Abstract
An elevated platelet count may contribute to significant thrombotic events and pose a risk for diabetic microvascular complications. Albuminuria, one of the hallmarks of diabetes, is thought to be a risk factor for endothelial dysfunction. In this study, we investigated the association between relative thrombocytosis and an increased urine albumin-to-creatinine ratio in healthy adult participants. Using multivariate analyses on data from the Korea National Health and Nutrition Examination Survey V–VI, 12,525 eligible native Koreans aged ≥ 20 were categorized into platelet count quintiles by sex. The highest platelet count quintile included younger, more obese participants with elevated white blood cell counts, poor lipid profiles, and a better estimated glomerular filtration rate. Restricted cubic spline regression analysis revealed significant associations between platelet count and fasting blood glucose, glycated hemoglobin, and urine albumin-to-creatinine ratio. Adjusted logistic regression models indicated that heightened fasting blood glucose and platelet count were linked to risk of microalbuminuria (fasting blood glucose, odds ratio = 1.026, 95%CI = 1.011–1.042; platelet count, odds ratio = 1.004, 95%CI = 1.002–1.006). Particularly, an increased platelet count was notably associated with microalbuminuria progression in subjects with impaired fasting glucose. These findings suggest that an elevated platelet count, even below diagnostic thrombocytosis levels, independently correlates with an increased risk of vascular endothelial dysfunction in patients with impaired fasting glucose.
1. Introduction
Diabetes mellitus (DM) can be accompanied by various systemic complications and is often caused by metabolic abnormalities in vascular functions and responses [1]. The risk of the development and progression of DM-related vascular complications increases with the duration and severity of hyperglycemia [2]. Several experimental studies on the pathogenesis of diabetic vascular complications demonstrated that increased glycemic exposure contributed to the overproduction of reactive oxygen species and alteration of various intracellular signaling pathways in vascular beds, which could be responsible for the derangement of endogenous vascular protective mechanisms and the initiation of various vascular endothelial dysfunctions [3,4].
In particular, diabetic kidney disease is progressed by hemodynamic changes and inflammations occurring in the microvascular unit and is characterized by albuminuria, that is, an increase in the urinary excretion rate of albumin. Microalbuminuria, which may be prominent several years before the diagnosis of overt diabetic kidney disease, may imply the presence of glomerular filtration barrier dysfunction, a significant feature of vascular endothelial injury related to pathologic inflammatory responses in patients with DM [5,6,7]. Recent evidence suggests that microalbuminuria is a strong and independent predictor of increased cardiovascular and all-cause mortality among individuals with and without DM [8,9]. Thus, identifying the risk of albuminuria is critical for improving the long-term prognosis of DM.
Platelet activation is one of many factors related to the pathogenesis of albuminuria in DM patients and is related to endothelial dysfunction, chronic vascular inflammation, intravascular thrombi, and microangiopathy. Platelets are anucleate cytoplasmic discs derived from megakaryocytes and circulate in the blood, playing critical roles in managing vascular integrity and regulating hemostasis [10,11,12]. Accumulating evidence shows that the deterioration of platelet structure and function in patients with DM could be responsible for the development of vascular complications [13,14]. Recently, some authors argued that not only long-term continuous hyperglycemic exposure but also short-term hyperglycemic spikes were deeply associated with thrombocytosis and platelet hyperactivity, which may contribute to vascular endothelial injury and blood clotting disorders [15,16,17,18,19].
To provide a mechanistic explanation of the relationship between platelets, vascular endothelial dysfunction, and diabetes, oxidative stress occurs due to insulin resistance and high blood sugar, which inhibits fibrinolysis in diabetes. Additionally, as the secretion of free fatty acids becomes excessive, endothelial dysfunction occurs, and platelet dysfunction occurs as metabolic abnormalities such as obesity and dyslipidemia occur. These processes ultimately lead to the risk of vascular complications due to changes in platelet function [20]. Although increased platelet counts (PCs) as well as platelet dysfunction could be related to microvascular complications of DM, there is little clinical evidence of an association between relative thrombocytosis and vascular endothelial dysfunction, especially in the prediabetic condition. Therefore, in this study, we aimed to evaluate whether relative thrombocytosis is associated with increased albuminuria through a possible interaction with mild hyperglycemia.
2. Materials and Methods
2.1. Study Population
Data were collected from public-use data sets from the Korean National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Centers for Disease Control and Prevention (KCDC) among non-institutionalized Korean civilians between 2011 and 2014. All participants were volunteers and provided written informed consent before enrollment. All data, except survey data, were anonymized before the analysis. This study was approved by the Institutional Review Board of the KCDC (No. 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C).
