The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword?
Abstract
1. Introduction
2. Definition and Etiology of Sarcopenia or Frailty
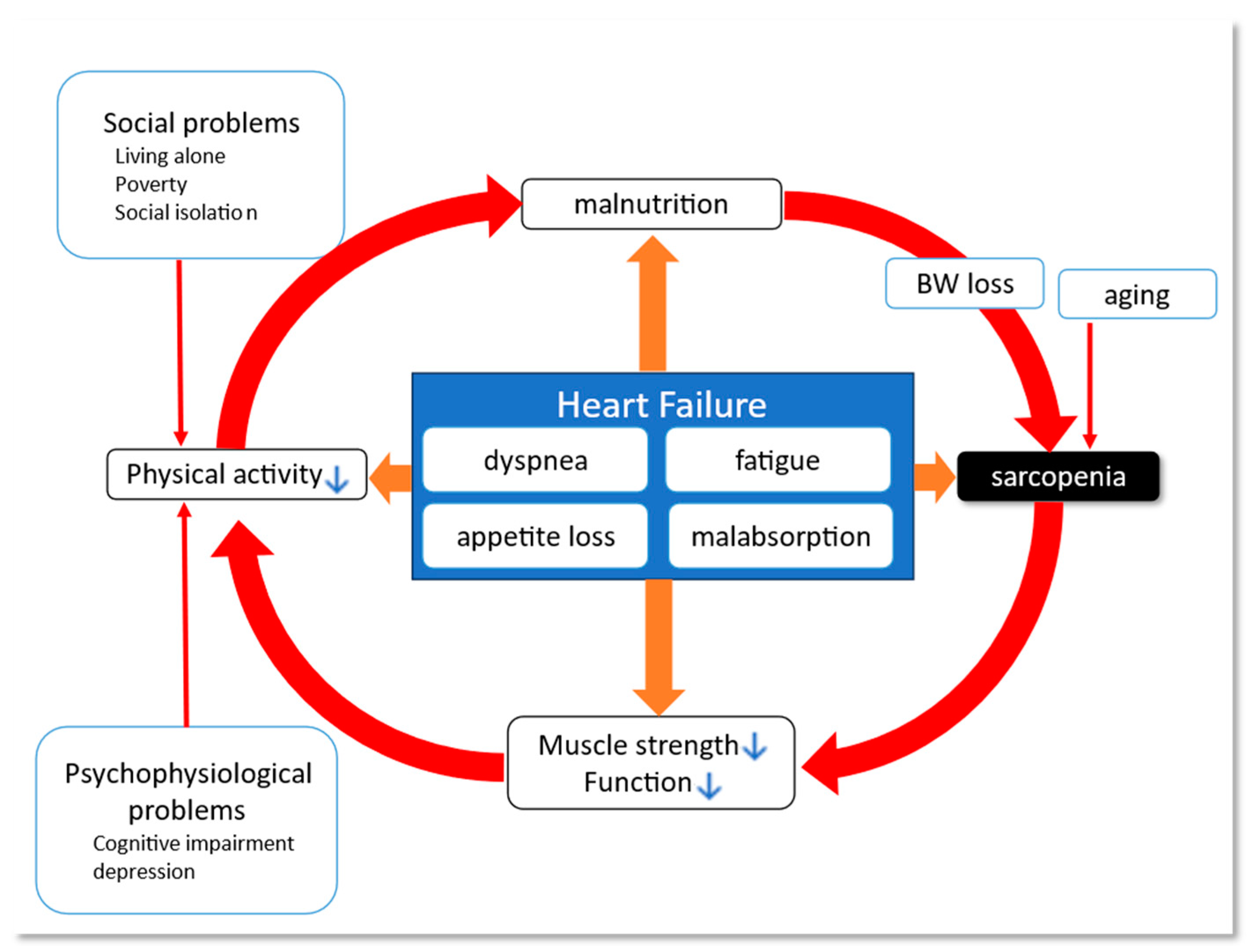
3. Heart Failure and Frailty/Sarcopenia
4. Safety and Efficacy of SGLT-2is for Sarcopenic or Frail Patients
5. Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diabetes Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef]
- Wojcik, C.; Warden, B.A. Mechanisms and Evidence for Heart Failure Benefits from SGLT2 Inhibitors. Curr. Cardiol. Rep. 2019, 21, 130. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes. Metab. 2018, 20, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Takashima, H.; Oguma, H.; Nakamura, Y.; Ohno, M.; Utsunomiya, K.; Furukawa, T.; Tei, R.; Abe, M. Canagliflozin Improves Erythropoiesis in Diabetes Patients with Anemia of Chronic Kidney Disease. Diabetes Technol. Ther. 2019, 21, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC. Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Ye, Y.; Bajaj, M.; Yang, H.C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Evans, M.; Morgan, A.R.; Davies, S.; Beba, H.; Strain, W.D. The role of sodium-glucose co-transporter-2 inhibitors in frail older adults with or without type 2 diabetes mellitus. Age Ageing 2022, 51, afac201. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Lupón, J.; González, B.; Santaeugenia, S.; Altimir, S.; Urrutia, A.; Más, D.; Díez, C.; Pascual, T.; Cano, L.; Valle, V. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev. Esp. De Cardiol. 2008, 61, 835–842. [Google Scholar] [CrossRef]
- Forman, D.E.; Fleg, J.L.; Kitzman, D.W.; Brawner, C.A.; Swank, A.M.; McKelvie, R.S.; Clare, R.M.; Ellis, S.J.; Dunlap, M.E.; Bittner, V. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J. Am. Coll. Cardiol. 2012, 60, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Tjam, E.Y.; Heckman, G.A.; Smith, S.; Arai, B.; Hirdes, J.; Poss, J.; McKelvie, R.S. Predicting heart failure mortality in frail seniors: Comparing the NYHA functional classification with the Resident Assessment Instrument (RAI) 2.0. Int. J. Cardiol. 2012, 155, 75–80. [Google Scholar] [CrossRef]
- Khan, H.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Newman, A.B.; Harris, T.B.; Rodondi, N.; Bauer, D.C.; Kritchevsky, S.B.; Butler, J. Frailty and risk for heart failure in older adults: The health, aging, and body composition study. Am. Heart J. 2013, 166, 887–894. [Google Scholar] [CrossRef]
- Gastelurrutia, P.; Lupón, J.; Altimir, S.; de Antonio, M.; González, B.; Cabanes, R.; Rodríguez, M.; Urrutia, A.; Domingo, M.; Zamora, E.; et al. Fragility is a key determinant of survival in heart failure patients. Int. J. Cardiol. 2014, 175, 62–66. [Google Scholar] [CrossRef]
- Lo, A.X.; Donnelly, J.P.; McGwin, G., Jr.; Bittner, V.; Ahmed, A.; Brown, C.J. Impact of gait speed and instrumental activities of daily living on all-cause mortality in adults ≥65 years with heart failure. Am. J. Cardiol. 2015, 115, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Fülster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschöpe, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Takahashi, T.; Yokoyama, M.; Kinoshita, D.; Honda, Y.; Funayama, A.; Nishiyama, S.; Takahashi, H.; et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur. J. Intern. Med. 2015, 26, 118–122. [Google Scholar] [CrossRef]
