The Impact of Positive Inotropic Therapy on Hemodynamics and Organ Function in Acute Heart Failure: A Differentiated View
Abstract
:1. Introduction
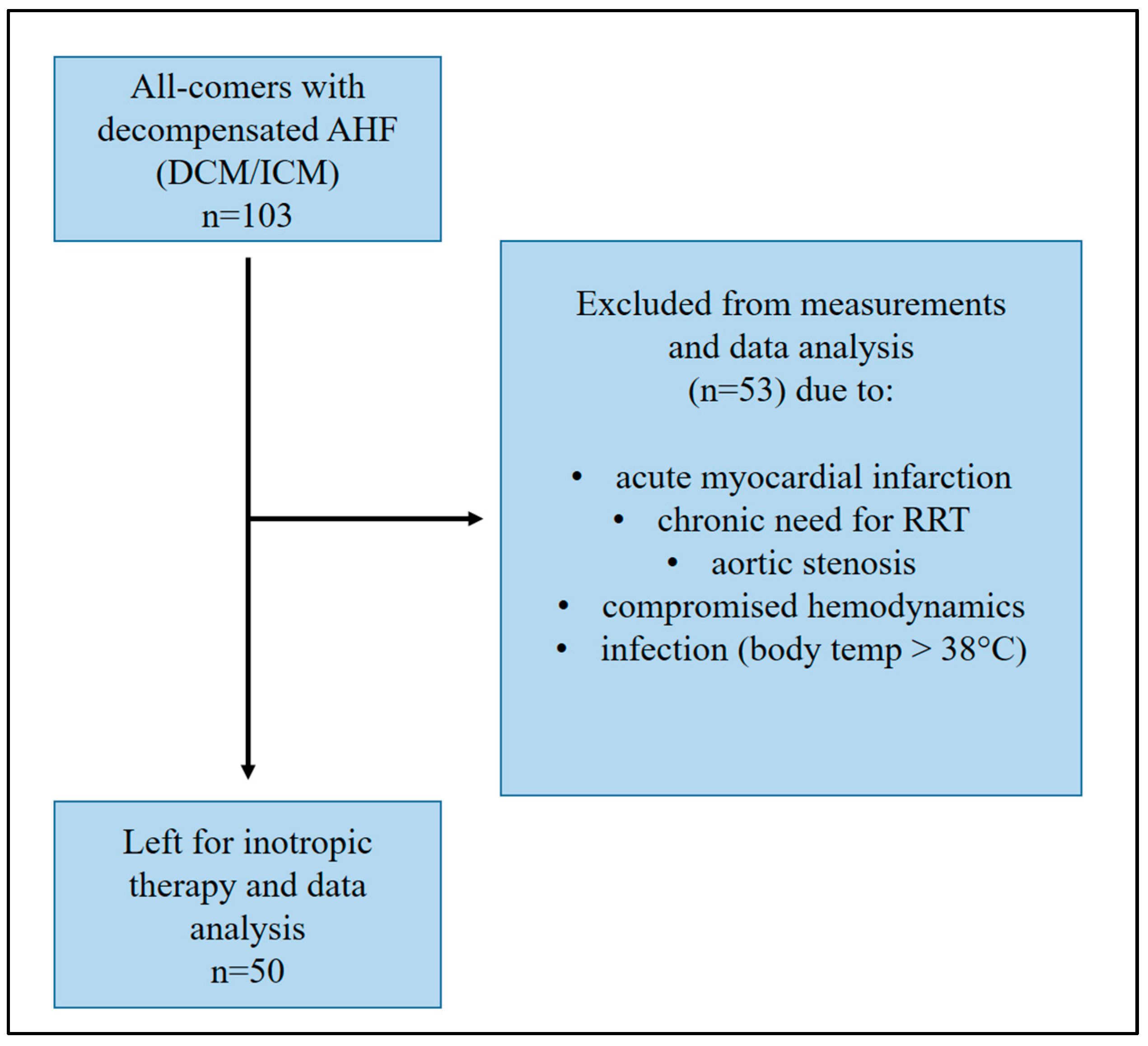
2. Materials and Methods
2.1. Patients and Measurements
2.2. NICaS® Device and Procedure
2.3. Statistical Analysis
2.4. Ethical Declaration
3. Results
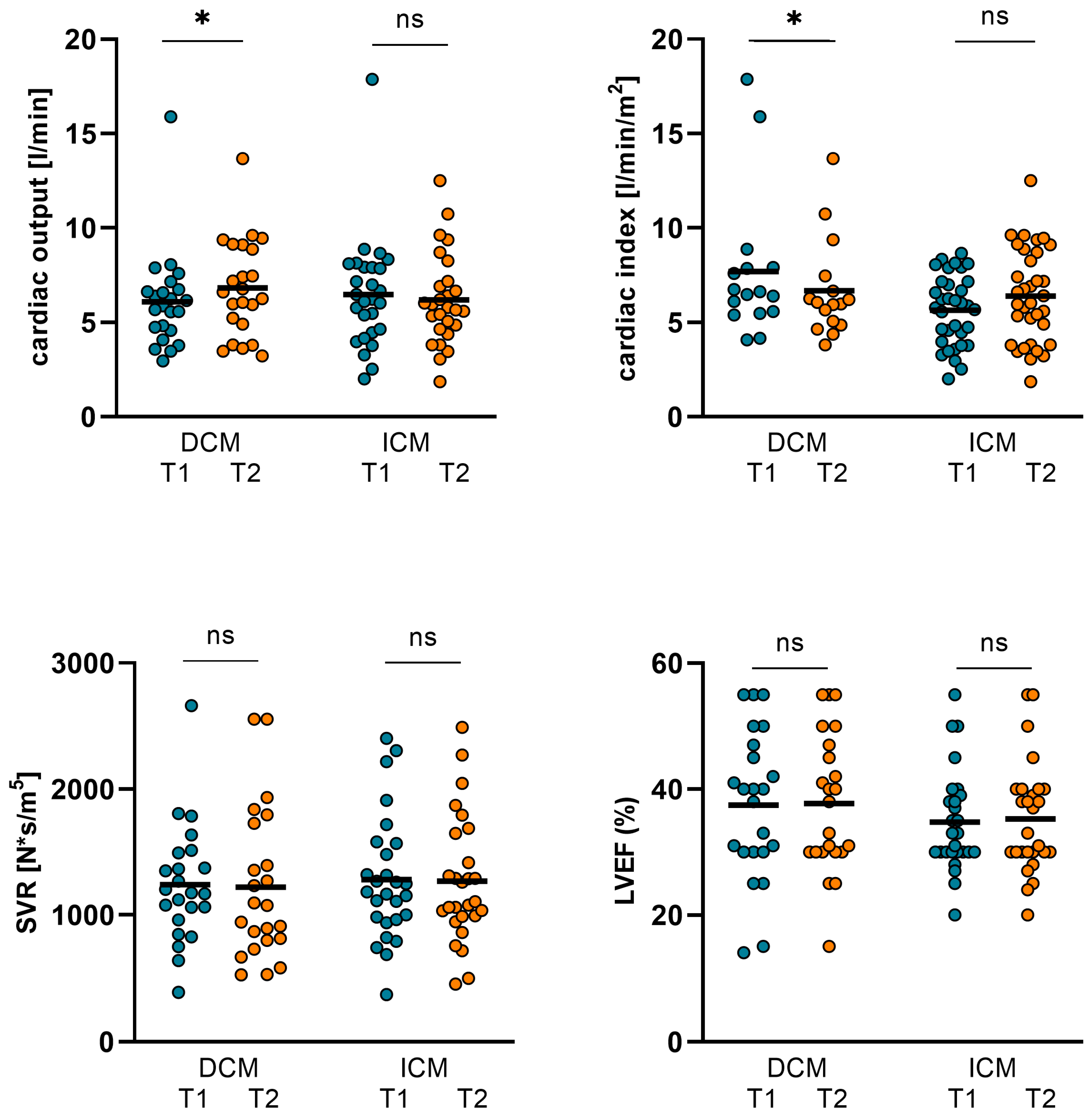
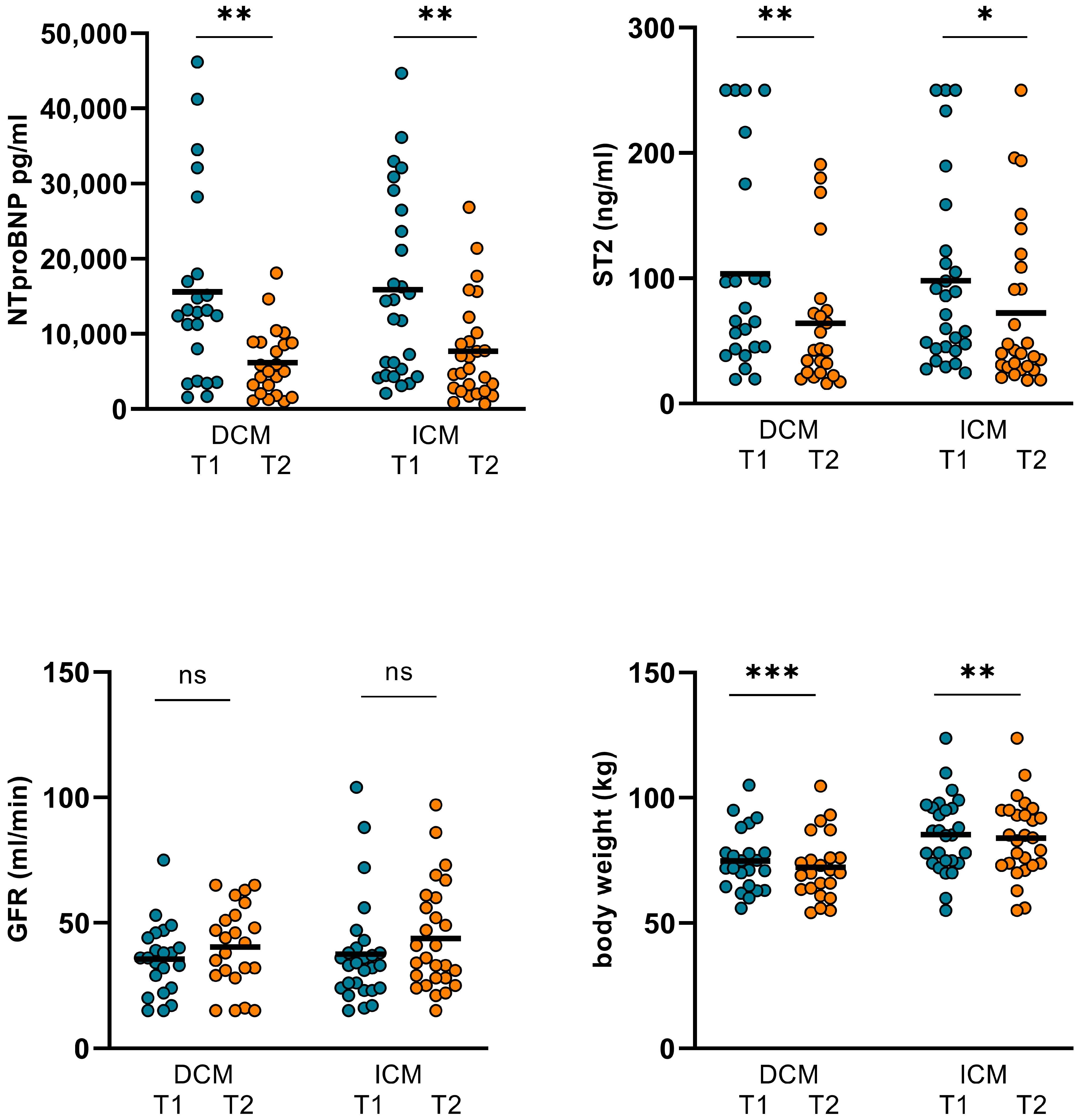
3.1. Changes in Hemodynamics, LVEF, and Biomarkers in ICM and DCM Subgroups
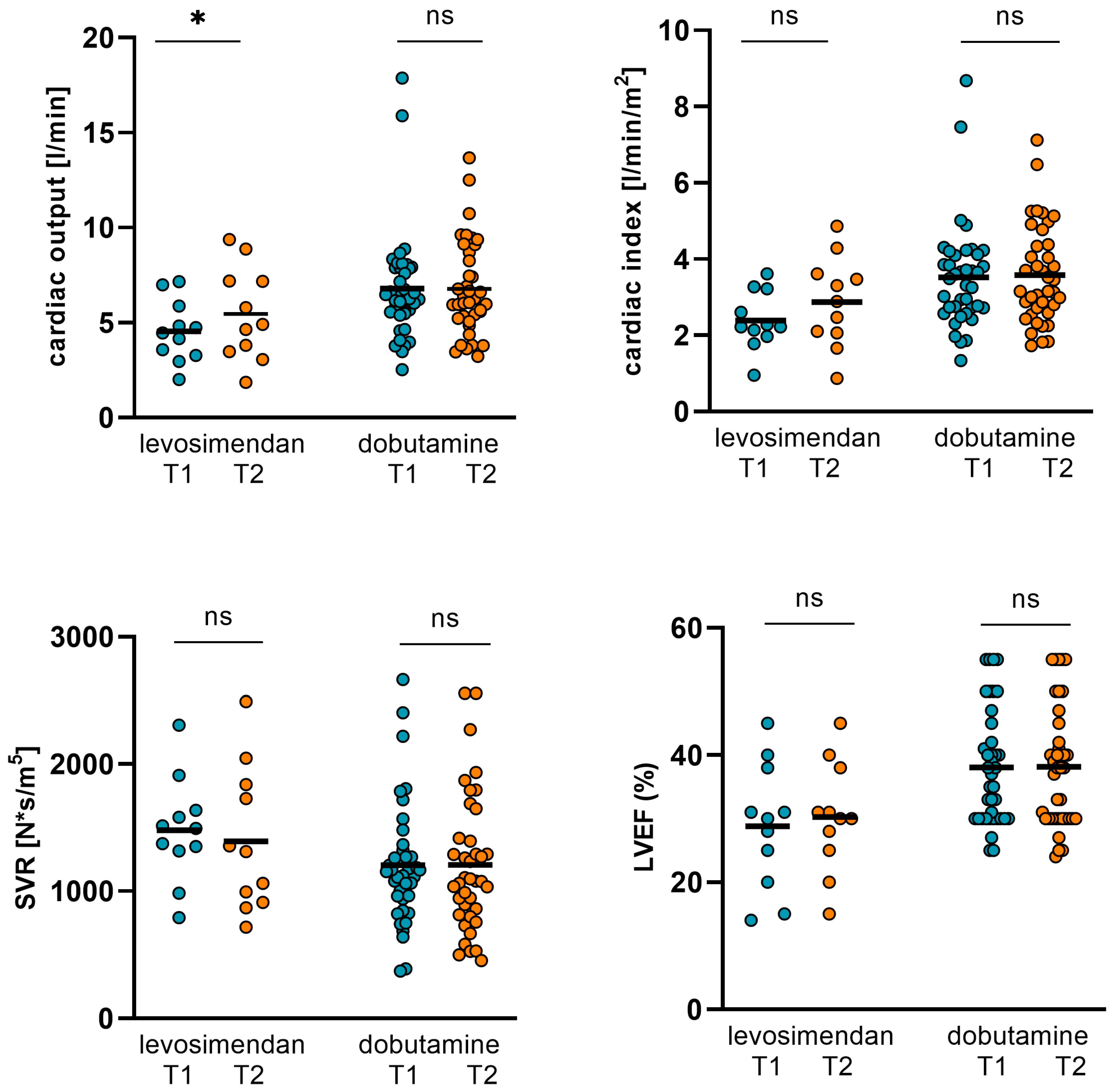
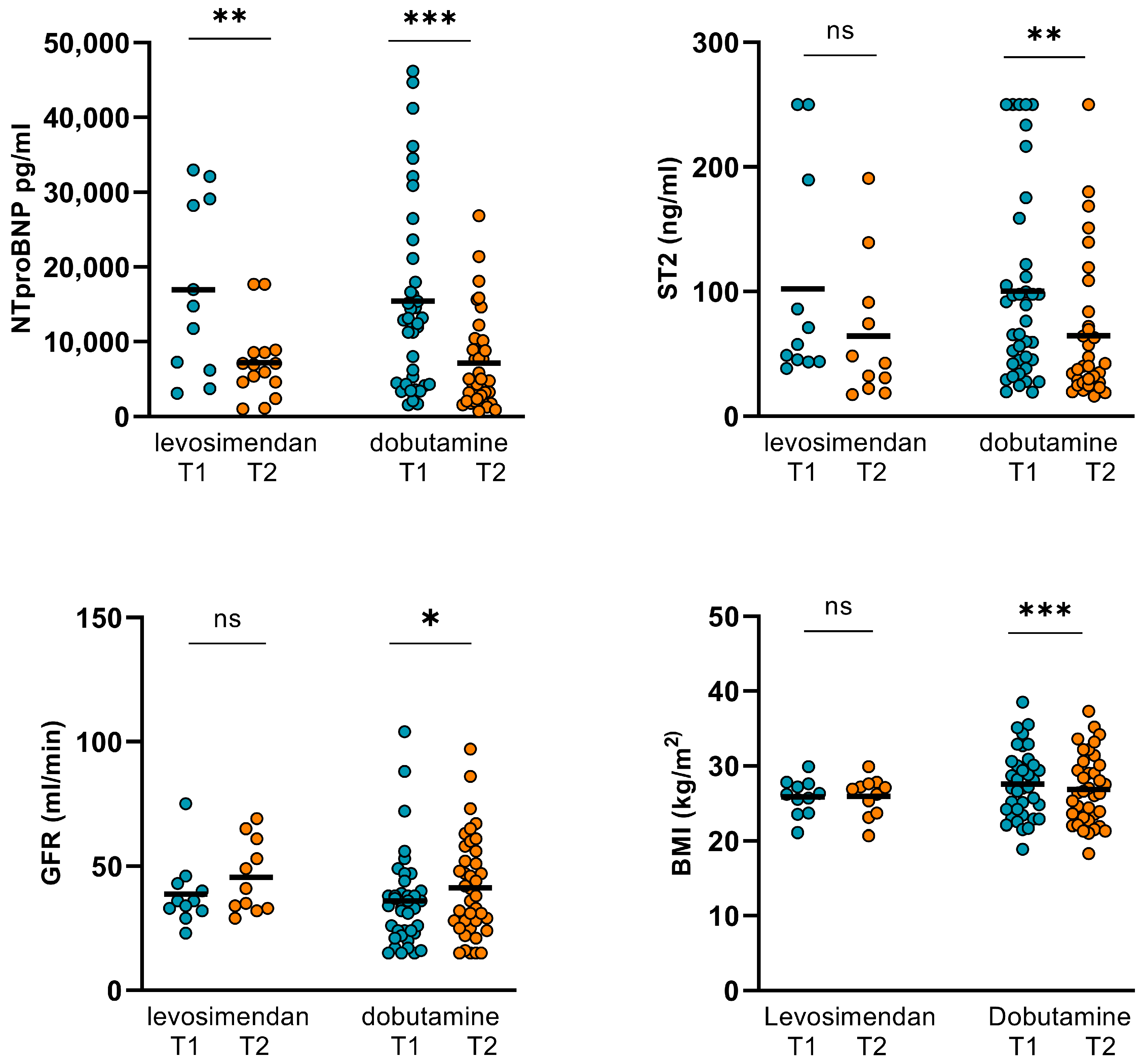
3.2. Changes in Hemodynamics, LVEF, and Biomarkers in Levosimendan and Dobutamine Subgroups
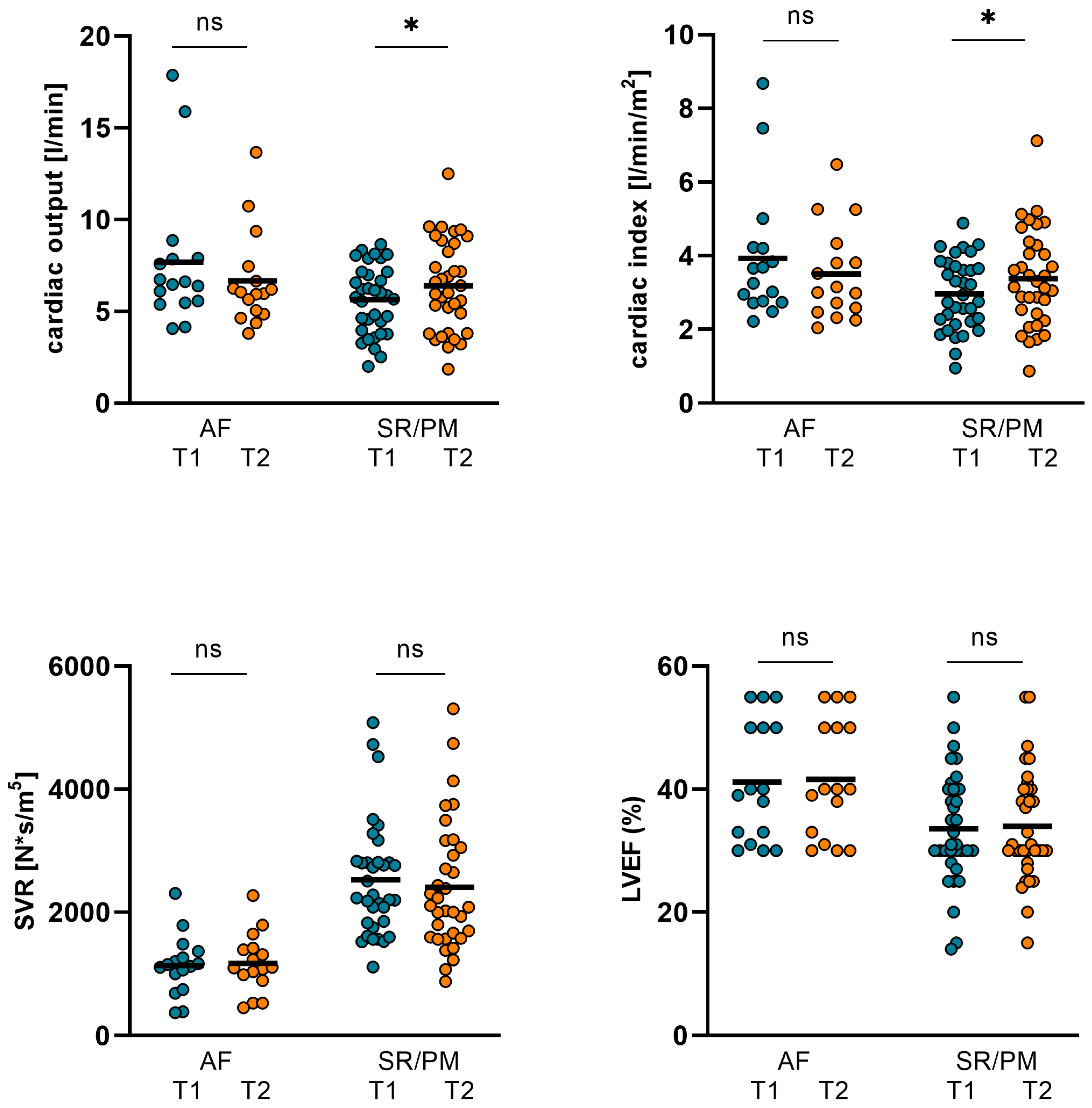
3.3. The Comparison of Changes in Hemodynamics, LVEF, and Biomarkers in Patients with Either AF or Sinus Rhythm/Continuous Pacemaker Stimulation (SR/PM)
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AF | atrial fibrillation |
| ANOVA | analysis of variance |
| AT | angiotensin |
| ARNI | angiotensin receptor/neprilysin inhibitor |
| CABG | coronary artery bypass graft |
| CI | cardiac index |
| CO | cardiac output |
| DAP | diastolic arterial pressure |