A total of 35954 individuals had participated in the KNHANES 2011–2014. The following participants were excluded from this study: those for whom data were incomplete (anthropometric or laboratory); those <20 years of age; currently pregnant women; or those who had any medical problem related to chronic cardiovascular disease, estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) < 60, urine albumin/creatinine ratio (UACR, mg/g creatinine) > 300, or plasma PC (103/µL) < 150 or >450. The final 12,525 participants were divided into five quintiles according to their PCs stratified by sex (Figure 1).
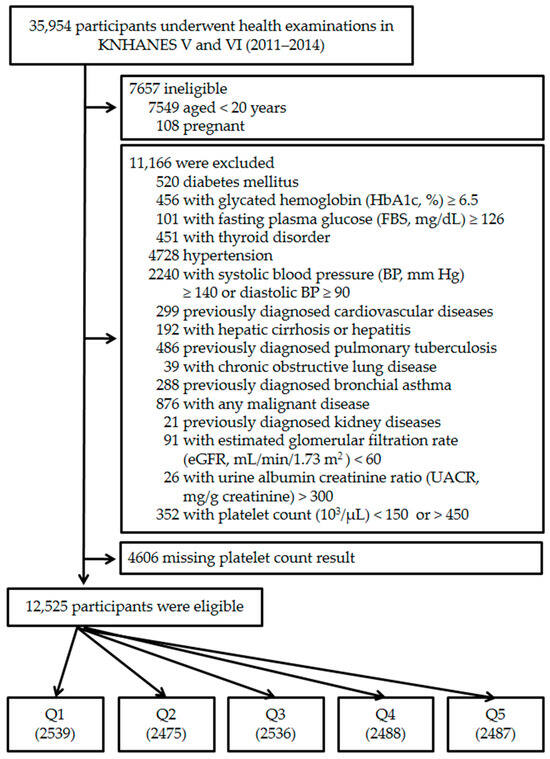
Figure 1.
Flow chart of the study group enrollment process. KNHANES, Korean National Health and Nutritional Examination Survey; Q, platelet count quintile.
2.2. Anthropometric and Clinical Measurements
Anthropometric measurements were made by well-trained examiners. The participants wore lightweight gowns or underwear. Height was measured to the nearest 0.1 cm using a portable stadiometer (Seriter, Bismarck, ND, USA). Weight was measured to the nearest 0.1 kg on a calibrated balance beam scale (Giant-150N; Hana, Seoul, Republic of Korea). Waist circumference (WC) was measured using a flexible tape at the narrowest point between the lowest border of the rib cage and the uppermost lateral border of the iliac crest at the end of normal expiration. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Smoking history was gathered through self-reported questionnaires. Smokers are individuals who smoked more than 100 cigarettes in their lifetime. Smoking duration (in years) and smoking dose (daily average) are multiplied to calculate smoking pack-years. Cut-off points of anthropometric variables are presented in the Supplemental Table S1.
Blood pressure (BP) was measured three times using a mercury sphygmomanometer (Baumanometer; Baum, Copiague, NY, USA) while subjects were in a sitting position following a 5 min rest period. The average values of the last 2 recorded systolic and diastolic BP values were used in the analysis.
2.3. Laboratory Tests
Venous blood samples were collected after 8 h overnight fasting. Platelets were counted using an automated hematology analyzer (Sysmex XE-2100D Hematology Analyzer, Sysmex Corporation, Kobe, Japan) in EDTA-anticoagulated whole blood samples. Fasting plasma concentrations of glucose, triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were determined by a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Glycated hemoglobin (HbA1c) levels were determined by high-performance liquid chromatography using an automated HLC-723G7 analyzer (Tosoh Corporation, Tokyo, Japan). Serum creatinine levels were measured colorimetrically (Hitachi Automatic Analyzer 7600) and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [21]. To obtain the UACR, urinary albumin was measured in spot urine using the immunoturbidimetric method and urinary creatinine was measured using the colorimetric method. Reference values of all laboratory tests completed are presented in the Supplemental Table S1.
2.4. Definitions
According to the American Diabetes Association standards of medical care from 2024 [22], mild hyperglycemia was defined as a fasting blood glucose (FBS) of 100–125 mg/dL or an HbA1c of 5.7–6.4% without the use of hypoglycemic medications. According to the KDIGO 2012 Clinical Practice Guideline [23], participants with microalbuminuria were defined as those with a UACR of 30–300 mg/g creatinine.