- Onoue, Y.; Izumiya, Y.; Hanatani, S.; Tanaka, T.; Yamamura, S.; Kimura, Y.; Araki, S.; Sakamoto, K.; Tsujita, K.; Yamamoto, E.; et al. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int. J. Cardiol. 2016, 215, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, T.; Nagatomo, Y.; Saji, M.; Iguchi, N.; Daida, H.; Yoshikawa, T. Low muscle mass assessed by psoas muscle area is associated with clinical adverse events in elderly patients with heart failure. PLoS ONE 2021, 16, e0247140. [Google Scholar] [CrossRef]
- Konishi, M.; Kagiyama, N.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Misumi, T.; Kitai, T.; Iwata, K.; et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 1022–1029. [Google Scholar] [CrossRef]
- Shimokawa, H.; Miura, M.; Nochioka, K.; Sakata, Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015, 17, 884–892. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ Can. Med. Assoc. J. = J. De L’association Med. Can. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 13. Older Adults: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S244–S257. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Richter, D.; Guasti, L.; Walker, D.; Lambrinou, E.; Lionis, C.; Abreu, A.; Savelieva, I.; Fumagalli, S.; Bo, M.; Rocca, B.; et al. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur. J. Prev. Cardiol. 2022, 29, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, E.Z.; Goyal, P.; Hummel, S.L.; Krishnaswami, A.; Goodlin, S.J.; Hart, L.L.; Forman, D.E.; Wenger, N.K.; Kirkpatrick, J.N.; Alexander, K.P. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J. Am. Coll. Cardiol. 2018, 71, 1921–1936. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Chokshi, N.B.K.; Karmakar, B.; Pathan, S.K.; Joshi, V.; Gohel, D.M.; Coulshed, D.S.; Negishi, K.; Pathan, F.K. A Systematic Review of Frailty Scores Used in Heart Failure Patients. Heart Lung Circ. 2023, 32, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Dewan, P.; Merkely, B.; Belohlávek, J.; Drożdż, J.; Kitakaze, M.; Inzucchi, S.E.; Kosiborod, M.N.; Martinez, F.A.; Tereshchenko, S.; et al. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure with Reduced Ejection Fraction: A Post Hoc Analysis of the DAPA-HF Trial. Ann. Intern. Med. 2022, 175, 820–830. [Google Scholar] [CrossRef]
- Butt, J.H.; Jhund, P.S.; Belohlávek, J.; de Boer, R.A.; Chiang, C.E.; Desai, A.S.; Drożdż, J.; Hernandez, A.F.; Inzucchi, S.E.; Katova, T.; et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients with Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation 2022, 146, 1210–1224. [Google Scholar] [CrossRef]
- To, T.L.; Doan, T.N.; Ho, W.C.; Liao, W.C. Prevalence of Frailty among Community-Dwelling Older Adults in Asian Countries: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 895. [Google Scholar] [CrossRef]
