Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Participants
2.2. Intervention
2.3. MpMRI Technique and Evaluation
2.4. Prostate Biopsy Procedure and Pathologic Analysis
2.5. Proclarix™ Assessment
2.6. BCN-RC 1 and BCN-RC 2 Risk of csPCa Assessment
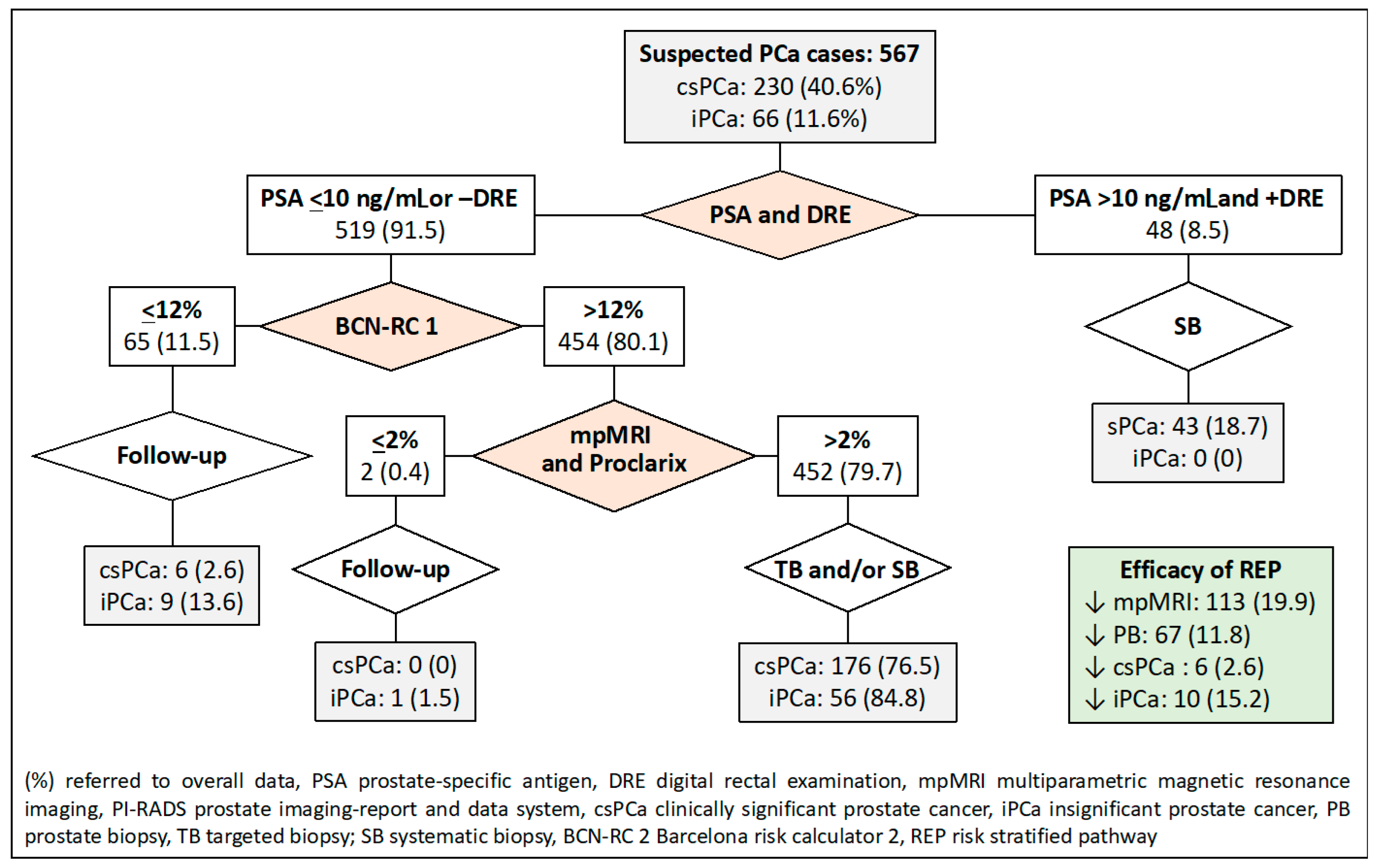
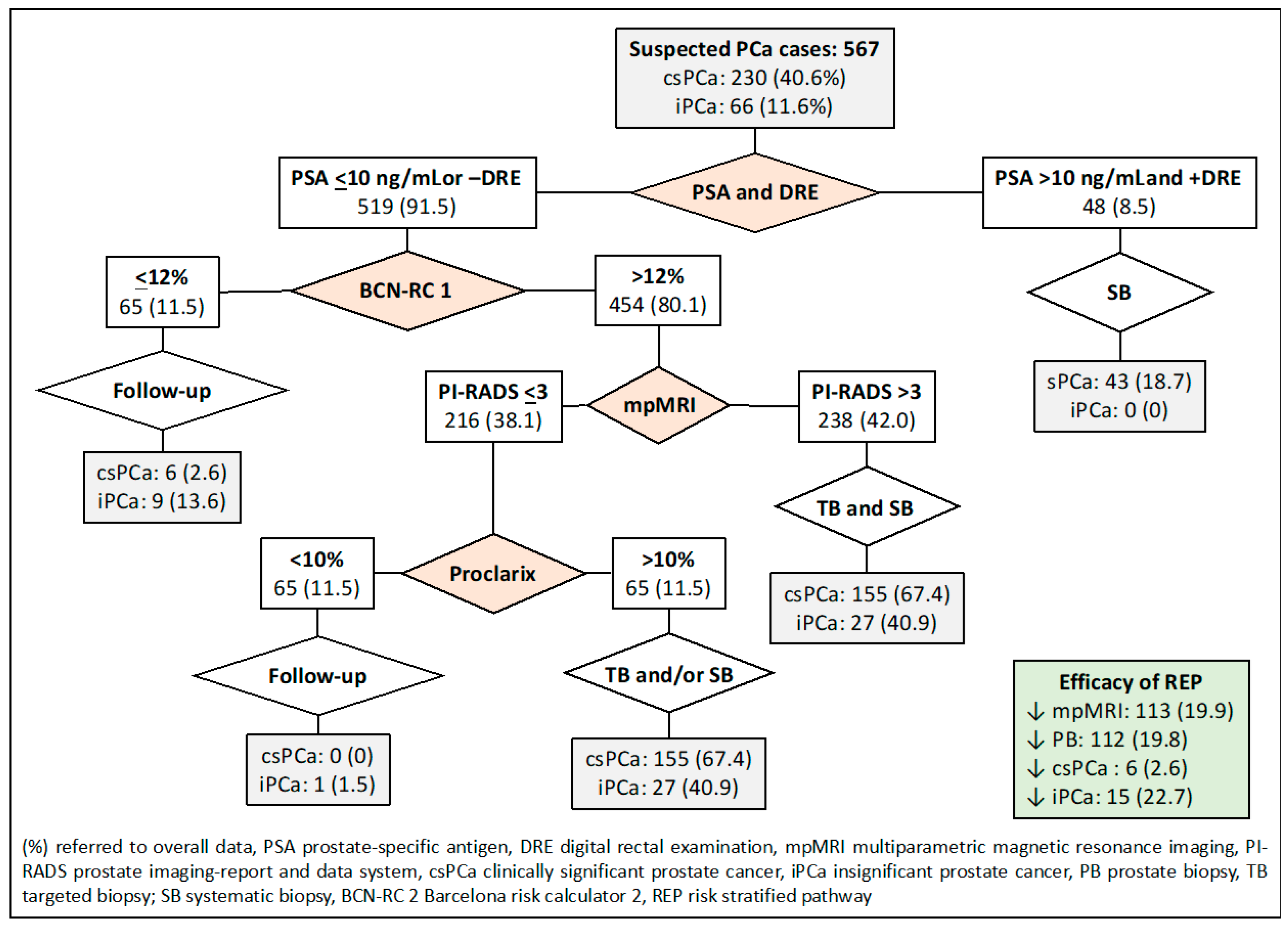
2.7. Proposed Risk-Stratified Pathways for Analysis
- (1)