2.5. Statistical Analysis
All data, including sociodemographic data, medical conditions, anthropometric and clinical measurements, and laboratory results, are presented as mean ± SE or frequencies (proportions). Data were analyzed using sampling weights to account for multistage and stratified sampling. The normality of the distribution was ascertained by the Kolmogorov–Smirnov test. If variables did not follow a normal distribution, logarithmic (log) transformation was applied before the statistical analysis. Participant characteristics were analyzed based on platelet count quintiles using weighted one-way analysis of variance (ANOVA) tests for continuous variables and weighted chi-square tests. The generalized linear model was used to compare quantitative variables and the chi-squared and Fisher’s exact test to compare proportions for the categorical variables. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated in multiple logistic regression models according to the presence of a dependent variable (case vs. control) after controlling for potential confounding factors within platelet count quintiles. Restricted cubic spline (RCS) regression analysis was used to find the possible non-linear dependency of the association between candidate risk factors and increased risk of dependent variable [24]. A two-tailed p < 0.05 was considered statistically significant. Finally, we repeated the statistical analysis and analyzed the significant results obtained. Statistical Analysis Software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all of the analyses.
3. Results
3.1. Baseline Characteristics
The participants (n = 12,525) comprised 4939 men and 7586 women with a mean age of 44.0 ± 14.5 years. They were divided into quintiles according to PCs stratified by sex. Participants in the highest PC quintile were likely to be younger and more obese and have increased white blood cell counts, greater glycemic exposure, a poor lipid profile, and better eGFR compared to those in the lower quintiles. However, there was a marginal difference in UACR among PC quintiles (Table 1).

Table 1.
General characteristics grouped according to blood platelet count.
3.2. Relation of Increased PC with Microalbuminuria and Related Risk Factors
In conventional linear regression analysis, we found that although PC was significantly related to BMI, white blood cell count, hemoglobin, eGFR, FBS, HbA1c, TG, LDL cholesterol, 25-vitamin D, and UACR, there was no significant relation of PC with WC, systolic BP, diastolic BP, and HDL cholesterol (Table 2).

Table 2.
Linear regression of platelet count (103/μL).
To evaluate the relationship pattern of PC with candidate risk factors of vascular endothelial dysfunction, we performed a RCS linear regression model with age, sex, and smoking history as covariates and found that PC had differing relationships with these risk factors: non-linear relationship with BPs, linear relationship with indicators of glycemic exposure, and potential J-shaped relationship with UACR (Figure 2).
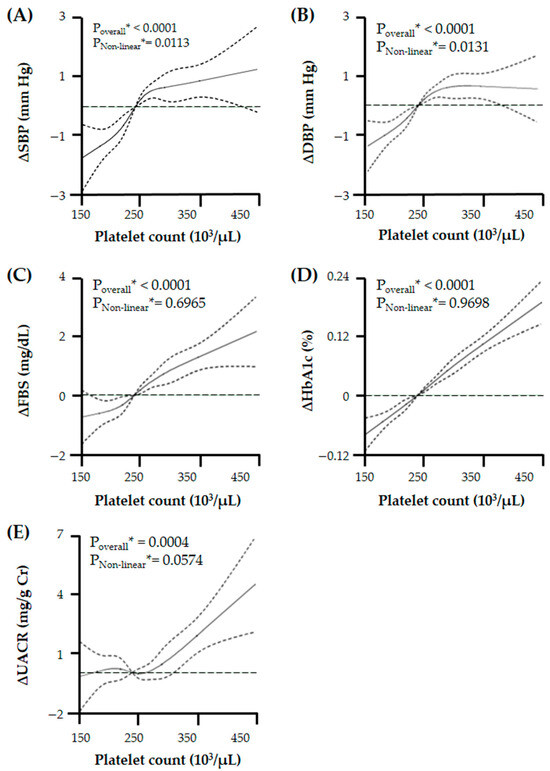
Figure 2.
Relationship of platelet count with changes in (A) systolic blood pressure (BP), (B) diastolic BP, (C) fasting blood glucose (FBS), (D) glycated hemoglobin (HbA1c), and (E) urine albumin/creatinine ratio (UACR) with a chosen reference platelet count of 240 × 103/μL. There is a varied relationship between platelet count and UACR or related clinical risk factors. Especially, platelet count has a J-shaped relation with UACR. Solid lines represent changes in microalbuminuria and related clinical risk factors and dashed lines represent 95% confidential intervals. * Calculated by restricted cubic spline (RCS) regression adjusted for age, sex, and smoking history.