| DCM | dilative cardiomyopathy |
| ESC | European Society of Cardiology |
| FDA | US food and drug administration |
| GGI | Granov Goor Index |
| GFR | glomerular filtration rate |
| HF | heart failure |
| HR | heart rate |
| ICM | ischemic cardiomyopathy |
| ICU | intensive care unit |
| LVEF | left ventricular ejection fraction |
| MAP | mean arterial pressure |
| NT-pro BNP | N-terminal pro-B-type natriuretic peptide |
| PM | pacemaker rhythm |
| SAP | systolic arterial pressure |
| SD | standard deviation |
| SGLT-II | sodium-glucose linked transporter 2 |
| SGLTi | sodium-glucose cotransporter inhibitor |
| ST2 | soluble suppressor of tumorigenicity 2 |
| STI | systolic time intervals |
| SR | sinus rhythm |
| SVR | systemic vascular resistance |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.D.; De Michieli, L.; Ola, O.; Akula, A.; Mehta, R.A.; Hodge, D.O.; Tak, T.; Cagin, C.; Gulati, R.; Jaffe, A.S.; et al. Diagnosis and Prognosis of Type 2 Myocardial Infarction Using Objective Evidence of Acute Myocardial Ischemia: A Validation Study. Am. J. Med. 2023, 136, 687–693.e2. