Rethinking Fluid Responsiveness during Septic Shock: Ameliorate Accuracy of Noninvasive Cardiac Output Measurements through Evaluation of Arterial Biomechanical Properties
Abstract
1. Introduction
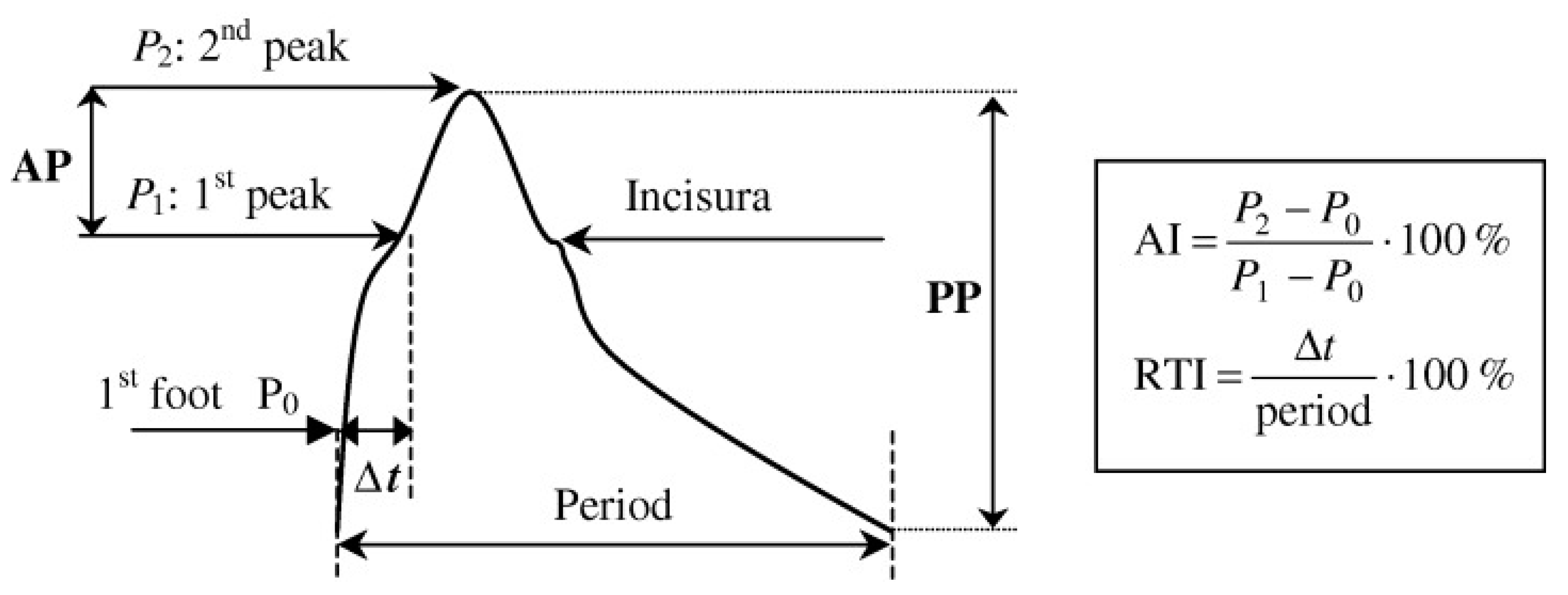
2. Arterial Biomechanics and Pulse Wave Velocity in Health and Disease
3. Limitations of Noninvasive Cardiac Output Measurement during Septic Shock
4. Rationale of Our Hypothesis
5. Testing Our Hypothesis with Specific Measurements: Mapping of Arterial Stiffness at Different Arterial Segments for More Reliable CO Estimation
- 1.
- To describe the trajectories of different arterial biomechanical indices during hemodynamic management of septic shock;
- 2.
- To evaluate, using statistical models, whether these domains of measurements, in multivariate analyses, predict outcomes of interest (i.e., fluid responsiveness) and therefore identify the incremental predictive value of such ‘physiomarkers’;
- 3.
- To evaluate, through statistical analyses, how profiles of such indices affect CO measurements.
- 1.
- To guide, personalize and optimize the diagnostic and therapeutic management of ICU patients with septic shock using a wide spectrum of vascular physiomarkers;
- 2.