3.3. Association of Relative Thrombocytosis with Microalbuminuria
We performed multiple logistic regression models, using age, sex, and smoking history as covariates, to find a possible association between PC and other candidate predictors for vascular endothelial dysfunction. When participants with microalbuminuria were compared to controls, we found that both PC and FBS were significantly associated with microalbuminuria, and further adjustment for systolic BP, FBS, aspartate aminotransferase, triglyceride, and LDL cholesterol as predictors did not attenuate these associations (PC, adjusted OR = 1.002, 95% CI = 1.001–1.004; FBS, adjusted OR = 1.026, 95% CI = 1.011–1.042; Table 3).

Table 3.
Multivariate logistic regression analysis of microalbuminuria *.
Our further analyses revealed that there was a J-shaped association between PC and the risk of microalbuminuria in the RCS analysis (Figure 3), and interestingly, the risk of microalbuminuria was increased with increasing PCs only in participants with mild fasting hyperglycemia, not in participants with mild elevated HbA1c (Figure 4).
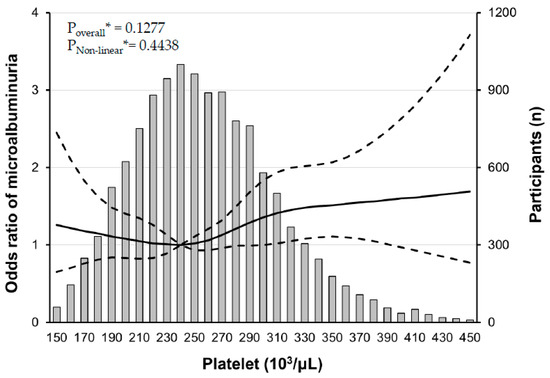
Figure 3.
Multiple logistic regression analysis of microalbuminuria#-specific risk with restricted cubic splines (RCS). There is a non-linear association between platelet count and the risk of microalbuminuria. The solid line represents the risk of albuminuria and dashed lines represent 95% confidential intervals. RCS plots are performed using a platelet count of 240 × 103/μL as a reference. * Calculated by RCS logistic regression model adjusted for age, sex, smoking history, systolic BP, AST, triglyceride, and LDL cholesterol. # defined as a urine albumin/creatinine ratio between 30 and 300 mg/g creatinine.
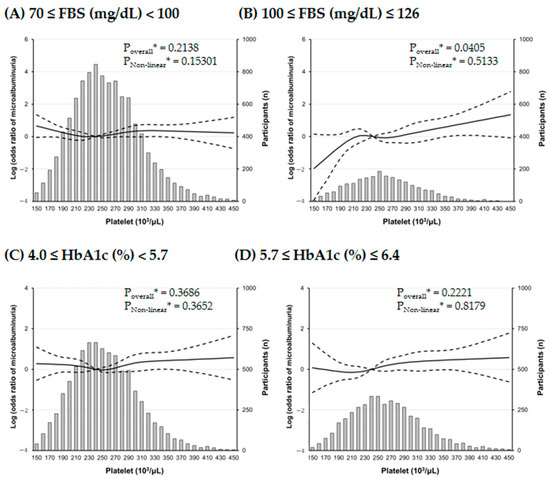
Figure 4.
Multiple logistic regression analysis of microalbuminuria#-specific risk with restricted cubic splines (RCS) regression. The effect of platelet count on microalbuminuria prominently increases in subjects with mild fasting hyperglycemia. Solid lines represent the risk of albuminuria and dashed lines represent 95% confidential intervals. RCS plots are performed using a platelet count of 240 × 103/μL as a reference. * Calculated by RCS logistic regression model adjusted for age, sex, smoking history, systolic BP, AST, triglyceride, and LDL cholesterol. # defined as a urine albumin/creatinine ratio between 30 and 300 mg/g creatinine.
4. Discussion
This study provides a comprehensive overview of the relationship between metabolic disturbance, relative thrombocytosis, and microvascular endothelial dysfunction, showing that an elevated PC in participants with mild hyperglycemia is associated with an increased risk of microalbuminuria. Such results point out that, in addition to the established risk factor of microalbuminuria, relative thrombocytosis could be an important indicator of predicting vascular endothelial dysfunction before the diagnosis of overt DM.