- Da Mata, F.A.; Pereira, P.P.; Andrade, K.R.; Figueiredo, A.C.; Silva, M.T.; Pereira, M.G. Prevalence of Frailty in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0160019. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Galluzzo, L.; Rodríguez-Laso, Á.; Van der Heyden, J.; Ranhoff, A.H.; Lamprini-Koula, M.; Ciutan, M.; López-Samaniego, L.; Carcaillon-Bentata, L.; Kennelly, S.; et al. Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: A systematic review and meta-analysis. Ann. Dell’istituto Super. Di Sanita 2018, 54, 226–238. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Lainscak, M.; Springer, J.; Anker, S.D. Cardiac cachexia: A systematic overview. Pharmacol. Ther. 2009, 121, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.; Becher, H.; Fassbender, K.; Chu, Q.; Baracos, V.E. Concurrent evolution of cancer cachexia and heart failure: Bilateral effects exist. J. Cachexia Sarcopenia Muscle 2014, 5, 95–104. [Google Scholar] [CrossRef]
- Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R.; Zhou, J.; Fried, L.P. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Talha, K.M.; Pandey, A.; Fudim, M.; Butler, J.; Anker, S.D.; Khan, M.S. Frailty and heart failure: State-of-the-art review. J. Cachexia Sarcopenia Muscle 2023, 14, 1959–1972. [Google Scholar] [CrossRef]
- Ijaz, N.; Buta, B.; Xue, Q.L.; Mohess, D.T.; Bushan, A.; Tran, H.; Batchelor, W.; deFilippi, C.R.; Walston, J.D.; Bandeen-Roche, K.; et al. Interventions for Frailty Among Older Adults with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 482–503. [Google Scholar] [CrossRef]
- Vitale, C.; Uchmanowicz, I. Frailty in patients with heart failure. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2019, 21, L12–L16. [Google Scholar] [CrossRef]
- Vitale, C.; Spoletini, I.; Rosano, G.M. Frailty in Heart Failure: Implications for Management. Card. Fail. Rev. 2018, 4, 104–106. [Google Scholar] [CrossRef]
- Pandey, A.; Kitzman, D.; Reeves, G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC. Heart Fail. 2019, 7, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.; Jackson, A.; Jhund, P.S.; Shen, L.; Ferreira, J.P.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.; et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—An analysis of PARADIGM-HF and ATMOSPHERE. Eur. J. Heart Fail. 2020, 22, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Segar, M.W.; Singh, S.; Reeves, G.R.; O’Connor, C.; Piña, I.; Whellan, D.; Kraus, W.E.; Mentz, R.J.; Kitzman, D.W. Frailty Status Modifies the Efficacy of Exercise Training Among Patients with Chronic Heart Failure and Reduced Ejection Fraction: An Analysis From the HF-ACTION Trial. Circulation 2022, 146, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Vidán, M.T.; Blaya-Novakova, V.; Sánchez, E.; Ortiz, J.; Serra-Rexach, J.A.; Bueno, H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur. J. Heart Fail. 2016, 18, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lupón, J.; Vidán, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayés-Genís, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef] [PubMed]
- Duggan, E.; Knight, S.P.; Xue, F.; Romero-Ortuno, R. Haemodynamic Parameters Underlying the Relationship between Sarcopenia and Blood Pressure Recovery on Standing. J. Clin. Med. 2023, 13, 18. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metab. Clin. Exp. 2023, 146, 155639. [Google Scholar] [CrossRef]