- To promptly alert physicians to change their therapeutic strategy based on time variation in these physiomarkers.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-pathophysiology and therapeutic concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Early management of severe sepsis. Chest 2014, 145, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.-J.; Joannes-Boyau, O.; Teboul, J.-L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four Ds and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B. Arterial functions: How to interpret the complex physiology. Nephrol. Dial. Transplant. 2010, 25, 3815–3823. [Google Scholar] [CrossRef]
- Bodys-Pelka, A.; Kusztal, M.; Boszko, M.; Glowczynska, R.; Grabowski, M. Non-invasive continuous measurement of hemodynamic parameters-clinical utility. J. Clin. Med. 2021, 10, 4929. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Mathioulakis, D.; Stamatelopoulos, K.S.; Gialafos, E.J.; Lekakis, J.P.; Nanas, J.; Stamatelopoulos, S.F.; Tsangaris, S.G. New aspects on the role of blood pressure and arterial stiffness in mechanical assistance by intra-aortic balloon pump: In-vitro data and their application in clinical practice. Artif. Organs 2004, 28, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Vardoulis, O.; Papaioannou, T.; Stergiopoulos, N. On the estimation of total arterial compliance from aortic pulse wave velocity. Ann. Biomed. Eng. 2012, 40, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Adji, A. An updated clinical primer on large artery mechanics: Implications of pulse waveform analysis and arterial tonometry. Curr. Opin. Cardiol. 2005, 20, 275–281. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, M.J.; Cui, Y.J.; Liang, L.; Zhou, M.-M.; Yang, Y.-W.; Huang, F. Carotid-femoral pulse wave velocity in the prediction of cardiovascular events and mortality: An updated systematic review and meta-analysis. Angiology 2018, 69, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E.; London, G.M. Stiffness of capacitive and conduit arteries: Prognostic significance for-end-stage renal disease patients. Hypertension 2005, 45, 592–596. [Google Scholar] [CrossRef]
- Lamia, B.; Teboul, J.L.; Monnet, X.; Osman, D.; Maizel, J.; Richard, C.; Chemla, D. Contribution of arterial stiffness and stroke volume to peripheral pulse pressure in ICU patients: An arterial tonometry study. Intensive Care Med. 2007, 33, 1931–1937. [Google Scholar] [CrossRef]
- Dufour, N.; Chemla, D.; Teboul, J.L.; Monnet, X.; Richard, C.; Osman, D. Changes in pulse pressure following fluid loading: A comparison between aortic root (non-invasive tonometry) and femoral artery (invasive recordings). Intensive Care Med. 2010, 37, 942–949. [Google Scholar] [CrossRef]
- Kazune, S.; Grabovskis, A.; Cescon, C.; Strike, E.; Vanags, I. Association between increased arterial stiffness and clinical outcomes in patients with early sepsis: A prospective observational cohort study. Intensive Care Med. Exp. 2019, 7, 26. [Google Scholar] [CrossRef]
- Nagayama, D.; Imamura, H.; Endo, K.; Saiki, A.; Sato, Y.; Yamaguchi, T.; Watanabe, Y.; Ohira, M.; Shirai, K.; Tatsuno, I. Marker of sepsis severity is associated with the variation in cardio-ankle vascular index (CAVI) during sepsis treatment. Vasc. Health Risk Manag. 2019, 15, 509–516. [Google Scholar] [CrossRef]
- Lamia, B.; Chemla, D.; Richard, C.; Teboul, J.L. Clinical review: Interpretation of arterial pressure wave in shock states. Crit. Care 2005, 9, 601–606. [Google Scholar] [CrossRef][Green Version]
- Jansen, J.R.; Schreuder, J.J.; Mulier, J.P.; Smith, N.T.; Settels, J.J.; Wesseling, K.H. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br. J. Anaesth. 2001, 87, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Hatib, F.; Jansen, J.R.C.; Pinsky, M. Peripheral vascular decoupling in porcine endotoxic shock. J. Appl. Physiol. 2011, 111, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Herpain, A.; Baselli, G.; Ferrario, M. Vascular decoupling in septic shock: The combined role of autonomic nervous system, arterial stiffness and peripheral vascular tone. Front. Physiol. 2020, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Niroumandi, S.; Alavi, R.; Wolfson, A.M.; Vaidya, A.S.; Pahlevan, N.M. Assessment of aortic characteristic impedance and arterial compliance from non-invasive carotid pressure waveform in the Framingham heart study. Am. J. Cardiol. 2023, 204, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; MacCallum, H.; Hupperetz, P.C.; van Thoor, C.J.; Cockcroft, J.R.; Webb, D.J. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J. Physiol. 2001, 530, 541–550. [Google Scholar] [CrossRef]