In the analysis based on platelet count quintiles, it became evident that there were recognizable differences in various baseline characteristics among the groups. An intriguing observation was that microalbuminuria exhibited a distinct pattern across these quintiles. Specifically, in quintile 1, microalbuminuria was higher compared to quintiles 2–4, and as platelet count quintiles increased, there was a gradual rise in microalbuminuria, reaching its highest level in quintile 5. Next, in the linear regression performed, it was confirmed that microalbuminuria had a positive correlation with platelet count. Additionally, not only glycemic index but also white blood cell count demonstrated a relatively strong positive correlation with platelet count in linear regression. Multivariate logistic regression analysis was performed to more clearly identify the risk factors for microalbuminuria. The results highlighted that systolic blood pressure, platelet count, and glycemic index were contributors to an increased risk of microalbuminuria. Interestingly, white blood cell count did not emerge as a significant risk factor for microalbuminuria in this analysis. Logistic regression model I indicated that an elevated systolic blood pressure increased the risk of albuminuria, a finding that aligns with expectations, considering microalbuminuria’s established status as a cardiovascular risk factor [25]. To explore the refined relationship between platelet count and microalbuminuria, this study employed restricted cubic splines, revealing a J curve. This non-linear relationship underscores the intricate nature of the association, implying the existence of a complex mechanism between platelet count and microalbuminuria.
Our study revealed that although a mild increase in glycemic exposure was related to an increased PC, it was more correlated with FBS than HbA1c. Long-term exposure to metabolic disturbances and the accumulation of pro-inflammatory mediators in diabetes can elicit pathologic platelet activation and cause microvascular complications [19,26,27,28,29]. Recent studies have demonstrated that an acute short-term hyperglycemic spike is also a sufficient stimulus to increase high shear stress-induced platelet activation in patients with type 2 DM [15,18]. Impaired fasting glucose due to inappropriate endogenous glucose production originates from hepatic insulin resistance, reduced hepatic glucose clearance, and dysfunction of glucose uptake and production [30]. Meanwhile, patients with an elevated HbA1c level reflecting mean glucose level during the past 2 to 3 months may have continuously worsening impaired fasting glucose, impaired glucose tolerance characterized by skeletal muscle resistance and beta cell dysfunction, or both [31]. Although little is known about whether mild fasting hyperglycemia is associated with relative thrombocytosis and related systemic complications, it can be assumed that increased PC has a greater impact on impaired fasting glucose in prediabetes rather than impaired glucose tolerance.
Our linear regression analysis showed that there was a close relationship between plasma PC and clinical parameters of chronic systemic inflammation, such as white blood cell and albuminuria, in the general population. Low-grade systemic inflammation can account for not only the activation of platelets in peripheral blood but also the proliferation and differentiation of megakaryocyte colony-forming units in bone marrow, which may affect the long-term process of atherosclerosis as well as the development of microvascular complications of various chronic illnesses [17,32]. Some authors argued that an increased PC might be one of the significant contributors to the development of microalbuminuria and overt diabetic nephropathy [16,33]. Such findings suggest that an elevated PC, even below the diagnostic criteria of thrombocytosis, could have a role in predicting the initiation of a systemic inflammatory response and related microvascular injury.
Unfortunately, we failed to find a possible relation between leukocytosis and the development of microalbuminuria in this study. This result was inconsistent with those of previous studies showing that neutrophils were glucose-sensitive inflammatory cells and played a critical role in platelet activation [18,26]. Because of the limitation of our cross-sectional study design, we did not have a differential count of white blood cells, which made it difficult to efficiently demonstrate the long-term harmful effects of neutrophil activation on the microvascular endothelium. Another possibility is that this pathologic process may result from the accumulation of more severe metabolic derangements or some confounding variables, such as other genetic factors or environmental conditions, which could have exerted a hidden effect on our research outcomes.
There were several limitations to our study. First, because this cross-sectional population-based study did not include other platelet indices, such as plateletcrit, mean platelet volume, or platelet distribution width, we had to use only PC as a surrogate marker. Second, because a very small number of participants had UACR > 300 mg/g creatinine, we could not include them in the study. This limitation made it impossible to investigate the relationship between metabolic disturbance and severely increased albuminuria. Third, our public-use data sets did not contain the results of an oral glucose tolerance test, insulin level, and associated adipokines, as well as details regarding a history of acute infection, recent surgeries, and medication usage such as corticosteroids. Consequently, we could not finely define the metabolic and inflammatory status of the participants. Fourth, there is a limitation to explaining the mechanism of an independent role of platelets in microvascular endothelial dysfunction due to a lack of data about thrombopoietin, C-reactive protein, pro-inflammatory cytokines, renin, angiotensin II, or aldosterone in this study. Furthermore, our findings could have been influenced by the anticoagulants used in the plasma samples. Fifth, because of a social desirability bias in the self-reporting of medical history, medication, and use of tobacco and alcohol, our results may conflict with those of previous studies. Finally, participants might have forgotten pertinent relevant details.