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Kellner, C.; Sörensen, N.A.; Lehmacher, J.; Toprak, B.; Schock, A.; Hartikainen, T.S.; Twerenbold, R.; Zeller, T.; Westermann, D.; et al. Long-term outcome of patients presenting with myocardial injury or myocardial infarction. Clin. Res. Cardiol. 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Kenchaiah, S.; Larson, M.G.; Benjamin, E.J.; Kupka, M.J.; Ho, K.K.; Murabito, J.M.; Vasan, R.S. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002, 347, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Piña, I.L.; Allen, L.A.; Desai, N.R. Managing the economic challenges in the treatment of heart failure. BMC Cardiovasc. Disord. 2021, 21, 612. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Raj, L.; Maidman, S.D.; Adhyaru, B.B. Inpatient management of acute decompensated heart failure. Postgrad. Med. J. 2020, 96, 33–42. [Google Scholar] [CrossRef]
- Heringlake, M.; Alvarez, J.; Bettex, D.; Bouchez, S.; Fruhwald, S.; Girardis, M.; Grossini, E.; Guarracino, F.; Herpain, A.; Toller, W.; et al. An update on levosimendan in acute cardiac care: Applications and recommendations for optimal efficacy and safety. Expert. Rev. Cardiovasc. Ther. 2021, 19, 325–335. