Associations of Amino Acids with the Risk of Prediabetes: A Case-Control Study from Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Data Collection and Measurement
2.3. Participants
2.4. Ethics Considerations
2.5. Variables
2.6. Diagnostic Criteria
2.7. Chemicals
2.7.1. Biochemistry
2.7.2. Gas Chromatography–Mass Spectrometry
2.7.3. Sample Preparation
2.7.4. GC–MS Analysis
2.7.5. Bias
2.7.6. Sample Size Calculation
2.7.7. Quantitative Variables
2.7.8. Statistical Methods
3. Results
3.1. Descriptive Data
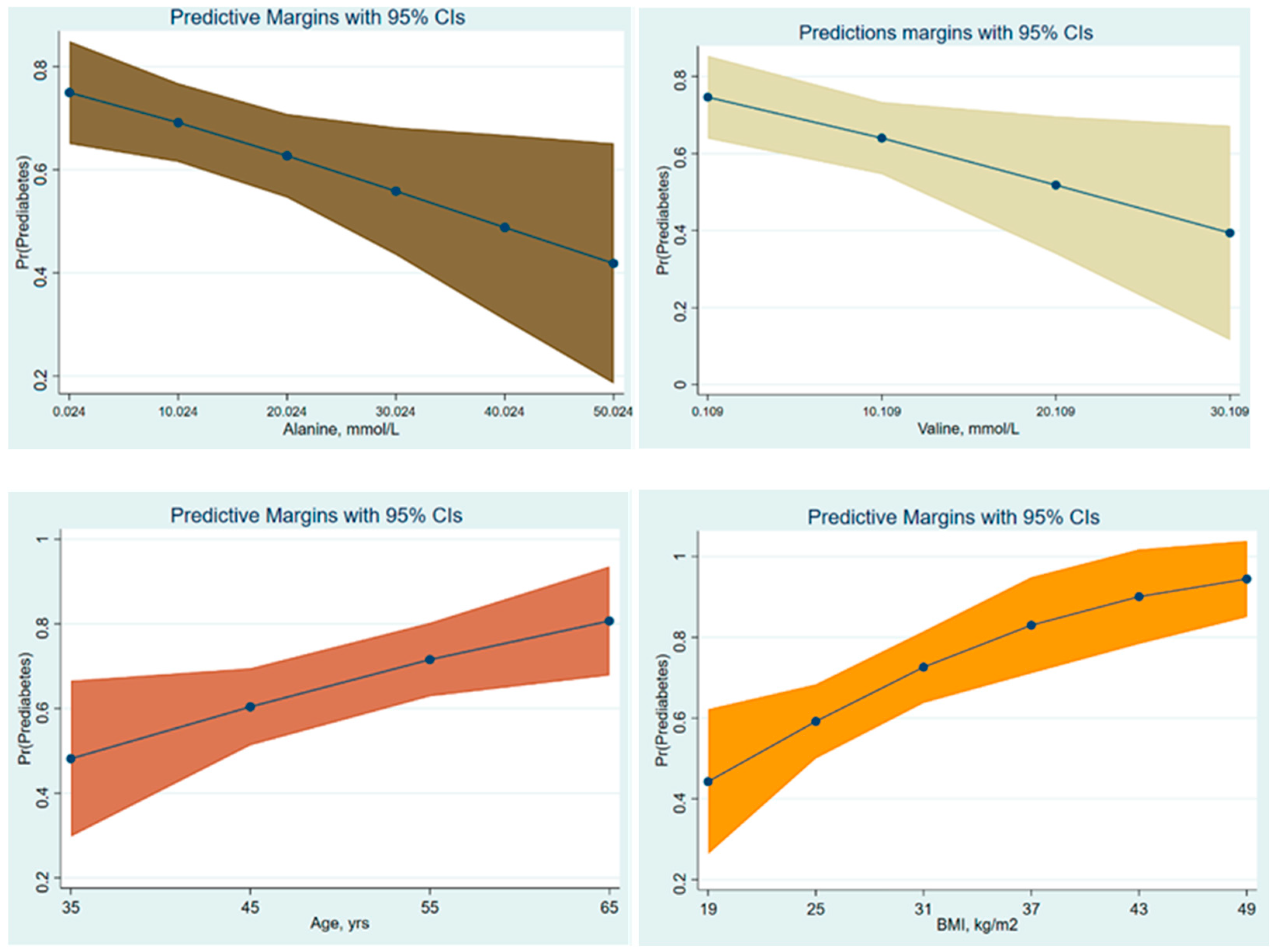
3.2. Amino Acids and the Risk of Prediabetes
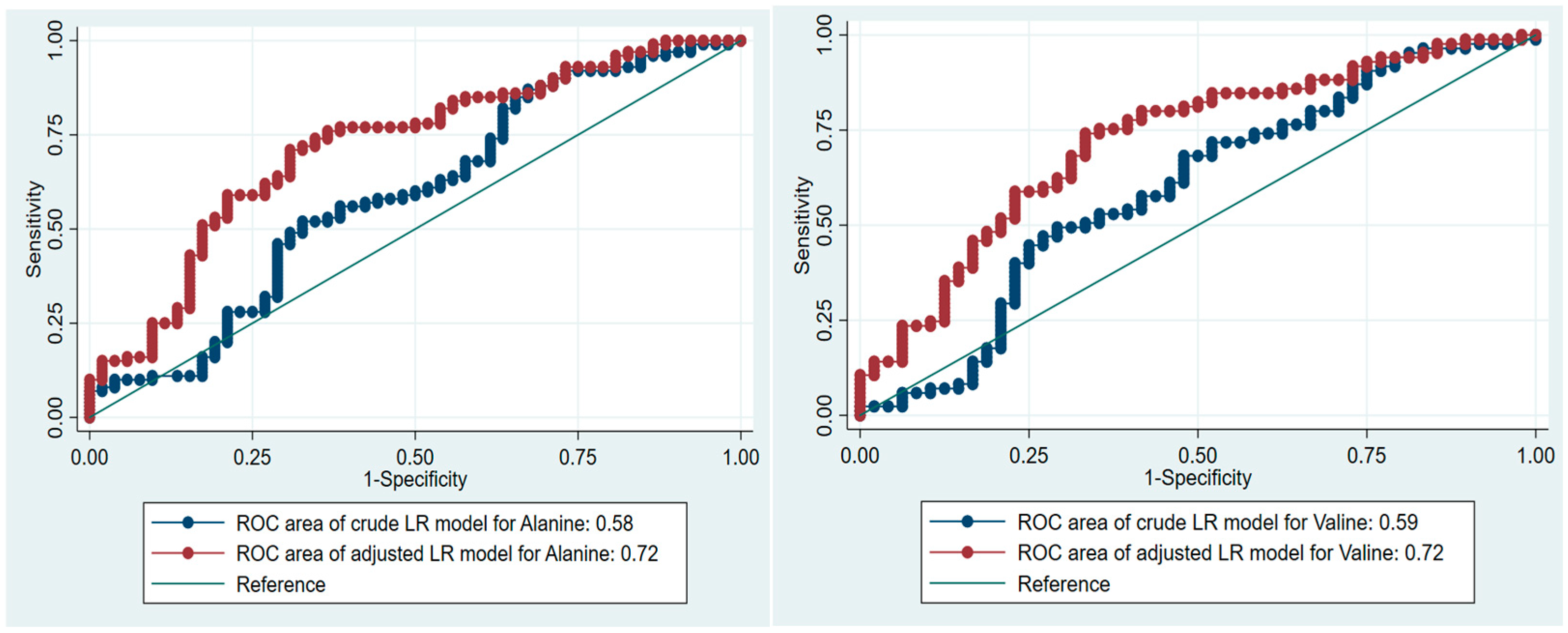
3.3. AAs and Prediction of Prediabetes
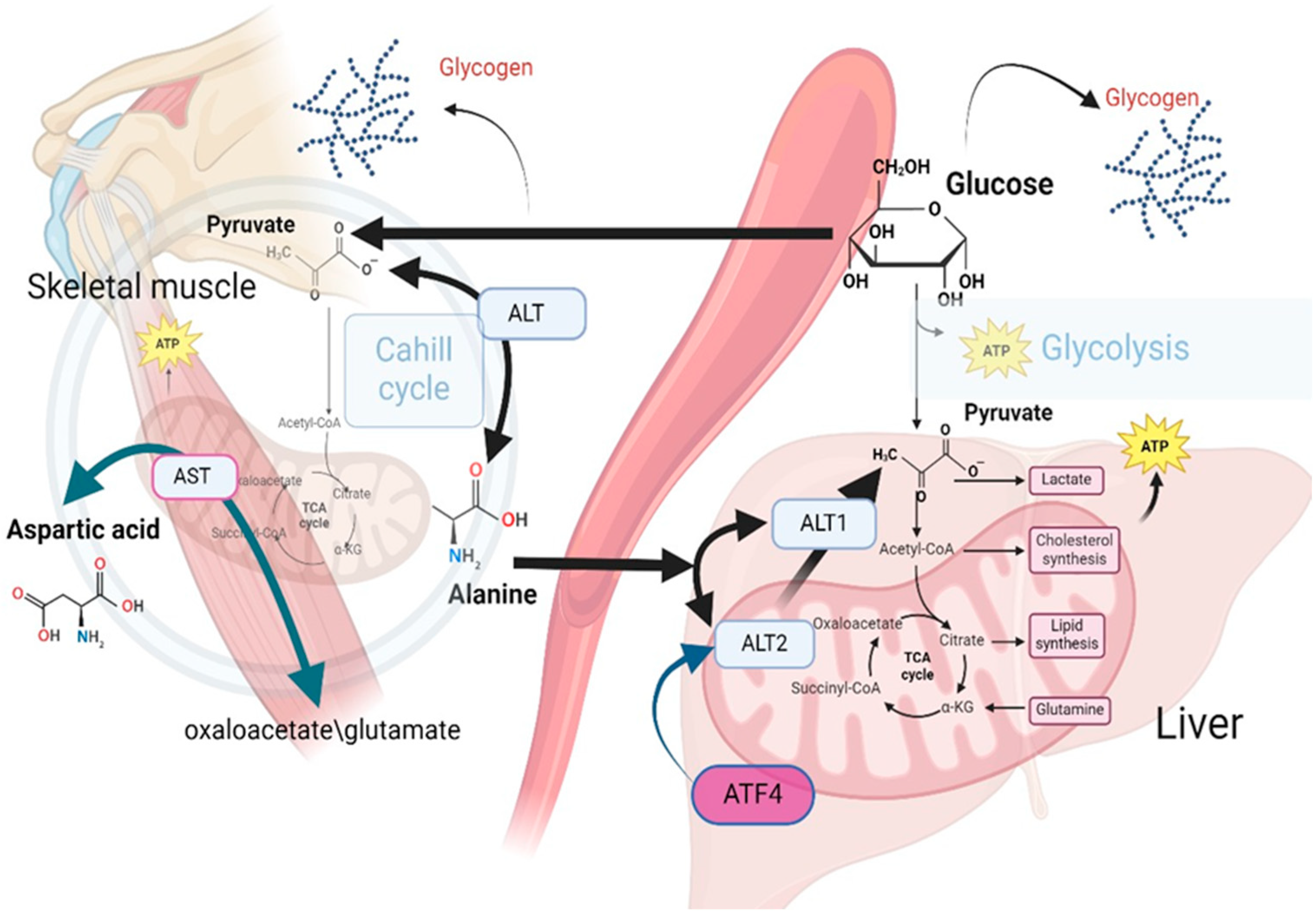
4. Discussion
Limitations
- The diagnosis of prediabetes in this study was exclusively based on fasting glucose and HbA1c levels, representing only a subset of potential diagnostic criteria. Incorporating the glucose tolerance test (GTT) could enhance the precision and accuracy of the classification of prediabetes within our cohort.
- About 75% of the study population consisted of individuals who were either overweight or obese. In this context, HOMA-IR and serum insulin levels, which were not specifically assessed in this study, could represent potential confounding factors in the observed association between AAs and the risk of prediabetes.
- The prevalence of obesity was approximately double in the case group compared to the control group, with 16.36% of controls classified as obese versus 39.45% of cases. However, the distribution of overweight individuals between the comparison groups was relatively comparable, with 41.82% in the control group and 42.20% in the case group. This similarity in overweight prevalence may help elucidate the lack of observed differences in BCAAs between comparison groups.
- The imbalance in the ratio of cases to controls presents a concern for diminished statistical power. To mitigate this issue, we increased the total sample size; however, this adjustment may not have entirely offset the potential limitations imposed by the original case–control ratio.
- The findings of this study are primarily applicable to populations of Kazakh ethnicity, thereby limiting the generalizability of the results to broader populations. As such, caution should be exercised when attempting to extrapolate these findings with other ethnic groups.