Despite the aforementioned limitations, our study was relatively successful in demonstrating a correlation between subtle changes in platelet count and the development of microalbuminuria in prediabetes. Our study results are particularly meaningful given that the literature on platelet dynamics in prediabetes is rare [34,35]. Previous studies on platelets in diabetes have revealed various pathological changes, such as changes in platelet hematocrit, platelet indices, increased intracellular calcium concentration, increased activation, adhesion, reactivity, and turnover of platelets, and increased reticular platelets [36,37,38]. These platelet abnormalities have a well-established association with the hyperglycemia, insulin resistance, and oxidative stress observed in diabetes, potentially leading to endothelial dysfunction, increased vascular inflammation, and increased risk of vascular complications [39,40,41,42]. These abnormal changes in platelets may play a major role in the development of microvascular complications of diabetes, and accumulated evidence in diabetic patients suggests that platelet changes may contribute to increased microalbuminuria [43,44,45]. Ultimately, we hope that our study will lead to a reassessment of the importance of platelet count testing in prediabetes, which has received relatively little attention.
5. Conclusions
In conclusion, the results of the present study showed that in the condition of impaired fasting glucose, a relative thrombocytosis was independently associated with increased risk of microalbuminuria even before the appearance of overt DM. Further study on the role of PC as a valuable predictor of diabetic vascular endothelial dysfunction is needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm14010089/s1, Table S1: Reference values for anthropometric variables and laboratory tests.
Author Contributions
Conceptualization, J.-S.P. and C.H.L.; methodology, J.-S.P.; software, J.-S.P.; validation, J.-S.P.; formal analysis, J.-S.P.; investigation, J.-S.P.; resources, J.-S.P.; data curation, J.-S.P.; writing—original draft preparation, J.W.C.; writing—review and editing, J.W.C. and T.H.K.; visualization, J.-S.P.; supervision, J.-S.P.; project administration, J.-S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Data were collected from public-use data sets from the Korean National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Centers for Disease Control and Prevention (KCDC) among non-institutionalized Korean civilians between 2011 and 2014. All data, except survey data, were anonymized before the analysis. This study was approved by the Institutional Review Board of the KCDC (No. 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The publicly archived datasets can be downloaded through link to the KCDC. https://knhanes.kdca.go.kr/knhanes/eng/index.do (accessed on 1 July 2020).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karalliedde, J.; Gnudi, L. Diabetes Mellitus, a Complex and Heterogeneous Disease, and the Role of Insulin Resistance as a Determinant of Diabetic Kidney Disease. Nephrol. Dial. Transpl. 2016, 31, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cintrón, M.; Flores-Tamez, V.A.; Le, T.; Baudel, M.M.; Navedo, M.F. Cellular and Molecular Effects of Hyperglycemia on Ion Channels in Vascular Smooth Muscle. Cell. Mol. Life Sci. 2021, 78, 31–61. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Cao, W.; Cui, J.; Li, S.; Zhang, D.; Guo, Y.; Li, Q.; Luan, Y.; Liu, X. Crocetin Restores Diabetic Endothelial Progenitor Cell Dysfunction by Enhancing NO Bioavailability via Regulation of PI3K/AKT-eNOS and ROS Pathways. Life Sci. 2017, 181, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Scurt, F.G.; Menne, J.; Brandt, S.; Bernhardt, A.; Mertens, P.R.; Haller, H.; Chatzikyrkou, C.; ROADMAP Steering Committee. Systemic Inflammation Precedes Microalbuminuria in Diabetes. Kidney Int. Rep. 2019, 4, 1373–1386. [Google Scholar] [CrossRef]