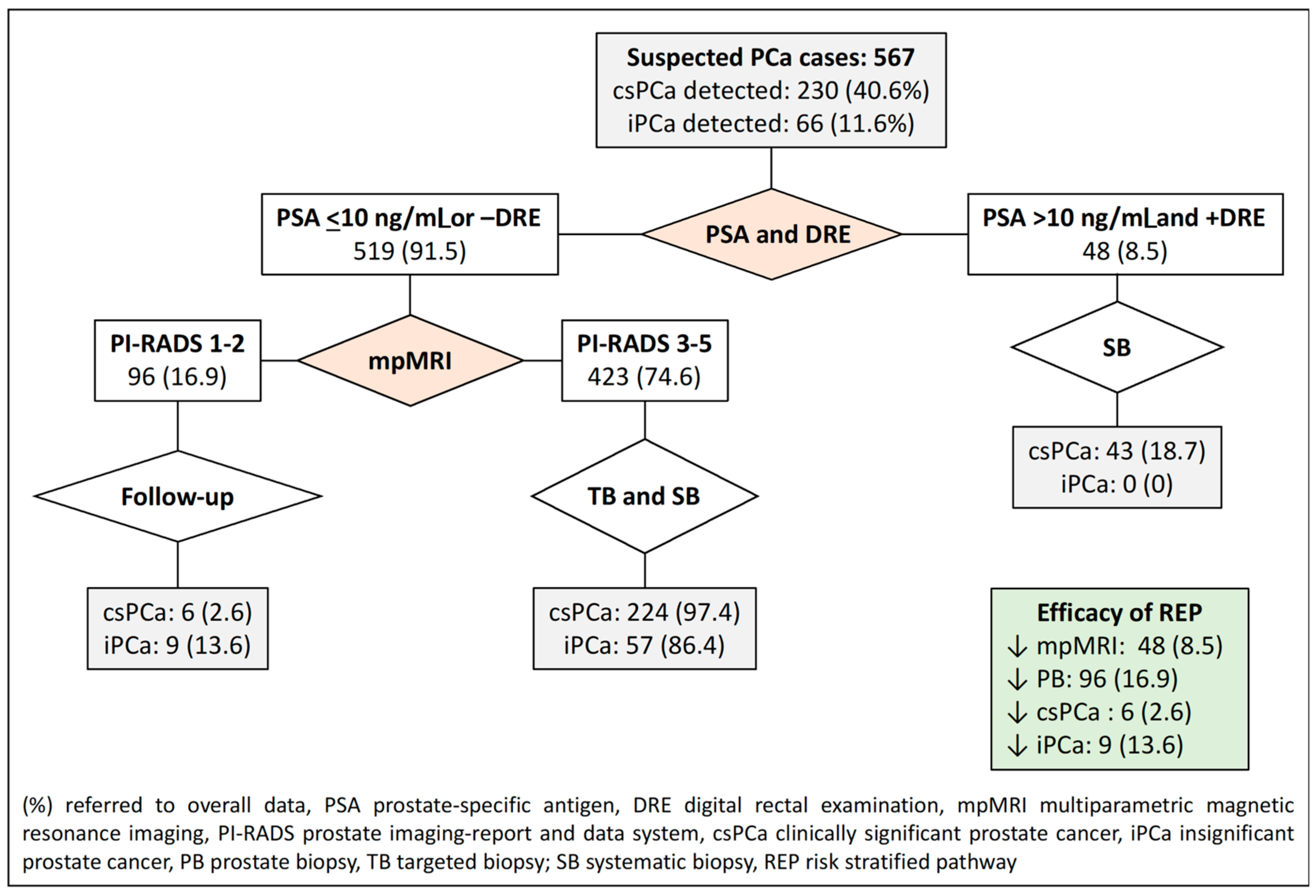
- The first RSP represents the commonly recommended pathway and serves as the control in our study. In this pathway, all individuals suspected of having PCa undergo mpMRI, and subsequent stratification is based on the PI-RADS score. A prostate biopsy is avoided if the PI-RADS score is less than 3. For men with a PI-RADS score greater than 3, targeted and systematic biopsies are conducted.