- Not all common 20 AAs were included in this study. Two AAs were excluded from the study. Glutamine exclusion was executed to maintain the study’s limited scope, while concentrating on the metabolic processes linked to the studied demographic’s high-protein, high-fat dietary patterns. Alanine and glutamine are the principal glucogenic amino acids, but Alanine plays a significant role during early-starvation, exposure to high-fat and high-protein diets, and diabetes [60,61,68,69]. Most research concerning the utilization of Alanine and glutamine in gluconeogenesis concludes that Alanine serves as the predominant AA for gluconeogenesis within the hepatic system. In contrast, glutamine assumes a principal role in the renal system and the small intestine [70,71,72]. Tryptophan was omitted because the baseline concentration of tryptophan in the bloodstream of healthy individuals may vary by a factor of five, and this particular AA does not exert a substantial influence on the mechanisms related to MS [46,73]. The detection of AAs in serum utilizing gas chromatography–mass spectrometry (GC–MS) presents notable challenges attributable to the chemical properties of amino acids and the inherent limitations of the GC–MS methodology. However, GC–MS offers the advantage of enabling simultaneous and precise quantification of fatty acids in conjunction with amino acids. This capability is particularly significant in the context of metabolic research.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abate, N.; Chandalia, M. Ethnicity and type 2 diabetes: Focus on Asian Indians. J. Diabetes Complicat. 2001, 1, 320–327. [Google Scholar] [CrossRef]
- Monika; Arora, P.; Kumar, V.; Popli, P. Conceptual Overview of Prevalence of Prediabetes. Curr. Diabetes Rev. 2024. [Google Scholar] [CrossRef]
- Kim, S.H. Reframing prediabetes: A call for better risk stratification and intervention. J. Intern. Med. 2024, 295, 735–747. [Google Scholar] [CrossRef]
- Committee, T.I.E. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327. [Google Scholar] [CrossRef]
- Piller, C. Dubious diagnosis. Science 2019, 363, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitis and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation, WHO, Jeneva. 2006. Available online: https://www.who.int/publications/i/item/definition-and-diagnosis-of-diabetes-mellitus-and-intermediate-hyperglycaemia (accessed on 23 September 2024).
- Genc, S.; Evren, B.; AYDIN, M.; Sahin, I. Evaluation of prediabetes patients in terms of metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2760–2769. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef]
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2018, 55, 21–32. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Knezek, S.; Engelen, M.; Ten Have, G.; Thaden, J.; Deutz, N. Prediabetes Is Associated with Specific Changes in Valine Metabolism. Curr. Dev. Nutr. 2022, 6, 34. [Google Scholar] [CrossRef]
- Wang, S.; Jung, S.; Ko, K.S. Effects of amino acids supplementation on lipid and glucose metabolism in HepG2 cells. Nutrients 2022, 14, 3050. [Google Scholar] [CrossRef]
- Formagini, T.; Brooks, J.V.; Roberts, A.; Bullard, K.M.; Zhang, Y.; Saelee, R.; O’Brien, M.J. Prediabetes prevalence and awareness by race, ethnicity, and educational attainment among US adults. Front. Public Health 2023, 11, 1277657. [Google Scholar] [CrossRef]
- Ding, Q.; Lu, Y.; Herrin, J.; Zhang, T.; Marrero, D.G. 1271-P: Associations of Combined Socioeconomic, Behavioral, and Metabolic Factors with Undiagnosed Diabetes and Prediabetes among Different Racial and Ethnic Groups. Diabetes 2023, 72 (Suppl. 1). [Google Scholar] [CrossRef]
- Adjei, N.K.; Samkange-Zeeb, F.; Kebede, M.; Saleem, M.; Heise, T.L.; Zeeb, H. Racial/ethnic differences in the prevalence and incidence of metabolic syndrome in high-income countries: A protocol for a systematic review. Syst. Rev. 2020, 9, 134. [Google Scholar] [CrossRef]
- Seah, J.Y.H.; Hong, Y.; Cichońska, A.; Sabanayagam, C.; Nusinovici, S.; Wong, T.Y.; Cheng, C.Y.; Jousilahti, P.; Lundqvist, A.; Perola, M.; et al. Circulating metabolic biomarkers consistently predict incident type 2 diabetes in Asian and European populations—A plasma metabolomics analysis of four ethnic groups. medRxiv 2021. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2005. Available online: https://idf.org/media/uploads/2023/05/attachments-30.pdf (accessed on 18 September 2024).