- Macdonald, S. Fluid resuscitation in patients presenting with sepsis: Current insights. Open Access Emerg. Med. 2022, 19, 633–638. [Google Scholar] [CrossRef]
- Marik, P.E.; Linde-Zwirble, W.T.; Bittner, E.A.; Sahatjian, J.; Hansell, D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: An analysis of a large national database. Intensive Care Med. 2017, 43, 625–632. [Google Scholar] [CrossRef]
- Pinsky, M.R. Functional hemodynamic monitoring. Crit. Care Clin. 2015, 31, 89–111. [Google Scholar] [CrossRef]
- Barbier, C.; Loubieres, Y.; Schmit, C.; Hayon, J.; Ricome, J.-L.; Jardin, F.; Vielliard-Baron, A. Respiratory changes in inferior vena cava diameter are helpful in prediciting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004, 30, 1740–1746. [Google Scholar] [CrossRef]
- Via, G.; Tavazzi, G.; Price, S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: A physiologically based point of view. Intensive Care Med. 2016, 42, 1164–1167. [Google Scholar] [CrossRef]
- Bentzer, P.; Griesdale, D.E.; Boyd, J.; MacLean, K.; Sirounis, D.; Ayas, N.T. Will this hemodynamically unstable patient respond to a bolus of intravenous fluid? JAMA 2016, 316, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.I.M.; Romero, M.G.; Cano, A.G.; Aya, H.D.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: A validation study. Crit. Care 2014, 18, 626. [Google Scholar] [CrossRef] [PubMed]
- Guinot, P.G.; Bernard, E.; Levrard, M.; Dupont, H.; Lorne, E. Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit. Care 2015, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Chemla, D.; Osman, D.; Anguel, N.; Richard, C.; Pinsky, M.R.; Teboul, J.-L. Measuring aortic diameter improves accuracy of esophageal Doppler in assessing fluid responsiveness. Crit. Care Med. 2007, 35, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Galeijnse, M.L.; Forster, T.; Soliman, O.I.; Ten Cate, F.J.; Csanády, M. Echocardiographic evaluation and clinical implications of aortic stiffness and coronary flow reserve and their relation. Clin. Cardiol. 2008, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Stratos, C.; Boudoulas, H.; Kourouklis, C.; Toutouzas, P. Distensibility of the ascending aorta: Comparison of invasive and noninvasive techniques in healthy men and in men with coronary artery disease. Eur. Heart J. 1990, 11, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExSUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine learning algorithm to predict hypotension based on high fidelity arterial pressure waveform analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef]
- Etemadi, M.; Hogue, C.W. Preventive intraoperative hypotension: Artificial intelligence versus augmented intelligence? Anesthesiology 2020, 133, 1170–1172. [Google Scholar] [CrossRef]
- Magder, S. Flow-directed vs goal directed strategy for management of hemodynamics. Curr. Opin. Crit. Care 2016, 22, 267–273. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Papaioannou, T.G.; Soulis, D.; Vardoulis, O.; Protogerou, A.; Sfikakis, P.S.; Stergiopoulos, N.; Stefanadis, C. First in vivo application and evaluation of a novel non-invasive estimation of cardiac output. Med. Eng. Phys. 2014, 36, 1352–1357. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, V.; Papaioannou, T. Rethinking Fluid Responsiveness during Septic Shock: Ameliorate Accuracy of Noninvasive Cardiac Output Measurements through Evaluation of Arterial Biomechanical Properties. J. Pers. Med. 2024, 14, 70. https://doi.org/10.3390/jpm14010070
Papaioannou V, Papaioannou T. Rethinking Fluid Responsiveness during Septic Shock: Ameliorate Accuracy of Noninvasive Cardiac Output Measurements through Evaluation of Arterial Biomechanical Properties. Journal of Personalized Medicine. 2024; 14(1):70. https://doi.org/10.3390/jpm14010070
Chicago/Turabian StylePapaioannou, Vasileios, and Theodoros Papaioannou. 2024. "Rethinking Fluid Responsiveness during Septic Shock: Ameliorate Accuracy of Noninvasive Cardiac Output Measurements through Evaluation of Arterial Biomechanical Properties" Journal of Personalized Medicine 14, no. 1: 70. https://doi.org/10.3390/jpm14010070
APA StylePapaioannou, V., & Papaioannou, T. (2024). Rethinking Fluid Responsiveness during Septic Shock: Ameliorate Accuracy of Noninvasive Cardiac Output Measurements through Evaluation of Arterial Biomechanical Properties. Journal of Personalized Medicine, 14(1), 70. https://doi.org/10.3390/jpm14010070






