A |
| 32 | Nirei et al. [32] | 2021 | Japan | 76 | M | Hematuria | No per anamnesis | 1.04 mg/dL | CT, MRU | Complete + ectopic upper moiety ureter opens to prostate verumontanum | Right | Upper moiety renal pelvis per imaging on diagnostic URS—to the ectopic ureteral opening beside the verumontanum confirmed no lower ureteral malignancy | Radical NU, because there was no obvious tumor around the ectopic ureter, lower ureter was blinded, and the prostate was preserved | Laparoscopic | High-grade pTa UC | 6 months follow-up cystoscopy to blinded ectopic ureter—no recurrence sign |
| 33 | Sarver et al. [33] | 2022 | United States | 76 | M | Hematuria | S/P irradiation therapy for prostate adenocarcinoma S/P BCG instillation therapy for bladder LG UC | N/A | CTU, diagnostic URS | Incomplete | Left | At the level of bifurcation | Laser ablation of UTUC following biopsy confirmed T1 LG UC | Minimally invasive endoscopic management | - | N/A |
| 34 | Case presentation | 2023 | Israel | 69 | F | Hematuria | No per anamnesis | 0.8 | CTU, diagnostic URS | Complete | Left | Upper moiety renal pelvis | Upper pole Hemi-NU with distal common sheath ureterectomy, including bladder cuff, and reimplantation of the lower moiety ureter | Robotic assisted laparoscopic | Mucinous HG UC pT3NXM0 | Continued adjuvant therapy 9 months follow-up NED |
| Patients | ||
| No. | 34 | |
| Bilateral tumor | 2 | |
| Bilateral DCS with bilateral tumor | 1 | |
| Unilateral DCS with bilateral tumor | 1 | |
| Overall UTUC in DCS | 35 units | |
| Year of report | ||
| Range | 1981–2023 | |
| Age, years | ||
| Mean (SD) | 65.8 | (11.1) |
| Median (IQR) | 67 | (62–73.5) |
| Region, no. (%) | ||
| Australia | 1 | (2.94) |
| China | 1 | (2.94) |
| Croatia | 1 | (2.94) |
| India | 1 | (2.94) |
| Israel | 1 | (2.94) |
| Japan | 18 | (52.94) |
| Malaysia | 1 | (2.94) |
| Taiwan | 3 | (8.82) |
| Tunisia | 1 | (2.94) |
| Turkey | 1 | (2.94) |
| United Kingdom | 1 | (2.94) |
| United States | 4 | (11.76) |
| Gender, no. (%) | ||
| Male | 21 | (61.75) |
| Female | 13 | (38.25) |
| Presentation, no. (%) | ||
| N/A | 6 | |
| Hematuria | 19 | |
| Flank pain | 1 | |
| Abdominal pain | 1 | |
| Hematuria + flank pain | 3 | |
| Hematuria + abdominal pain | 1 | |
| Non-specific | 3 | |
| Total cases with hematuria | 23 | (67.5%) |
| Exposure, no. | ||
| N/A | 17 | |
| None | 10 | |
| Smoking | 3 | |
| Radio/chemotherapy | 3 | |
| Other | 1 | |
| Creatinine (mg/dL), no. | ||
| N/A | 23 | |
| Normal | 8 | |
| Abnormal | 3 | |
| Diagnosis, no. | ||
| N/A | 5 | |
| CTU alone | 6 | |
| CTU combined with IVU/RPG/US/MRI | 14 | |
| RPG alone | 1 | |
| RPG/URS combined with other modalities | 14 | |
| Anatomy, no. (%) | ||
| N/A | 1 | |
| Complete | 15 | (36.42%) |
| Incomplete | 18 | (51.42%) |
| Blind ending bifid ureter | 1 | |
| Side, no. (%) | ||
| Right | 18 | (51.42%) |
| Left | 17 | (48.58%) |
| Bilateral with DCS | 1 | |
| Location, no. (%) | ||
| Pelvis, upper moiety | 10 | (28.5%) |
| Pelvis, lower moiety | 4 | (11.5%) |
| Proximal ureter, upper moiety | 1 | (2.85%) |
| Proximal ureter, lower moiety | 2 | (5.7%) |
| Bifurcation | 8 | (22.5%) |
| Distal ureter, upper moiety | 4 | (11.4%) |
| Distal ureter, lower moiety | 3 | (8.5%) |
| Distal ureter, common sheath | 2 | (5.7%) |
| Ectopic ureter | 1 | (2.85%) |
| Management, no. (%) | ||
| N/A | 6 | (17.14%) |
| Radical NU | 12 | (34.28%) |
| Radical NU with bladder cuff/partial cystectomy | 9 | (25.71%) |
| Heminephroureterectomy | 5 | (14.28%) |
| Other | 3 | (8.57%) |
| Approach, no. | ||
| N/A | 13 | |
| Open | 16 | |
| Laparoscopic | 3 | |
| Robotic | 1 | |
| Endoscopic | 1 | |
| Other | 1 | |
| Pathology, no. | ||
| N/A | 11 | |
| Undefined grade TCC | 7 | |
| Low-grade UC | 3 | |
| High-grade UC | 8 | |
| Sarcomatoid type UC | 2 | |
| Mucinous type UC | 1 | |
| Squamous cell carcinoma | 1 | |
| Poorly differentiated | 1 | |
| Follow-up, no. | ||
| N/A | 17 | |
| 2 years: NED | 5 | |
| Up to 2 years: recurrence | 3 | |
| Up to 1 year: NED | 2 | |
| Up to 1 year: recurrence | 2 | |
| Up to 6 months: NED | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zohar, Y.; Sivan, B.; Mintz, I.; Hefer, B.; Rouvinov, K.; Shani Shrem, N.; Mabjeesh, N.J. Management of Upper Tract Urothelial Carcinoma in a Double Collecting System Kidney. J. Pers. Med. 2024, 14, 158. https://doi.org/10.3390/jpm14020158
Zohar Y, Sivan B, Mintz I, Hefer B, Rouvinov K, Shani Shrem N, Mabjeesh NJ. Management of Upper Tract Urothelial Carcinoma in a Double Collecting System Kidney. Journal of Personalized Medicine. 2024; 14(2):158. https://doi.org/10.3390/jpm14020158
Chicago/Turabian StyleZohar, Yarden, Bezalel Sivan, Ishai Mintz, Ben Hefer, Keren Rouvinov, Noa Shani Shrem, and Nicola J. Mabjeesh. 2024. "Management of Upper Tract Urothelial Carcinoma in a Double Collecting System Kidney" Journal of Personalized Medicine 14, no. 2: 158. https://doi.org/10.3390/jpm14020158
APA StyleZohar, Y., Sivan, B., Mintz, I., Hefer, B., Rouvinov, K., Shani Shrem, N., & Mabjeesh, N. J. (2024). Management of Upper Tract Urothelial Carcinoma in a Double Collecting System Kidney. Journal of Personalized Medicine, 14(2), 158. https://doi.org/10.3390/jpm14020158