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Assiri, M.A.; Ahamad, S.R.; Aljumayi, S.R.; Alotaibi, W.H.; Alhamdan, M.M.; Alhazzani, K.; Alharbi, M.; Alqahtani, F.; Alasmari, A.F. Serum metabolomic analysis of male patients with cannabis or amphetamine use disorder. Metabolites 2022, 12, 179. [Google Scholar] [CrossRef]
- Shi, S.; Yi, L.; Yun, Y.; Zhang, X.; Liang, Y. A combination of GC-MS and chemometrics reveals metabolic differences between serum and plasma. Anal. Methods 2015, 7, 1751–1757. [Google Scholar] [CrossRef]
- Yao, H.; Shi, P.; Zhang, L.; Fan, X.; Shao, Q.; Cheng, Y. Untargeted metabolic profiling reveals potential biomarkers in myocardial infarction and its application. Mol. BioSyst. 2010, 6, 1061–1070. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 7. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Puricelli, L.; Millioni, R.; Vedovato, M.; Tiengo, A. Insulin resistance of amino acid and protein metabolism in type 2 diabetes. Clin. Nutr. 2011, 30, 267–272. [Google Scholar] [CrossRef]
- Long, J.; Yang, Z.; Wang, L.; Han, Y.; Peng, C.; Yan, C.; Yan, D. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Weng, L.; Quinlivan, E.; Gong, Y.; Beitelshees, A.L.; Shahin, M.H.; Turner, S.T.; Chapman, A.B.; Gums, J.G.; Johnson, J.A.; Frye, R.F.; et al. Association of branched and aromatic amino acids levels with metabolic syndrome and impaired fasting glucose in hypertensive patients. Metab. Syndr. Relat. Disord. 2015, 13, 195–202. [Google Scholar] [CrossRef]
- Owei, I.; Umekwe, N.; Stentz, F.; Wan, J.; Dagogo-Jack, S. Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019, 98, 76–83. [Google Scholar] [CrossRef]
- Tom, A.; Nair, K.S. Assessment of branched-chain amino acid status and potential for biomarkers. J. Nutr. 2006, 136, 324S–330S. [Google Scholar] [CrossRef]
- Sun, L.; Hu, C.; Yang, R.; Lv, Y.; Yuan, H.; Liang, Q.; He, B.; Pang, G.; Jiang, M.; Dong, J.; et al. Association of circulating branched-chain amino acids with cardiometabolic traits differs between adults and the oldest-old. Oncotarget 2017, 8, 88882. [Google Scholar] [CrossRef]
- Everman, S.; Meyer, C.; Tran, L.; Hoffman, N.; Carroll, C.C.; Dedmon, W.L.; Katsanos, C.S. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E671–E677. [Google Scholar] [CrossRef]
- O’Connor, E.M.; O’Herlihy, E.A.; O’Toole, P.W. Gut microbiota in older subjects: Variation, health consequences and dietary intervention prospects. Proc. Nutr. Soc. 2014, 73, 441–451. [Google Scholar] [CrossRef]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Kaiser, J.C.; Heinrichs, D.E.; Garsin, D.A. Branching out: Alterations in bacterial physiology and virulence due to branched-chain amino acid deprivation. mBio 2018, 9, e01188-18. [Google Scholar] [CrossRef]
- Mansfeld, J.; Urban, N.; Priebe, S.; Groth, M.; Frahm, C.; Hartmann, N.; Gebauer, J.; Ravichandran, M.; Dommaschk, A.; Schmeisser, S.; et al. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun. 2015, 6, 10043. [Google Scholar] [CrossRef]
- Canfield, C.A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Ribeiro, R.; Senior, A.; Hsu, B.; Hirani, V.; Blyth, F.M.; Waite, L.M.; Simpson, S.J.; Naganathan, V.; Cumming, R.G.; et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: The Concord Health and Ageing in Men Project. J. Gerontol. Ser. A 2020, 75, 1805–1810. [Google Scholar] [CrossRef]
- Bancks, M.P.; Bertoni, A.G.; Carnethon, M.; Chen, H.; Cotch, M.F.; Gujral, U.P.; Kandula, N.R. Association of Diabetes Subgroups with Race/Ethnicity, Risk Factor Burden and Complications: The MASALA and MESA Studies. J. Clin. Endocrinol. Metab. 2021, 106, e2106–e2115. [Google Scholar] [CrossRef]