- (2)
- The second RSP involves an initial stratification of men based on their serum PSA level and DRE characteristics (normal vs. suspicious). In this pathway, men with a PSA level exceeding 10 ng/mL and suspicious DRE skip mpMRI and proceed directly to systematic biopsies. On the other hand, men with a serum PSA level of 10 or lower or a normal DRE undergo mpMRI. Prostate biopsies are avoided for those with a PI-RADS score of less than 3, while targeted and systematic biopsies are performed for those with a PI-RADS score greater than 3.
- (3)
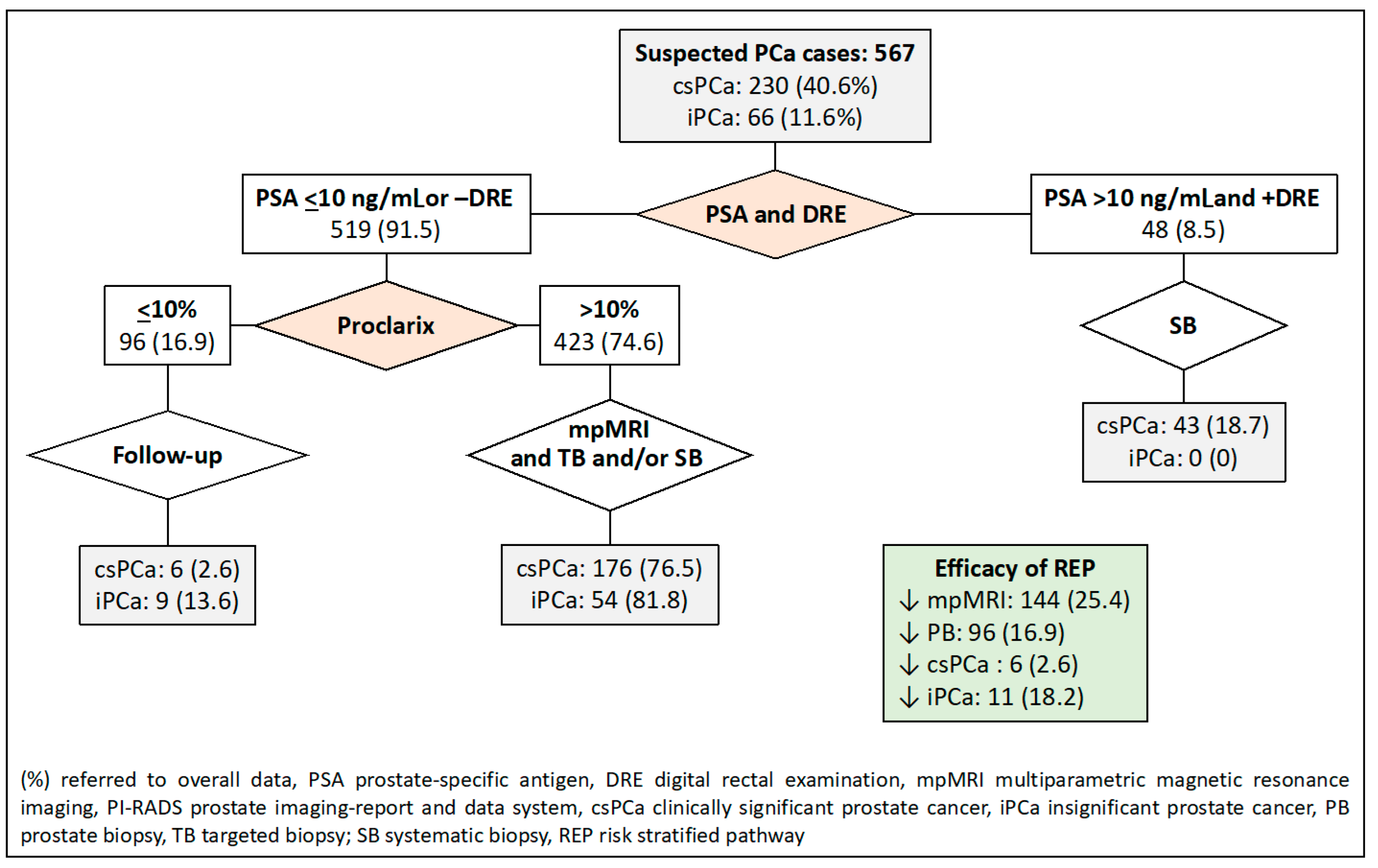
- In the third RSP, men are initially stratified based on their serum PSA level and DRE characteristics, similar to the first RSP. The second stratification is then conducted using Proclarix™ for men with a serum PSA level less than 10 ng/mL or a normal DRE. In this pathway, individuals with Proclarix™ scores of 10% or less undergo follow-up, while those with scores exceeding 10% proceed to mpMRI and targeted and/or systematic biopsies in cases where the PI-RADS score is less than 3.