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. ESC Guidelines for the diagnosis and treatment ofacute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Tang, W.H.; Wu, Y.; Grodin, J.L.; Hsu, A.P.; Hernandez, A.F.; Butler, J.; Metra, M.; Voors, A.A.; Felker, G.M.; Troughton, R.W.; et al. Prognostic Value of Baseline and Changes in Circulating Soluble ST2 Levels and the Effects of Nesiritide in Acute Decompensated Heart Failure. JACC Heart Fail. 2016, 4, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Manzano-Fernández, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdés, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Al Younis, S.M.; Hadjileontiadis, L.J.; Stefanini, C.; Khandoker, A.H. Non-invasive technologies for heart failure, systolic and diastolic dysfunction modeling: A scoping review. Front. Bioeng. Biotechnol. 2023, 11, 1261022. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Milo, O.; Kaluski, E.; Vered, Z.; Cotter, G. Whole-body electrical bio-impendance is accurate in non invasive determination of cardiac output: A thermodilution controlled, prospective, double blind evaluation. J. Card. Fail. 2004, 10, S38–S39. [Google Scholar] [CrossRef]
- Paredes, O.L.; Shite, J.; Shinke, T.; Watanabe, S.; Otake, H.; Matsumoto, D.; Imuro, Y.; Ogasawara, D.; Sawada, T.; Yokoyama, M. Impedance cardiography for cardiac output estimation: Reliability of wrist-to-ankle electrode configuration. Circ. J. 2006, 70, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Shite, J.; Paredes, O.L.; Shinke, T.; Ogasawara, D.; Sawada, T.; Kawamori, H.; Miyoshi, N.; Kato, H.; Yoshino, N.; et al. Whole body bioimpedance monitoring for outpatient chronic heart failure follow up. Circ. J. 2009, 73, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Emoto, N.; Miyagawa, K.; Nakayama, K.; Kinutani, H.; Tanaka, H.; Shinke, T.; Hirata, K.-I. Noninvasive and simple assessment of cardiac output and pulmonary vascular resistance with whole-body impedance cardiography is useful for monitoring patients with pulmonary hypertension. Circ. J. 2013, 77, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Website of European Society of Cardiology. Facts and Figures: Heart Failure. Available online: https://www.escardio.org/Journals/ESC-Journal-Family/European-Journal-of-Heart-Failure (accessed on 10 October 2023).