- Vanweert, F.; Boone, S.C.; Brouwers, B.; Mook-Kanamori, D.O.; de Mutsert, R.; Rosendaal, F.R.; Lamb, H.J.; Schrauwen-Hinderling, V.B.; Schrauwen, P.; Hesselink, M.K.; et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity. Int. J. Obes. 2021, 45, 1510–1520. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef]
- Park, J.E.; Lim, H.R.; Kim, J.W.; Shin, K.H. Metabolite changes in risk of type 2 diabetes mellitus in cohort studies: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 140, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Wedyan, M.; Qnais, E.; Jawarneh, H.; McClements, L. Plasma Amino Acids Metabolomics’ Important in Glucose Management in Type 2 Diabetes. Front. Pharmacol. 2021, 12, 695418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nogueira-Ferreira, R.; Oliveira, P.F.; Ferreira, R. Liver metabolism: The pathways underlying glucose utilization and production. In Glycolysis; Academic Press: New York, NY, USA, 2024; pp. 141–156. [Google Scholar] [CrossRef]
- Gautier-Stein, A.; Chilloux, J.; Soty, M.; Thorens, B.; Place, C.; Zitoun, C.; Duchampt, A.; Da Costa, L.; Rajas, F.; Lamaze, C.; et al. A caveolin-1 dependent glucose-6-phosphatase trafficking contributes to hepatic glucose production. Mol. Metab. 2023, 70, 101700. [Google Scholar] [CrossRef]
- Burgess, S.C.; Leone, T.C.; Wende, A.R.; Croce, M.A.; Chen, Z.; Sherry, A.D.; Malloy, C.R.; Finck, B.N. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-deficient mice. J. Biol. Chem. 2006, 281, 19000–19008. [Google Scholar] [CrossRef]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Méndez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 53, 1080–1092. [Google Scholar] [CrossRef]
- Felig, P.; Pozefsk, T.; Marlis, E.; Cahill, G.F. Alanine: Key role in gluconeogenesis. Science 1970, 167, 1003–1004. [Google Scholar] [CrossRef]
- Waterhouse, C.; Keilson, J. The contribution of glucose to alanine metabolism in man. J. Lab. Clin. Med. 1978, 92, 803–812. [Google Scholar]
- Holeček, M. Origin and roles of Alanine and glutamine in Gluconeogenesis in the liver, kidneys, and small intestine under physiological and pathological conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef]
- Ryan, P.J.; Riechman, S.E.; Fluckey, J.D.; Wu, G. Interorgan metabolism of amino acids in human health and disease. In Amino Acids in Nutrition and Health: Amino Acids in Gene Expression, Metabolic Regulation, and Exercising Performance; Springer: Berlin/Heidelberg, Germany, 2021; pp. 129–149. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Yiew, N.K.; Vazquez, J.H.; Martino, M.R.; Kennon-McGill, S.; Price, J.R.; Allard, F.D.; Yee, E.U.; Layman, A.J.; James, L.P.; McCommis, K.S.; et al. Hepatic pyruvate and alanine metabolism are critical and complementary for maintenance of antioxidant capacity and resistance to oxidative insult. Mol. Metab. 2023, 77, 101808. [Google Scholar] [CrossRef]
- Yang, R.Z.; Park, S.; Reagan, W.J.; Goldstein, R.; Zhong, S.; Lawton, M.; Rajamohan, F.; Qian, K.; Liu, L.; Gong, D.W. Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009, 49, 598–607. [Google Scholar] [CrossRef]
- DeRosa, G.; Swick, R.W. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J. Biol. Chem. 1975, 250, 7961–7967. [Google Scholar] [CrossRef]