- (4)
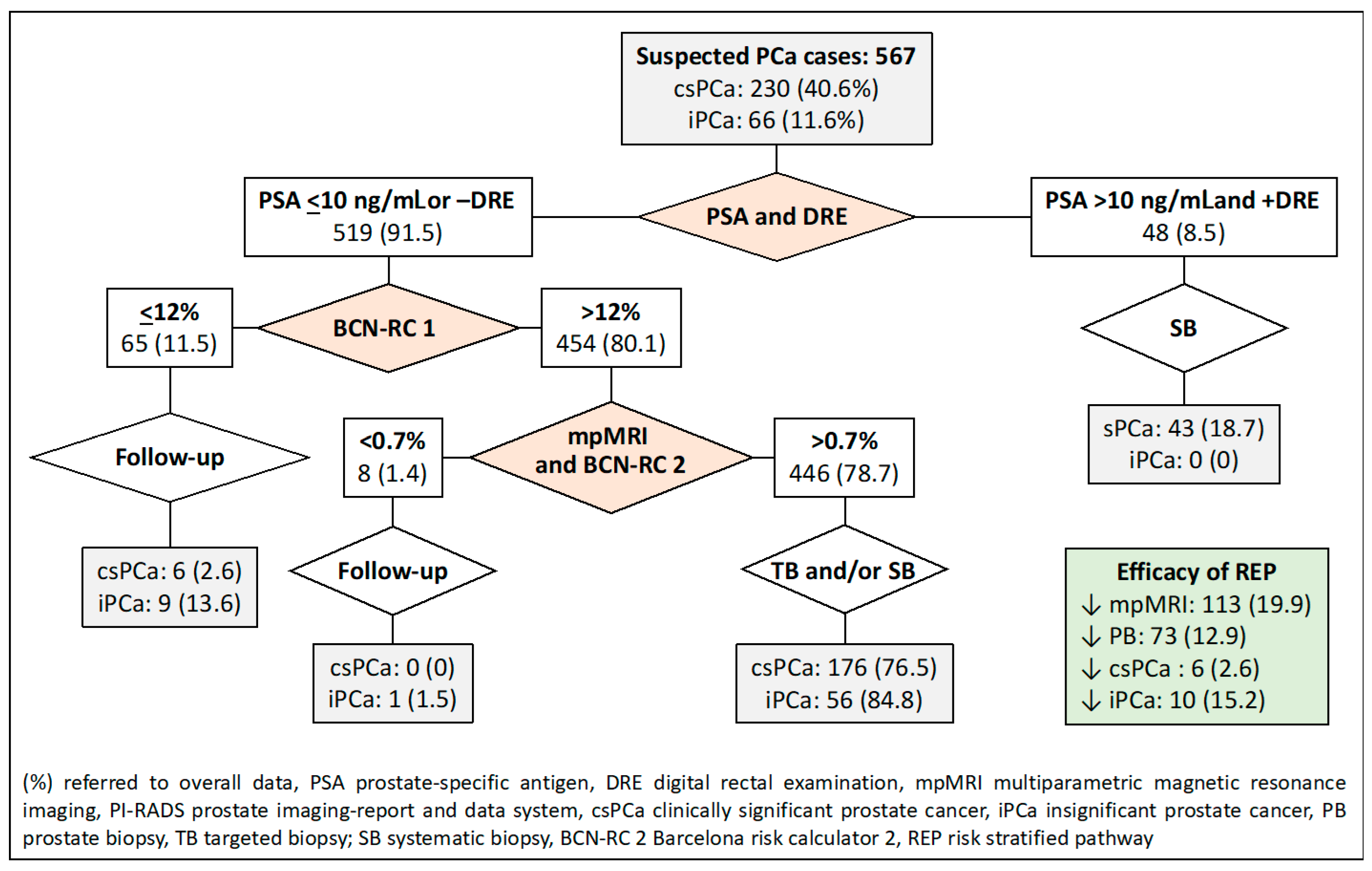
- The fourth RSP stratifies men based on serum PSA levels and DRE, similar to the first RSP. The second stratification is carried out using BCN-RC 1 in men with serum PSA levels of 10 or lower ng/mL or normal DRE. The chosen threshold of 12% was determined to maintain sensitivity, resulting in the same misdiagnosis rate of csPCa as the control pathway. Men with a csPCa risk of 12% or less undergo follow-up, while those with a risk greater than 12% undergo mpMRI, leading to a third stratification from BCN-RC 2. In this stage, a threshold of 0.7% was selected due to its 100% sensitivity in detecting csPCa. Consequently, men with a csPCa risk of 0.7 or less undergo follow-up, whereas those with a risk greater than 0.7% undergo targeted and/or systematic biopsies.
- (5)
- The fifth RSP incorporates the initial two stratifications from PSA-DRE and Procla-rix™. Men with Proclarix™ scores of 10% or greater undergo mpMRI, and a subsequent third stratification from BCN-RC 2 is performed. In this instance, the threshold with 100% sensitivity for csPCa was determined to be 0.6%. Accordingly, individuals with a risk of 0.6% or less undergo follow-up, while those with a risk exceeding 0.6% undergo targeted and/or systematic biopsy.
- (6)
- The sixth RSP stratifies men based on PSA-DRE and BCN-RC 1. Once the mpMRI is conducted, the third stratification is executed using Proclarix™. The threshold of Proclarix™ ensuring 100% sensitivity for csPCa in this context was determined to be 2%. Consequently, individuals with a csPCa risk of 2% or less undergo follow-up, while those with a risk exceeding 2% undergo targeted and/or systematic biopsy.