- Ding, J.; Kritchevsky, S.B.; Newman, A.B.; Taaffe, D.R.; Nicklas, B.J.; Visser, M.; Lee, J.S.; Nevitt, M.; Tylavsky, F.A.; Rubin, S.M.; et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am. J. Clin. Nutr. 2007, 85, 405–410. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Upadhya, B.; Haykowsky, M.J.; Eggebeen, J.; Kitzman, D.W. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2015, 12, 205–214. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Brown, N.F. Skeletal muscle lipid and its association with insulin resistance: What is the role for exercise? Exerc. Sport Sci. Rev. 2005, 33, 150–154. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr. Res. 2015, 35, 1031–1039. [Google Scholar] [CrossRef]
- Fonseca, G.; Dos Santos, M.R.; de Souza, F.R.; Takayama, L.; Rodrigues Pereira, R.M.; Negrão, C.E.; Alves, M.N.N. Discriminating sarcopenia in overweight/obese male patients with heart failure: The influence of body mass index. ESC Heart Fail. 2020, 7, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Matsue, Y.; Kamiya, K.; Kagiyama, N.; Maeda, D.; Endo, Y.; Ueno, H.; Yoshioka, K.; Mizukami, A.; Saito, K.; et al. Sarcopenic obesity is associated with impaired physical function and mortality in older patients with heart failure: Insight from FRAGILE-HF. BMC Geriatr. 2022, 22, 556. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Del Buono, M.G.; Canada, J.M.; Kim, Y.; Damonte, J.I.; Trankle, C.R.; Halasz, G.; Mihalick, V.; Vecchié, A.; Markley, R.R.; et al. Sarcopenic Obesity Is Associated with Reduced Cardiorespiratory Fitness Compared with Nonsarcopenic Obesity in Patients with Heart Failure with Reduced Ejection Fraction. Circulation. Heart Fail. 2022, 15, e009518. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Rådholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results from the CANVAS Program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients with Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.F.; Jhund, P.S.; Anand, I.; Bengtsson, O.; Böhm, M.; de Boer, R.A.; DeMets, D.L.; Desai, A.S.; Drozdz, J.; Howlett, J.; et al. Effect of Dapagliflozin on Outpatient Worsening of Patients with Heart Failure and Reduced Ejection Fraction: A Prespecified Analysis of DAPA-HF. Circulation 2020, 142, 1623–1632. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Anker, S.D.; Khan, M.S.; Butler, J.; Ofstad, A.P.; Peil, B.; Pfarr, E.; Doehner, W.; Sattar, N.; Coats, A.J.S.; Filippatos, G.; et al. Weight change and clinical outcomes in heart failure with reduced ejection fraction: Insights from EMPEROR-Reduced. Eur. J. Heart Fail. 2023, 25, 117–127. [Google Scholar] [CrossRef]
- Adamson, C.; Jhund, P.S.; Docherty, K.F.; Bělohlávek, J.; Chiang, C.E.; Diez, M.; Drożdż, J.; Dukát, A.; Howlett, J.; Ljungman, C.E.A.; et al. Efficacy of dapagliflozin in heart failure with reduced ejection fraction according to body mass index. Eur. J. Heart Fail. 2021, 23, 1662–1672. [Google Scholar] [CrossRef]
- Adamson, C.; Kondo, T.; Jhund, P.S.; de Boer, R.A.; Cabrera Honorio, J.W.; Claggett, B.; Desai, A.S.; Alcocer Gamba, M.A.; Al Habeeb, W.; Hernandez, A.F.; et al. Dapagliflozin for heart failure according to body mass index: The DELIVER trial. Eur. Heart J. 2022, 43, 4406–4417. [Google Scholar] [CrossRef]