- McCommis, K.S.; Chen, Z.; Fu, X.; McDonald, W.G.; Colca, J.R.; Kletzien, R.F.; Burgess, S.C.; Finck, B.N. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 2015, 22, 682–694. [Google Scholar] [CrossRef]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Martino, M.R.; Gutiérrez-Aguilar, M.; Yiew, N.K.; Lutkewitte, A.J.; Singer, J.M.; McCommis, K.S.; Ferguson, D.; Liss, K.H.; Yoshino, J.; Renkemeyer, M.K.; et al. Silencing alanine transaminase 2 in diabetic liver attenuates hyperglycemia by reducing gluconeogenesis from amino acids. Cell Rep. 2022, 39, 110773. [Google Scholar] [CrossRef]
- Sherman, K.E. Alanine aminotransferase in clinical practice: A review. Arch. Intern. Med. 1991, 151, 260–265. [Google Scholar] [CrossRef]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925. [Google Scholar] [CrossRef]
- Najafi, F.; Mohseni, P.; Pasdar, Y. The association between dietary amino acid profile and the risk of type 2 diabetes: Ravansar non-communicable disease cohort study. BMC Public Health 2023, 23, 2284. [Google Scholar] [CrossRef]
- Natarajan, V.; Chawla, R.; Mah, T.; Vivekanandan, R.; Tan, S.Y.; Sato, P.Y.; Mallilankaraman, K. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics 2020, 20, 1800404. [Google Scholar] [CrossRef]
- Shahram, N.; Sungbo, C.; Eugeni, R. Alanine-specific appetite in slow growing chickens is associated with impaired glucose transport and TCA cycle. BMC Genom. 2022, 23, 393. [Google Scholar] [CrossRef]
- Onyango, A.N. Excessive gluconeogenesis causes the hepatic insulin resistance paradox and its sequelae. Heliyon 2022, 8, 12294. [Google Scholar] [CrossRef]
- Moon, J.; Koh, G. Clinical evidence and mechanisms of high-protein diet-induced weight loss. J. Obes. Metab. Syndr. 2020, 29, 166. [Google Scholar] [CrossRef]
- Mutel, E.; Gautier-Stein, A.; Abdul-Wahed, A.; Amigó-Correig, M.; Zitoun, C.; Stefanutti, A.; Houberdon, I.; Tourette, J.A.; Mithieux, G.; Rajas, F. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: Induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 2011, 60, 3121–3131. [Google Scholar] [CrossRef]
- Treberg, J.R.; Banh, S.; Pandey, U.; Weihrauch, D. Intertissue differences for the role of glutamate dehydrogenase in metabolism. Neurochem. Res. 2014, 39, 516–526. [Google Scholar] [CrossRef]
- Owen, O.E.; Reichle, F.A.; Mozzoli, M.A.; Kreulen, T.; Patel, M.S.; Elfenbein, I.B.; Golsorkhi, M.; Chang, K.H.; Rao, N.S.; Sue, H.S.; et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J. Clin. Investig. 1981, 68, 240–252. [Google Scholar] [CrossRef]
- Valle, D.L.; Antonarakis, S.; Ballabio, A.; Beaudet, A.L.; Mitchell, G.A. (Eds.) Part 8: Amino Acids. In The Online Metabolic and Molecular Bases of Inherited Disease; McGraw-Hill: New York, NY, USA, 2019; Available online: https://ommbid.mhmedical.com/book.aspx?bookID=2709#225069340. (accessed on 22 April 2024).
- Pasquali, M.; Longo, N. Amino acids. In Physician’s Guide to the Diagnosis, Treatment and Follow-up of Inherited Metabolic Diseases, 2nd ed.; Blau, N., Dionisi Vici, C., Ferreira, C.R., Vianey-Saban, C., van Karnebeek, C.D.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–50. [Google Scholar]

| Characteristics | Number (%) | Prediabetes, % | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Gender, male | 75 (44.12) | 70.83 | 29.17 | 0.367 |
| Age (years), mean (SD) | 50.55 (7.47) | 51.56 (7.45) | 48.35 (7.07) | 0.008 |
| ≤39 | 10 (5.88) | 4.46 | 9.09 | 0.04 |
| 40–49 | 70 (41.18) | 35.71 | 52.73 | |
| 50–59 | 70 (41.18) | 44.64 | 32.73 | |
| ≥60 | 20 (11.76) | 15.18 | 5.45 | |
| BMI categories (kg/m2) | ||||
| ≤24.9 | 43 (25.75) | 18.35 | 41.82 | 0.001, 0.0002 * |
| 25–29.9 | 70 (41.92) | 42.20 | 41.82 | |
| ≥30.0 | 54 (32.34) | 39.45 | 16.36 | |
| WC, cm, mean (SD) | 97.57(13.29) | 99.96 (13.60) | 92.75 (11.71) | 0.001 |