- Stringhini, S.; Zaninotto, P.; Kumari, M.; Kivimäki, M.; Batty, G.D. Lifecourse Socioeconomic Status and Type 2 Diabetes: The Role of Chronic Inflammation in the English Longitudinal Study of Ageing. Sci. Rep. 2016, 6, 24780. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Lees, J.S.; Welsh, C.E.; Celis-Morales, C.A.; Mackay, D.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Cleland, J.G.; Gill, J.M.R.; Jhund, P.S.; et al. Glomerular Filtration Rate by Differing Measures, Albuminuria and Prediction of Cardiovascular Disease, Mortality and End-Stage Kidney Disease. Nat. Med. 2019, 25, 1753–1760. [Google Scholar] [CrossRef]
- Fangel, M.V.; Nielsen, P.B.; Kristensen, J.K.; Larsen, T.B.; Overvad, T.F.; Lip, G.Y.; Jensen, M.B. Albuminuria and Risk of Cardiovascular Events and Mortality in a General Population of Patients with Type 2 Diabetes without Cardiovascular Disease: A Danish Cohort Study. Am. J. Med. 2020, 133, e269–e279. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef]
- Sim, X.; Poncz, M.; Gadue, P.; French, D.L. Understanding Platelet Generation from Megakaryocytes: Implications for in Vitro-Derived Platelets. Blood 2016, 127, 1227–1233. [Google Scholar] [CrossRef]
- Zamora, C.; Cantó, E.; Vidal, S. The Dual Role of Platelets in the Cardiovascular Risk of Chronic Inflammation. Front. Immunol. 2021, 12, 625181. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, Endothelial Stress, Inflammation, and the Metabolic Syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Buch, A.; Kaur, S.; Nair, R.; Jain, A. Platelet Volume Indices as Predictive Biomarkers for Diabetic Complications in Type 2 Diabetic Patients. J. Lab. Physicians 2017, 9, 84–88. [Google Scholar] [CrossRef]
- Spectre, G.; Stålesen, R.; Östenson, C.G.; Hjemdahl, P. Meal-Induced Platelet Activation in Diabetes Mellitus Type 1 or Type 2 is Related to Postprandial Insulin rather than Glucose Levels. Thromb. Res. 2016, 141, 93–97. [Google Scholar] [CrossRef]
- Gresele, P.; Guglielmini, G.; De Angelis, M.; Ciferri, S.; Ciofetta, M.; Falcinelli, E.; Lalli, C.; Ciabattoni, G.; Davì, G.; Bolli, G.B. Acute, Short-Term Hyperglycemia Enhances Shear Stress-Induced Platelet Activation in Patients with Type II Diabetes Mellitus. J. Am. Coll. Cardiol. 2003, 41, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Desilles, J.P.; Syvannarath, V.; Ollivier, V.; Journé, C.; Delbosc, S.; Ducroux, C.; Boisseau, W.; Louedec, L.; Di Meglio, L.; Loyau, S.; et al. Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation. Stroke 2017, 48, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, M.J.; Lee, M.K.; Al-Sharea, A.; Dragoljevic, D.; Barrett, T.J.; Montenont, E.; Basu, D.; Heywood, S.; Kammoun, H.L.; Flynn, M.; et al. Neutrophil-Derived S100 Calcium-Binding Proteins A8/A9 Promote Reticulated Thrombocytosis and Atherogenesis in Diabetes. J. Clin. Investig. 2017, 127, 2133–2147. [Google Scholar] [CrossRef]
- Shaked, I.; Foo, C.; Mächler, P.; Liu, R.; Cui, Y.; Ji, X.; Broggini, T.; Kaminski, T.; Suryakant Jadhav, S.; Sundd, P.; et al. A Lone Spike in Blood Glucose Can Enhance the Thrombo-Inflammatory Response in Cortical Venules. J. Cereb. Blood Flow. Metab. 2023, 271678X231203023. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial Dysfunction and Platelet Hyperactivity in Type 2 Diabetes Mellitus: Molecular Insights and Therapeutic Strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Diagnosis and Classification of Diabetes: Standards of Medical Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-Response Analyses Using Restricted Cubic Spline Functions in Public Health Research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H. Albuminuria, Estimated Glomerular Filtration Rate, and Traditional Predictors for Composite Cardiovascular and Kidney Outcome: A Population-based Cohort Study in Korea. Kidney Res. Clin. Pract. 2022, 41, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Bergmeier, W. Sugar Makes Neutrophils RAGE: Linking Diabetes-Associated Hyperglycemia to Thrombocytosis and Platelet Reactivity. J. Clin. Investig. 2017, 127, 2040–2043. [Google Scholar] [CrossRef]