- (7)
- Finally, the seventh RSP consists of four stratifications. The initial two are determined by PSA-DRE and BCN-RC 1. The third stratification is contingent upon the mpMRI results, where individuals with a PI-RADS greater than 3 undergo targeted and systematic biopsies, whereas those with a PI-RADS score of less than 3 are stratified based on Proclarix™. Men with Proclarix™ scores of 10% or less are scheduled for follow-up, while those with scores of 10% or higher undergo targeted and/or systematic biopsies.
2.8. Endpoint Variables
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
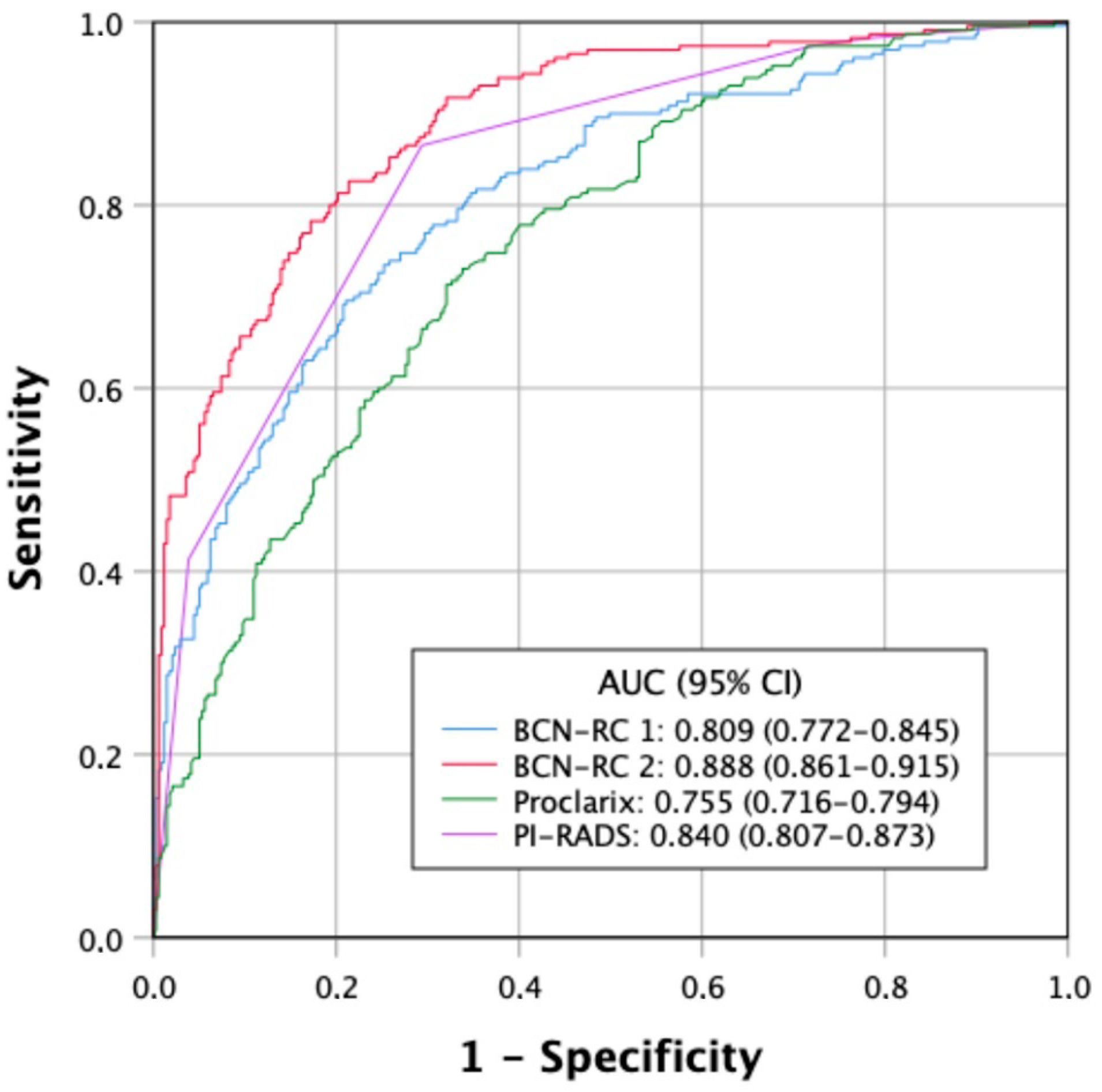
3.2. Discrimination Ability for csPCa of Tools Used for Stratifications of Men Suspected of Having PCa
3.3. Behavior and Clinical Effectiveness of Proposed RSPs
3.4. Efficacy of Proposed RSPs
3.5. Cost-Effectiveness Approximative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of Digital Rectal Examination and Serum Prostate Specific Antigen in the Early Detection of Prostate Cancer: Results of a Multicenter Clinical Trial of 6,630 Men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. Early Detection of Prostate Cancer in 2020 and Beyond: Facts and ecommendations for the European Union and the European Commission. Eur. Urol. 2020, 79, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.F.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific Antigen. Testing as Part. of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: Past, present and future. Nat. Rev. Urol. 2022, 19, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Croswell, J.M.; Dana, T.; Bougatsos, C.; Blazina, I.; Fu, R.; Gleitsmann, K.; Koenig, H.C.; Lam, C.; Maltz, A.; et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 762–771. [Google Scholar] [CrossRef]
- Gómez Rivas, J.; Leenen, R.C.A.; Venderbos, L.D.F.; Helleman, J.; de la Parra, I.; Vasilyeva, V.; Moreno-Sierra, J.; Basu, P.; Chandran, A.; van den Bergh, R.C.N.; et al. Navigating through the Controversies and Emerging Paradigms in Early Detection of Prostate Cancer: Bridging the Gap from Classic RCTs to Modern Population-Based Pilot Programs. J. Pers. Med. 2023, 13, 1677. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chandran, A. Early Detection of Prostate Cancer in the European Union: Combining Forces with PRAISE-U. Eur. Urol. 2023, 84, 519–522. [Google Scholar] [CrossRef]
- Osses, D.F.; Roobol, M.J.; Schoots, I.G. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2019, 20, 1637. [Google Scholar] [CrossRef]
- Alterbeck, M.; Järbur, E.; Thimansson, E.; Wallström, J.; Bengtsson, J.; Björk-Eriksson, T.; Bjartell, A.; Bratt, O.; Jiborn, T.; Arnsrud Godtman, R. Designing and Implementing a Population-based Organised Prostate Cancer Testing Programme. Eur. Urol. Focus 2022, 8, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Triquell, M.; Campistol, M.; Celma, A.; Regis, L.; Cuadras, M.; Planas, J.; Trilla, E.; Morote, J. Magnetic Resonance Imaging-Based Predictive Models for Clinically Significant Prostate Cancer: A Systematic Review. Cancers 2022, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Falagario, U.G.; Martini, A.; Wajswol, E.; Treacy, P.J.; Ratnani, P.; Jambor, I.; Anastos, H.; Lewis, S.; Haines, K.; Cormio, L.; et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-specific Antigen Density, 4Kscore and Risk Calculators. Eur. Urol. Oncol. 