- Kutz, A.; Kim, D.H.; Wexler, D.J.; Liu, J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Comparative Cardiovascular Effectiveness and Safety of SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors According to Frailty in Type 2 Diabetes. Diabetes Care 2023, 46, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Shiki, K.; Homma, G.; Meinicke, T.; Ogura, Y.; Seino, Y. Efficacy and safety of the sodium-glucose co-transporter-2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: A randomized, double-blind, placebo-controlled, 52-week clinical trial (EMPA-ELDERLY). Diabetes Obes. Metab. 2023, 25, 3538–3548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Z.; Wang, Y.; Song, D.; Zhu, D. Effect of sodium-glucose transporter 2 inhibitors on sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1203666. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Pennells, D.; Abdelhafiz, A.H. Hypoglycaemic therapy in frail older people with type 2 diabetes mellitus-a choice determined by metabolic phenotype. Aging Clin. Exp. Res. 2022, 34, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Peuker, H.; Staron, R.S. The impact of biochemical methods for single muscle fibre analysis. Acta Physiol. Scand. 1999, 166, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef]
- Sano, M.; Meguro, S.; Kawai, T.; Suzuki, Y. Increased grip strength with sodium-glucose cotransporter 2. J. Diabetes 2016, 8, 736–737. [Google Scholar] [CrossRef] [PubMed]
| Population | SGLT-2is | Trial | Primary Endpoint |
|---|---|---|---|
| T2DM and high risk of CVD | Empagliflozin | EMPA-REG OUTCOME [14] | MACE, HR 0.86 [95%CI, 0.74–0.99] |
| Canagliflozin | CANVAS program [15] | MACE, HR 0.86 [95%CI, 0.75–0.97] | |
| Dapagliflozin | DECLARE-TIIM 58 [16] | The composite of CV death and hospitalization for HF, HR 0.83 [95%CI, 0.73–0.95] | |
| HFrEF | Empagliflozin | EMPEROR-Reduced [18] | The composite of CV death and hospitalization for HF, HR 0.75 [95%CI, 0.65–0.86] |
| Dapagliflozin | DAPA-HF [17] | The composite of CV death and hospitalization or urgent intravenous therapy for HF, HR 0.74 [95%CI, 0.65–0.85] | |
| T2DM and HF | Sotagliflozin | SOLOIST-WHF [19] | The composite of CV death and hospitalization or urgent visit for HF, HR 0.67 [95%CI, 0.52–0.85] |
| HFpEF | Dapagliflozin | DELIVER [20] | The composite of CV death and hospitalization for HF, HR 0.82 [95%CI, 0.73–0.92] |
| Empagliflozin | EMPEROR-Preserved [21] | The composite of CV death and hospitalization for HF, HR 0.79 [95%CI, 0.69–0.90] | |
| Acute HF | Empagliflozin | EMPULSE [26] | The composite of all-cause death, worsening HF event, and KCCQ-TSS, stratified win ratio 1.36 [95%CI, 1.09–1.68] |
| Frailty or Sarcopenia | Assessment | Measure | Description |
|---|---|---|---|
| Sarcopenia | Muscle mass | Skeletal muscle mass index (SMI) (appendicular skeletal muscle mass/height2) | Various cutoffs employed by studies |
| Muscle strength | Hand grip | Various cutoffs employed by studies | |
| Sarcopenia/Frailty | Physical function | Gait speed | |
| Physical function | Short Physical Performance Battery (SPPB) [40] | A summation of scores on three tests: balance, gait speed and chair stand | |
| Physical function | Timed-Up and Go test (TUG) [41] | ||
| Frailty | Multidimensional | Rockwood frailty index [42] | Accumulation of symptoms, function, comorbidities, clinical laboratory abnormalities, and impaired quality of life are assessed using 93 variables |