- Moore, S.F.; Williams, C.M.; Brown, E.; Blair, T.A.; Harper, M.T.; Coward, R.J.; Poole, A.W.; Hers, I. Loss of the Insulin Receptor in Murine Megakaryocytes/Platelets Causes Thrombocytosis and Alterations in IGF Signalling. Cardiovasc. Res. 2015, 107, 9–19. [Google Scholar] [CrossRef]
- Ferreiro, J.L.; Gómez-Hospital, J.A.; Angiolillo, D.J. Platelet Abnormalities in Diabetes Mellitus. Diabetes Vasc. Dis. Res. 2010, 7, 251–259. [Google Scholar] [CrossRef]
- Jung, S.W.; Moon, J.Y. The Role of Inflammation in Diabetic Kidney Disease. Korean J. Intern. Med. 2021, 36, 753–766. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Perreault, L.; Færch, K. Approaching Pre-Diabetes. J. Diabetes Complicat. 2014, 28, 226–233. [Google Scholar] [CrossRef]
- Ni, H. The Platelet “Sugar High” in Diabetes. Blood 2012, 119, 5949–5951. [Google Scholar] [CrossRef] [PubMed]
- Magri, C.J.; Calleja, N.; Buhagiar, G.; Fava, S.; Vassallo, J. Factors Associated with Diabetic Nephropathy in Subjects with Proliferative Retinopathy. Int. Urol. Nephrol. 2012, 44, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Urbano, F.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Miano, N.; Coppolino, G.; L’Episcopo, G.; Leggio, S.; Scicali, R.; et al. Increased Platelet Reactivity and Proinflammatory Profile Are Associated with Intima-Media Thickness and Arterial Stiffness in Prediabetes. J. Clin. Med. 2022, 11, 2870. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Niwa, T.; Nakajima, K.; Kobayashi, M.; Hanyu, N.; Nakayama, T. Correlation between Mean Platelet Volume and Fasting Plasma Glucose Levels in Prediabetic and Normoglycemic Individuals. Cardiovasc. Diabetol. 2013, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Wicik, Z.; Keshwani, D.; Aziz, F.; Aberer, F.; Pferschy, P.N.; Tripolt, N.J.; Sourij, C.; Prietl, B.; Prüller, F.; et al. Alteration of Circulating Platelet-related and Diabetes-related MicroRNAs in Individuals with Type 2 Diabetes Mellitus: A Stepwise Hypoglycaemic Clamp Study. Cardiovasc. Diabetol. 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, L.; Thomson, G.J.A.; Adams, R.C.M.; Nell, T.A.; Laubscher, W.A.; Pretorius, E. Platelet Activity and Hypercoagulation in Type 2 Diabetes. Cardiovasc. Diabetol. 2018, 17, 141. [Google Scholar] [CrossRef]
- Pretorius, E. Platelets as Potent Signaling Entities in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2019, 30, 532–545. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.L.; Gong, Y.X.; Wang, G.H.; Hu, Z.B.; Liu, L.; Lu, J.; Chen, P.P.; Lu, C.C.; Ruan, X.Z.; et al. Platelet Microparticles Mediate Glomerular Endothelial Injury in Early Diabetic Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2671–2695. [Google Scholar] [CrossRef]
- Vaidya, A.R.; Wolska, N.; Vara, D.; Mailer, R.K.; Schröder, K.; Pula, G. Diabetes and Thrombosis: A Central Role for Vascular Oxidative Stress. Antioxidants 2021, 10, 706. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Wang, C.; Liu, Y.; Naruse, K.; Takahashi, K. The Mechanisms of the Development of Atherosclerosis in Prediabetes. Int. J. Mol. Sci. 2021, 22, 4108. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Chung, W.Y.; Moon, M.K. Peripheral Arterial Endothelial Dysfunction Predicts Future Cardiovascular Events in Diabetic Patients with Albuminuria: A Prospective Cohort Study. Cardiovasc. Diabetol. 2020, 19, 82. [Google Scholar] [CrossRef]
- Razak, M.K.A.; Akif, A.M.; Nakeeb, N.; Rasheed, J.I. The Relationship between Mean Platelet Volume and Albuminuria in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. 2019, 13, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic Immune-inflammation Index is Associated with Diabetic Kidney Disease in Type 2 Diabetes Mellitus Patients: Evidence from NHANES 2011-2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Sun, L.; Zhang, C.; Wu, L.; Nie, G.; Huang, Z.; Xing, C.; Zhang, B.; Yuan, Y. Association of Platelet-to-Lymphocyte Ratio with Kidney Clinicopathologic Features and Renal Outcomes in Patients with Diabetic Kidney Disease. Int. Immunopharmacol. 2021, 93, 107413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).