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Crea, F. Epidemiology and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 329–332. [Google Scholar] [CrossRef]
- Zulkifly, H.; Lip, G.Y.H.; Lane, D.A. Epidemiology of atrial fibrillation. Int. J. Clin. Pract. 2018, 72, e13070. [Google Scholar] [CrossRef] [PubMed]
- Algalarrondo, V.; Extramiana, F. Épidémiologie et physiopathologie de la fibrillation atriale [Epidemiology and pathophysiology of atrial fibrillation]. Rev. Prat. 2020, 70, 894–898. (In French) [Google Scholar] [PubMed]
- Ramadan, M.; Refaat, M.M. Cardiac resynchronization therapy in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2403–2404. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, L.; Yang, X.; Chen, M.; Gao, Y. The common characteristics and mutual effects of heart failure and atrial fibrillation: Initiation, progression, and outcome of the two aging-related heart diseases. Heart Fail. Rev. 2022, 27, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, F.; Huang, H.; Wu, X.; Tian, W.; Yu, T. Is there any difference in the therapeutic effects of Levosimendan on advanced HFrEF patients with sinus rhythm or atrial fibrillation? Front. Cardiovasc. Med. 2023, 10, 1084300. [Google Scholar] [CrossRef] [PubMed]
- Abacilar, A.F.; Dogan, O.F. Levosimendan use decreases atrial fibrillation in patients after coronary artery bypass grafting: A pilot study. Heart Surg. Forum. 2013, 16, E287–E294. [Google Scholar] [CrossRef] [PubMed]
- Yontar, O.C.; Yilmaz, M.B.; Yalta, K.; Tandoğan, I. Efficacy of levosimendan in patients with chronic heart failure: Does rhythm matter? Anadolu Kardiyol. Derg. 2010, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P.C.; Singh, B.N.; Ellenbogen, K.; Fisher, S.; Fletcher, R.; Singh, S.N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: Observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). Dep. Veterans Aff. CHF-STAT Investig. Circ. 1998, 98, 2574–2579. [Google Scholar] [CrossRef]
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Trivedi, C.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistoclakis, S.; Russo, A.D.; et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef]
- Vecchio, N.; Ripa, L.; Orosco, A.; Tomas, L.; Mondragón, I.; Acosta, A.; Talavera, L.; Rivera, S.; Albina, G.; Diez, M.; et al. Atrial Fibrillation in Heart Failure Patients with Preserved or Reduced Ejection Fraction. Prognostic significance of Rhythm control strategy with Catheter Ablation. J. Atr. Fibrillation 2019, 11, 2128. [Google Scholar] [CrossRef]