2020, 3, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Granapragasam, V.J.; Burling, K.; George, A.; Stearn, S.; Warren, A.; Barrett, T.; Koo, B.; Gallagher, F.A.; Doble, A.; Kastner, C.; et al. The Prostate Health Index adds predictive value to multi-parametric MRI in detecting significant prostate cancers in a repeat biopsy population. Sci. Rep. 2016, 6, 35364. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, R.J.; van der Leest, M.M.G.; Israël, B.; Hannink, G.; YantiSetiasti, A.; Cornel, E.B.; Hulsbergen-van de Kaa, C.A.; Klaver, O.S.; Sedelaar, J.P.M.; Van Criekinge, W.; et al. Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: A prospective, multicenter study in biopsy-naïve men. Prostate Cancer Prostatic Dis. 2021, 24, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Del Giudice, F.; Falagario, U.G.; Cocci, A.; Russo, G.I.; Di Mauro, M.; Sepe, G.S.; Galasso, F.; Leonardi, R.; Iacona, G.; et al. SelectMDx and Multiparametric Magnetic Resonance Imaging of the Prostate for Men Undergoing Primary Prostate Biopsy: A Prospective Assessment in a Multi-Institutional Study. Cancers 2021, 13, 2047. [Google Scholar] [CrossRef]
- Nordström, T.; Picker, W.; Aly, M.; Jäderling, F.; Adolfsson, J.; Ström, P.; Haug, E.S.; Eklund, M.; Carlsson, S.; Grönberg, H. Detection of Prostate Cancer Using a Multistep Approach with Prostate-specific Antigen, the Stockholm 3 Test, and Targeted Biopsies: The STHLM3 MRI Project. Eur. Urol. Focus 2017, 3, 526–528. [Google Scholar] [CrossRef]
- Ström, P.; Nordström, T.; Aly, M.; Egevad, L.; Grönberg, H.; Eklund, M. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur. Urol. 2018, 74, 204–210. [Google Scholar] [CrossRef]
- Roobol, M.J.; van Vugt, H.A.; Loeb, S.; Zhu, X.; Bul, M.; Bangma, C.H.; van Leenders, A.G.; Steyerberg, E.W.; Schröder, F.H. Prediction of prostate cancer risk: The role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur. Urol. 2012, 61, 577–583. [Google Scholar] [CrossRef]
- Morote, J.; Borque-Fernando, Á.; Triquell, M.; Campistol, M.; Celma, A.; Regis, L.; Abascal, J.M.; Servian, P.; Planas, J.; Mendez, O.; et al. A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand. Cancers 2022, 14, 5100. [Google Scholar] [CrossRef]
- Alberts, A.R.; Roobol, M.J.; Verbeek, J.F.M.; Schoots, I.G.; Chiu, P.K.; Osses, D.F.; Tijsterman, J.D.; Beerlage, H.P.; Mannaerts, C.K.; Schimmöller, L.; et al. Prediction of High-grade Prostate Cancer Following Multiparametric Magnetic Resonance Imaging: Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculators. Eur. Urol. 2019, 75, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Borque-Fernando, A.; Triquell, M.; Celma, A.; Regis, L.; Escobar, M.; Mast, R.; de Torres, I.M.; Semidey, M.E.; Abascal, J.M.; et al. The Barcelona Predictive Model of Clinically Significant Prostate Cancer. Cancers 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Roche, S.; Mast, R.; Santamaría, A.; Planas, J.; et al. The Efficacy of Proclarix to Select Appropriate Candidates for Magnetic Resonance Imaging and Derived Prostate Biopsies in Men with Suspected Prostate Cancer. World J. Men’s Health 2022, 40, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Triquell, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaria, A.; Planas, J.; et al. Improving the Early Detection of Clinically Significant Prostate Cancer in Men in the Challenging Prostate Imaging-Reporting and Data System 3 Category. Eur. Urol. Open Sci. 2022, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Roobol, M.J.; Schröder, F.H.; Hugosson, J.; Jones, J.S.; Kattan, M.W.; Klein, E.A.; Hamdy, F.; Neal, D.; Donovan, J.; Parekh, D.J.; et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: Results from the prostate biopsy collaborative group. World J. Urol. 2012, 30, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Picola, N.; Paesano, N.; Celma, A.; Muñoz-Rodriguez, J.; Asiain, I.; Ruiz-Plazas, X.; Muñoz-Rivero, M.V.; García de Manuel, G.G.; Servian, P.; et al. Are magnetic resonance imaging and targeted biopsies needed in men with serum prostate-specific antigen over 10 ng/mL and an abnormal digital rectal examination. Urol. Oncol. 