| Phenotype model | Barthel index [43] | Score is calculated based on several daily activities (feeding, bathing, grooming, dressing, bowel and bladder control, toilet use capability, transfer from bed to chair and vice-versa, mobility on level surfaces, and capability to climb stairs) | |
| Medical domain | Clinical frailty scale [44] | A semi-quantitative global judgement | |
| Medical domain and physical function | Fried frailty phenotype [45] | Weight loss, weakness of hand grip, exhaustion, slowness, and low activity |
| Population | Study | Topics of Interest (Assessment Tool) | Main Findings |
|---|---|---|---|
| HFrEF | DAPA-HF sub-analysis [57] | Frailty (Frailty index) | The efficacy of dapagliflozin for HFrEF patients was consistent across the range of frailty, and the absolute reductions were larger in more frail patients. |
| DAPA-HF sub-analysis [94] | BMI | The efficacy of dapagliflozin for HFrEF patients was consistent across the spectrum of BMI. | |
| EMPEROR-Reduced sub-analysis [93] | BMI | The efficacy of dapagliflozin for HFrEF patients was consistent across the spectrum of BMI, and weight loss was associated with higher all-cause mortality regardless of BMI groups. | |
| HFpEF | DELIVER sub-analysis [58] | Frailty (Frailty index) | The benefit of dapagliflozin for HFpEF patients was consistent across the range of frailty and the improvement of QOL with medication was greater in those with a higher level of frailty. |
| DELIVER sub-analysis [95] | BMI | The benefit of dapagliflozin for HFpEF patients was consistent across the spectrum of BMI. | |
| DM | Kutz et al. (2023) [96] | Frailty (Frailty index) | Medicare beneficiaries with type 2 DM showed greater cardiovascular effectiveness associated with SGLT-2is and GLP-1 receptor agonists than DPP-4 inhibitors. |
| EMPA-ELDERLY [97] | Elderly (≥65) | Empagliflozin for elderly T2DM reduced body weight without compromising muscle mass or strength. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, A.; Nagatomo, Y.; Kawai, A.; Yukino-Iwashita, M.; Nakazawa, R.; Taruoka, A.; Takefuji, A.; Yasuda, R.; Toya, T.; Ikegami, Y.; et al. The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword? J. Pers. Med. 2024, 14, 141. https://doi.org/10.3390/jpm14020141
Naito A, Nagatomo Y, Kawai A, Yukino-Iwashita M, Nakazawa R, Taruoka A, Takefuji A, Yasuda R, Toya T, Ikegami Y, et al. The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword? Journal of Personalized Medicine. 2024; 14(2):141. https://doi.org/10.3390/jpm14020141
Chicago/Turabian StyleNaito, Ayami, Yuji Nagatomo, Akane Kawai, Midori Yukino-Iwashita, Ryota Nakazawa, Akira Taruoka, Asako Takefuji, Risako Yasuda, Takumi Toya, Yukinori Ikegami, and et al. 2024. "The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword?" Journal of Personalized Medicine 14, no. 2: 141. https://doi.org/10.3390/jpm14020141
APA StyleNaito, A., Nagatomo, Y., Kawai, A., Yukino-Iwashita, M., Nakazawa, R., Taruoka, A., Takefuji, A., Yasuda, R., Toya, T., Ikegami, Y., Masaki, N., Ido, Y., & Adachi, T. (2024). The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword? Journal of Personalized Medicine, 14(2), 141. https://doi.org/10.3390/jpm14020141