| Patient Characteristics | Overall Cohort | DCM Cohort (n = 23/46%) | ICM Cohort (n = 27/54%) | Comparison of ICM/DCM Subgroups (p-Value) |
|---|---|---|---|---|
| Age (years) | 78 ± 11 | 82.2 ± 8.7 | 76.6 ± 12.5 | 0.132 |
| Female (%) | 28 | 35 | 22 | 0.002 |
| Male (%) | 72 | 65 | 78 | 0.002 |
| Body weight (kg) (at hospital admission) | 80.45 ± 14.87 | 72.36 ± 17.53 | 85.04 ± 15.41 | 0.01 |
| SAP (mmHg) (at hospital admission) | 128.22 ± 27.16 | 125.13 ± 26.03 | 130.85 ± 28.8 | 0.468 |
| DAP (mmHg) (at hospital admission) | 67.46 ± 17.06 | 64.74 ± 19.78 | 69.56 ± 14.62 | 0.328 |
| MAP (mmHg) (at hospital admission) | 94.08 ± 27.01 | 94.93 ± 19.97 | 100.2 ± 20.01 | 0.358 |
| GFR mL/min (at hospital admission) | 36.2 ± 17.99 | 35.6 ± 14.2 | 37.5 ± 21 | 0.709 |
| LVEF (%) (at hospital admission) | 36 ± 10 | 37.48 ± 11.82 | 34.78 ± 8.08 | 0.345 |
| Levosimendan dosage (mg/24 h) (n = 11) | 12 | 12 | 12 | - |
| Dobutamine dosage (µg/kg/min) (n = 39) | 10.5 | 19.12 ± 3.64 | 18.95 ± 4.28 | 0.899 |
| NT-proBNP (pg/mL) (at hospital admission) | 15765 ± 12246 | 15616 ± 12611 | 15891 ± 12167 | 0.938 |
| ST2 (ng/mL) (at hospital admission) | 100.8 ± 77.6 | 103.7 ± 82.7 | 92.3 ± 74.4 | 0.805 |
| Comorbidities | ||||
| Chronic kidney disease (%) | 76.9 | 73.9 | 85.2 | 0.321 |
| Anemia (%) | 34.6 | 30.4 | 40.7 | 0.042 |
| COPD (%) | 32.7 | 26.9 | 40.7 | 0.019 |
| CABG (%) | 11.5 | 4.3 | 18.5 | 0.124 |
| Diabetes (%) | 73.1 | 65.2 | 85.2 | 0.099 |
| Dyslipidemia (%) | 46.2 | 30.4 | 63 | 0.022 |
| Peripheral artery disease (%) | 30.8 | 13 | 48.1 | 0.008 |
| Arterial hypertension (%) | 78.8 | 60.9 | 100 | <0.001 |
| AF paroxysmal (%) | 32.7 | 26.1 | 40.7 | 0.513 |
| AF persistent (%) | 28.8 | 30.4 | 29.6 | 0.951 |
| Duration of in-hospital stay (days) | 13.6 ± 6 | 13 ± 5 | 14.1 ± 6.8 | 0.533 |
| Patient Characteristics | Overall Cohort | AF Cohort (n = 16/52%) | SR/PM Cohort (n = 34/68%) | Comparison of AF/ SR-PM Subgroups (p-Value) |
|---|---|---|---|---|
| Age (years) | 78 ± 11 | 83.8 ± 3.3 | 77.7 ± 11.7 | 0.06 |
| Female (%) | 28 | 31.25 | 26.47 | 0.726 |
| Male (%) | 72 | 68.75 | 73.53 | 0.726 |
| Body weight (kg) (at hospital admission) | 80.45 ± 14.87 | 82.27 ± 15.13 | 79.6 ± 14.9 | 0.56 |