2023, 41, 299–301. [Google Scholar] [CrossRef]
- Morote, J.; Triquell, M.; Campistol, M.; Abascal, J.M.; Servian, P.; Trilla, E. Stratifying the initial prostate cancer suspicion to avoid magnetic resonance exams by sequencing men according to serum prostate-specific antigen, digital rectal examination and the prostate-specific antigen density based on digital rectal prostate volume category. BJUI Compass 2023, 4, 266–268. [Google Scholar] [PubMed]
- Remmers, S.; Kasivisvanathan, V.; Verbeek, J.F.M.; Moore, C.M.; Roobol, M.J.; ERSPC Rotterdam Study Group PRECISION Investigators Group. Reducing Biopsies and Magnetic Resonance Imaging Scans During the Diagnostic Pathway of Prostate Cancer: Applying the Rotterdam Prostate Cancer Risk Calculator to the PRECISION Trial Data. Eur. Urol. Open Sci. 2022, 36, 1–8. [Google Scholar] [CrossRef]
- Eldred-Evans, D.; Tam, H.; Sokhi, H.; Padhani, A.R.; Connor, M.; Price, D.; Gammon, M.; Klimowska-Nassar, N.; Burak, P.; Day, E.; et al. An Evaluation of Screening Pathways Using a Combination of Magnetic Resonance Imaging and Prostate-specific Antigen: Results from the IP1-PROSTAGRAM Study. Eur. Urol. Oncol. 2023, 6, 295–302. [Google Scholar] [CrossRef]
- Gibbons, M.; Simko, J.P.; Carroll, P.R.; Noworolski, S.M. Prostate cancer lesion detection, volume quantification and high-grade cancer differentiation using cancer risk maps derived from multiparametric MRI with histopathology as the reference standard. Magn. Reson. Imaging 2023, 99, 48–57. [Google Scholar] [CrossRef]
- Green, H.D.; Merriel, S.W.D.; Oram, R.A.; Ruth, K.S.; Tyrrell, J.; Jones, S.E.; Thirlwell, C.; Weedon, M.N.; Bailey, S.E.R. Applying a genetic risk score for prostate cancer to men with lower urinary tract symptoms in primary care to predict prostate cancer diagnosis: A cohort study in the UK Biobank. Br. J. Cancer 2022, 127, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Ruzgas, T.; Venclovas, Z.; Jievaltas, M.; Joniau, S. Impact of Grade Groups on Prostate Cancer-Specific and Other-Cause Mortality: Competing Risk Analysis from a Large Single Institution Series. Cancers 2021, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- Tesar, E.C.; Mikolasevic, I.; Skocilic, I.; Redjovic, A.; Vucinic, D.; Marusic, J.; Djordjevic, G. Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease. J. Pers. Med. 2023, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, J.; Gandhe, A.; Nelson, B.; Hu, J.C.; Gulani, V.; Margolis, D.; Schackman, B.R.; Jalali, A. Cost-Effectiveness of Annual Prostate MRI and Potential MRI-Guided Biopsy After Prostate-Specific Antigen Test Results. JAMA Netw. Open 2023, 6, e2344856. [Google Scholar] [CrossRef]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 697–773. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Confort, P.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU—EANM—ESTRO—ESUR—ISUP—SIOG Guidelines on Prostate Cancer. 2023. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 22 November 2023).