| Hypertension | 50 (29.94) | 33.94 | 21.82 | 0.109 |
| Antihypertensive therapy | 37 (22.56) | 78.38 | 21.62 | 0.08 |
| Pulse, beats per minute, mean (SD) | 74.22 (8.82) | 73.83 (8.53) | 74.65 (9.36) | 0.57 |
| Smoking | ||||
| No | 113 (67.66) | 69.72 | 63.64 | 0.03 |
| Quit | 34 (20.36) | 15.60 | 30.91 | |
| Yes | 20 (11.98) | 14.68 | 5.45 | |
| High fasting glucose (≥5.6) | 23 (13.61) | 73.91 | 26.09 | 0.452 |
| Lipid profile (mmol/L) | ||||
| High LDL-C (≥3.3) | 73 (42.94) | 70.83 | 29.17 | 0.367 |
| Low HDL-C (<1.03 in males, <1.29 in females) | 57 (33.53) | 75.44 | 24.56 | 0.097 |
| High TG (≥1.7) | 46 (27.06) | 79.55 | 20.45 | 0.04 |
| MS | 82 (50.31) | 66.67 | 18.18 | 0.0001 |
| Creatinine, mcmol/L, mean (SD) | 76.22 (24.53) | 74.28 (23.54) | 77.18 (19.62) | 0.43 |
| AAs, mmol/L | ORcrude | 95% CI | p-Value |
|---|---|---|---|
| Lysine | 0.98 | 0.94; 1.05 | 0.72 |
| Tyrosine | 0.99 | 0.98; 1.00 | 0.22 |
| Alanine | 0.96 | 0.94; 0.99 | 0.019 |
| Valine | 0.94 | 0.89; 0.99 | 0.015 |
| Leucine | 1.02 | 0.99; 1.05 | 0.26 |
| Isoleucine | 1.01 | 0.98; 1.04 | 0.55 |
| Proline | 1.01 | 0.98; 1.03 | 0.53 |
| Serine | 0.96 | 0.8; 1.16 | 0.70 |
| Threonine | 1.03 | 0.82; 1.28 | 0.82 |
| Methionine | 1.09 | 0.95; 1.25 | 0.23 |
| Aspartic | 0.96 | 0.88; 1.05 | 0.38 |
| Glutamic | 0.92 | 0.74; 1.14 | 0.43 |
| Phenylalanine | 0.95 | 0.85; 1.07 | 0.40 |
| Number of Observations | OR of Prediabetes | 95% CI | p-Value | Adjusted for | Model |
|---|---|---|---|---|---|
| Alanine mmol/L | |||||
| 156 | 0.96 | 0.94; 0.99 | 0.019 | Crude | 1 |
| 156 | 0.97 | 0.94; 0.99 | 0.022 | Age α | 2 |
| 153 | 0.97 | 0.94; 0.99 | 0.033 | Age + BMI β | 3 |
| Valine, mmol/L | |||||
| 136 | 0.94 | 0.90; 0.99 | 0.015 | Crude | 4 |
| 136 | 0.94 | 0.90; 0.99 | 0.014 | Age α | 5 |
| 133 | 0.95 | 0.91; 0.99 | 0.049 | Age + BMI β | 6 |
| LR Models | AUC, 95% CI | Cut-off Point, mmol/L | Sensitivity of the Adjusted LR Models, % | Specificity of the Adjusted LR Models, % | |
|---|---|---|---|---|---|
| Crude | Adjusted for Age and BMI | ||||
| Alanine | 0.58 (0.49; 0.69) | 0.72 (0.63; 0.81) | 6.234 | 86.00 | 30.77 |
| Valine | 0.60 (0.48; 0.70) | 0.72 (0.63; 0.81) | 2.267 | 84.71 | 41.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurtazina, A.; Voitsekhovskiy, I.; Kanapiyanov, B.; Toishimanov, M.; Dautov, D.; Karibayev, K.; Smail, Y.; Kozhakhmetova, D.; Dyussupov, A. Associations of Amino Acids with the Risk of Prediabetes: A Case-Control Study from Kazakhstan. J. Pers. Med. 2024, 14, 1067. https://doi.org/10.3390/jpm14101067
Nurtazina A, Voitsekhovskiy I, Kanapiyanov B, Toishimanov M, Dautov D, Karibayev K, Smail Y, Kozhakhmetova D, Dyussupov A. Associations of Amino Acids with the Risk of Prediabetes: A Case-Control Study from Kazakhstan. Journal of Personalized Medicine. 2024; 14(10):1067. https://doi.org/10.3390/jpm14101067
Chicago/Turabian StyleNurtazina, Alma, Ivan Voitsekhovskiy, Bakyt Kanapiyanov, Maxat Toishimanov, Daulet Dautov, Kairat Karibayev, Yerbol Smail, Dana Kozhakhmetova, and Altay Dyussupov. 2024. "Associations of Amino Acids with the Risk of Prediabetes: A Case-Control Study from Kazakhstan" Journal of Personalized Medicine 14, no. 10: 1067. https://doi.org/10.3390/jpm14101067
APA StyleNurtazina, A., Voitsekhovskiy, I., Kanapiyanov, B., Toishimanov, M., Dautov, D., Karibayev, K., Smail, Y., Kozhakhmetova, D., & Dyussupov, A. (2024). Associations of Amino Acids with the Risk of Prediabetes: A Case-Control Study from Kazakhstan. Journal of Personalized Medicine, 14(10), 1067. https://doi.org/10.3390/jpm14101067