- Oerther, B.; Engel, H.; Bamberg, F.; Sigle, A.; Gratzke, C.; Benndorf, M. Cancer detection rates of the PI-RADSv2.1 assessment categories: Systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis. 2022, 25, 256–263. [Google Scholar] [CrossRef]
- Moldovan, P.C.; Van den Broeck, T.; Sylvester, R.; Marconi, L.; Bellmunt, J.; van den Bergh, R.C.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Fossati, N.; et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Schröder, F.H.; Kranse, R.; Rietbergen, J.; Hoedemaeke, R.; Kirkels, W. The European Randomized Study of Screening for Prostate Cancer (ERSPC): An update. Members of the ERSPC, Section Rotterdam. Eur. Urol. 1999, 35, 539–543. [Google Scholar]
- Schröder, F.H. Detection of prostate cancer: The impact of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Can. J. Urol. 2005, 12 (Suppl. S1), 2–6. [Google Scholar] [PubMed]
- Roobol, M.J.; Vedder, M.M.; Nieboer, D.; Houlgatte, A.; Vincendeau, S.; Lazzeri, M.; Guazzoni, G.; Stephan, C.; Semjonow, A.; Haese, A.; et al. Comparison of Two Prostate Cancer Risk Calculators that Include the Prostate Health Index. Eur. Urol. Focus 2015, 1, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Risør, B.W.; Tayyari Dehbarez, N.; Fredsøe, J.; Sørensen, K.D.; Pedersen, B.G. Cost-Effectiveness Analysis of Stockholm 3 Testing Compared to PSA as the Primary Blood Test in the Prostate Cancer Diagnostic Pathway: A Decision Tree Approach. Appl. Health Econ. Health Policy 2022, 20, 867–880. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Measurement |
|---|---|
| Number of cases | 567 |
| Median age, years (IQR) | 69 (63–74) |
| Median total PSA, ng/mL (IQR) | 7.0 (4.9–11.2) |
| Abnormal DRE, n (%) | 109 (19.2) |
| Median free PSA, ng/mL (IQR) | 1.1 (0.7–1.7) |
| Median prostate volume, ml (IQR) | 55 (40–76) |
| Median percent free PSA, % (IQR) | 15.1 (10.7–20.6) |
| Median PSA density, ng/mL/cc (IQR) | 0.13 (0.09–0.21) |
| Repeat biopsy, n (%) | 133 (23.5) |
| Family history of PCa, n (%) | 48 (8.6%) |
| PI-RADS, n (%) | |
| 1–2 | 100 (17.6) |
| 3 | 169 (29.8) |
| 4 | 190 (33.5) |
| 5 | 108 (19.0) |
| Overall PCa detection, n (%) | 296 (52.2) |
| csPCa detection, n (%) | 230 (40.6) |
| iPCa detection, n (%) | 66 (11.6) |
| Pathway According to Stratifications | Thresholds | mpMRIs, n (%) | Prostate Biopsies, n (%) | csPCa Detection, n (%) | Over-Detection iPCa, n (%) |
|---|---|---|---|---|---|
| 1. mpMRI (currently recommended) | PI-RADS 2 | 0 (0) | 100 (17.6) | 6 (2.6) | 9 (13.6) |
| 2. PSA-DRE, and mpMRI | PSA 10 and +DRE; PI-RADS 2 | 48 (8.5) | 96 (16.9) | 6 (2.6) | 9 (13.6) |
| 3. PSA-DRE, Proclarix™ | PSA 10 and +DRE; Proclarix 10 | 144 (25.4) | 96 (16.9) | 6 (2.6) | 12 (18.2) |
| 4. PSA-DRE, BCN-RC1, and BCN-RC2 | PSA 10 and +DRE, BCN-RC1 12, BCN-RC2 0.7 | 113 (19.9) | 73 (12.9) | 6 (2.6) | 10 (15.2) |
| 5. PSA-DRE, Proclarix™, and BCN-RC2 | PSA 10 and +DRE, Proclarix 10, BCN-RC2 0.6 | 144 (25.4) | 107 (18.9) | 6 (2.6) | 13 (19.7) |
| 6. PSA-DRE, BCN-RC1, and Proclarix™ | PSA 10 and +DRE, BCN-RC1 12, Proclarix 10 | 113 (19.9) | 67 (11.8) | 6 (2.6) | 10 (15.2) |
| 7. PSA-DRE, BCN-RC1, mpMRI, and Proclarix™ | PSA 10 and +DRE, BCN-RC1 12, PI-RADS 3, Proclarix 10 | 113 (19.9) | 112 (19.8) | 6 (2.6) | 15 (22.7) |
| Pathway According to Stratifications | Avoided mpMRIs | mpMRI Savings | Avoided Prostate Biopsies | Prostate Biopsy Savings | MRI and Prostate Biopsy Savings | Proclarix™ Use | Proclarix™ Cost | Total Savings |
|---|---|---|---|---|---|---|---|---|
| 1. mpMRI (currently recommended) | 0 | 0 | 100 | 120,000 | 120,000 | 0 | 0 | 120,000 |
| 2. PSA-DRE, and mpMRI | 48 | 13,440 | 96 | 115,200 | 128,400 | 0 | 0 | 128,640 |
| 3. PSA-DRE, Proclarix™ | 144 | 40,320 | 96 | 115,200 | 155,520 | 519 | 103,800 | 51,900 |
| 4. PSA-DRE, BCN-RC1, and BCN-RC2 | 113 | 31,840 | 73 | 37,600 | 119,240 | 0 | 0 | 119,240 |
| 5. PSA-DRE, Proclarix™, and BCN-RC2 | 144 | 40,320 | 107 | 128,400 | 168,720 | 519 | 103.800 | 64.920 |
| 6. PSA-DRE, BCN-RC1, and Proclarix™ | 113 | 31,640 | 67 | 80,400 | 112,040 | 454 | 90,800 | 21,240 |
| 7. PSA-DRE, BCN-RC1, mpMRI, and Proclarix™ | 113 | 31,640 | 112 | 134,400 | 166,040 | 216 | 43,200 | 122,840 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morote, J.; Borque-Fernando, Á.; Esteban, L.M.; Celma, A.; Campistol, M.; Miró, B.; Méndez, O.; Trilla, E. Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer. J. Pers. Med. 2024, 14, 130. https://doi.org/10.3390/jpm14020130
Morote J, Borque-Fernando Á, Esteban LM, Celma A, Campistol M, Miró B, Méndez O, Trilla E. Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer. Journal of Personalized Medicine. 2024; 14(2):130. https://doi.org/10.3390/jpm14020130
Chicago/Turabian StyleMorote, Juan, Ángel Borque-Fernando, Luis M. Esteban, Ana Celma, Miriam Campistol, Berta Miró, Olga Méndez, and Enrique Trilla. 2024. "Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer" Journal of Personalized Medicine 14, no. 2: 130. https://doi.org/10.3390/jpm14020130
APA StyleMorote, J., Borque-Fernando, Á., Esteban, L. M., Celma, A., Campistol, M., Miró, B., Méndez, O., & Trilla, E. (2024). Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer. Journal of Personalized Medicine, 14(2), 130. https://doi.org/10.3390/jpm14020130