| SAP (mmHg) (at hospital admission) | 128.22 ± 27.16 | 130.6 ± 23.8 | 127.1 ± 29.25 | 0.675 |
| DAP (mmHg) (at hospital admission) | 67.46 ± 17.06 | 72.88 ± 15.84 | 64.74 ± 17.38 | 0.119 |
| MAP (mmHg) (at hospital admission) | 94.08 ± 27.01 | 101.75 ± 16.85 | 95.91 ± 21.25 | 0.34 |
| GFR mL/min (at hospital admission) | 36.2 ± 17.99 | 39.75 ± 25.74 | 35.12 ± 13.18 | 0.407 |
| LVEF (%) (at hospital admission) | 36 ± 10 | 41.18 ± 9.8 | 33.6 ± 9.2 | 0.01 |
| Levosimendan dosage (mg/24 h) (n = 11) | 12 | 12 | 12 | - |
| Dobutamine dosage (µg/kg/min) (n = 39) | 10.5 | 18.9 | 20.2 | 0.085 |
| NT-proBNP (pg/mL) (at hospital admission) | 15765 ± 12246 | 15183 ± 11474 | 16038 ± 12752 | 0.821 |
| ST2 (ng/mL) (at hospital admission) | 100.8 ± 77.6 | 90.48 ± 68.34 | 105.6 ± 82.05 | 0.526 |
| Comorbidities | ||||
| Chronic kidney disease (%) | 76.9 | 87.5 | 73.98 | 0.309 |
| Anemia (%) | 34.6 | 25 | 42.11 | 0.288 |
| COPD (%) | 32.7 | 43.75 | 26.32 | 0.279 |
| CABG (%) | 11.5 | 6.25 | 10.53 | 0.653 |
| Diabetes (%) | 73.1 | 75 | 73.68 | 0.929 |
| Dyslipidemia (%) | 46.2 | 62.5 | 42.11 | 0.229 |
| Peripheral artery disease (%) | 30.8 | 25 | 21.05 | 0.782 |
| Arterial hypertension (%) | 78.8 | 93.75 | 73.68 | 0.117 |
| AF paroxysmal (%) | 32.7 | - | - | - |
| AF persistent (%) | 28.8 | - | - | - |
| Duration of in-hospital stay (days) | 13.6 ± 6 | 14.63 ± 7.06 | 13.09 ± 5.46 | 0.403 |
| HF Pharmacological Treatment | At Admission (n = 50) (n/%) | At Discharge (n = 43) (n/%) |
|---|---|---|
| Beta blockers | 28/56 | 30/69.7 |
| Spironolactone | 16/32 | 27/62.8 |
| ACEI/ARB | 29/58 | 24/55.8 |
| Diuretics | 44/88 | 41/95.3 |
| Other vasodilators | 16/32 | 10/23.3 |
| ARNI | 11/22 | 15/35 |
| SGLTi | 17/34 | 33/77 |
| Baseline (T1) | Follow-Up (T2) | p Value | |
|---|---|---|---|
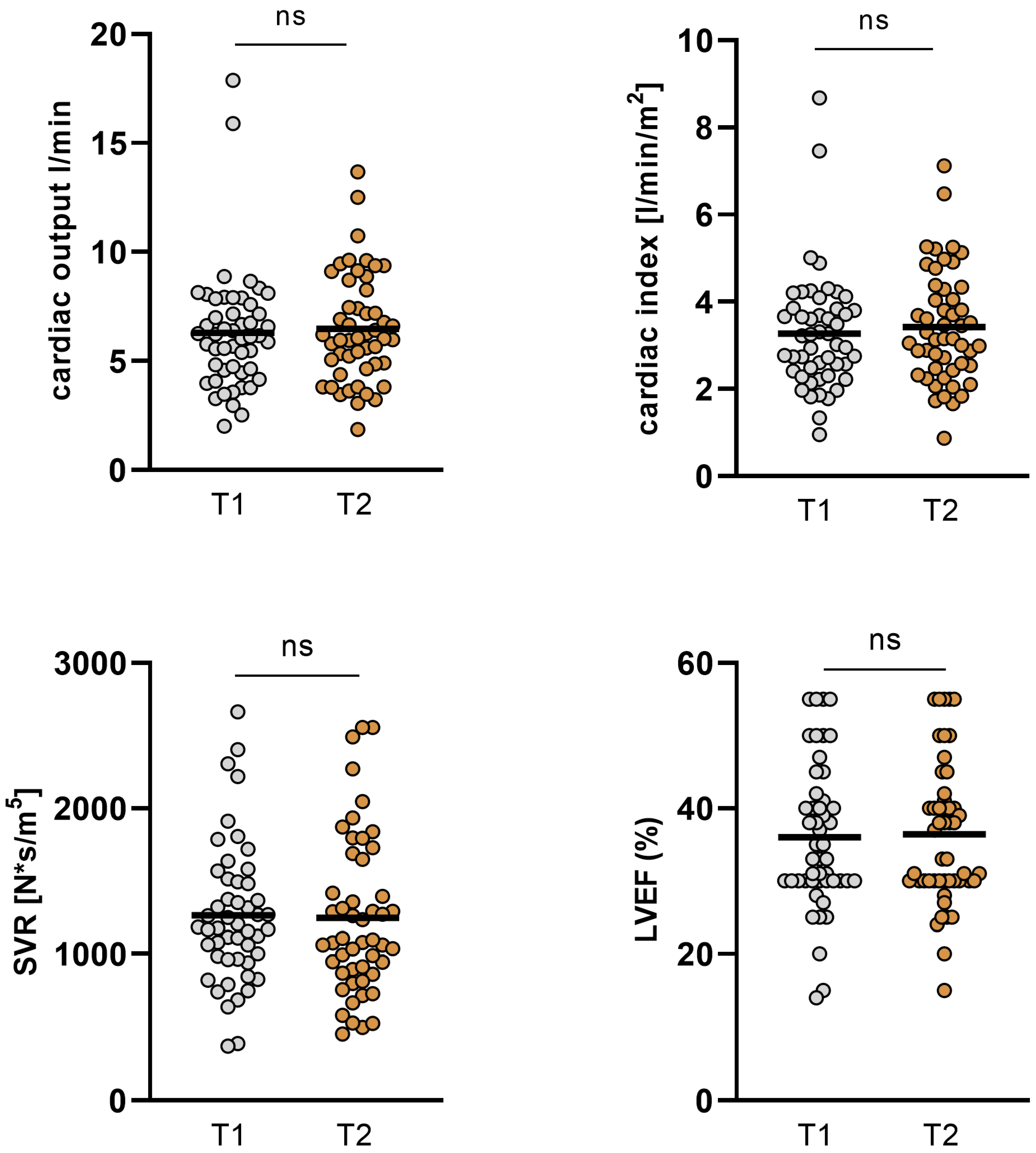
| CO (L/min) | 6.3 ± 2.78 | 6.48 ± 2.49 | 0.245 |
| CI (L/min/m2) | 3.27 ± 1.35 | 3.42 ± 1.29 | 0.173 |
| SVR (N*s/m5) | 1263 ± 480 | 1247 ± 542 | 0.84 |
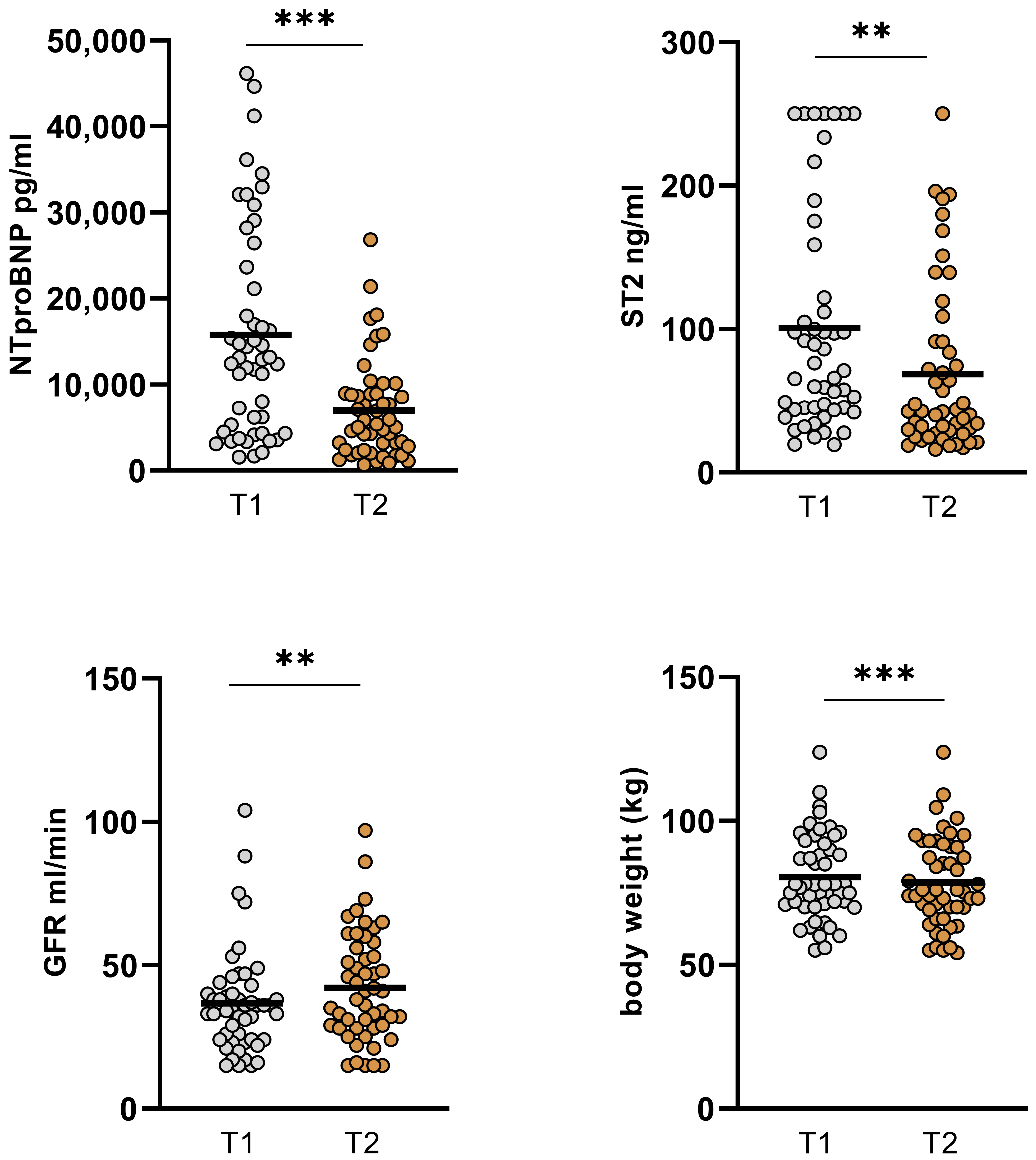
| GFR (mL/min) | 36.63 ± 18.1 | 42.8 ± 18.82 | 0.01 |
| NT-proBNP (pg/mL) | 15765 ± 12246 | 6984 ± 5775 | <0.001 |
| ST2 (ng/mL) | 102.76 ± 77.55 | 68.59 ± 58.94 | <0.001 |
| body weight (kg) | 80.45 ± 14.87 | 78.58 ± 15.5 | <0.001 |
| LVEF (%) | 36.02 ± 9.96 | 36.42 ± 9.82 | 0.498 |
| DCM (T1) n = 23 | DCM (T2) n = 23 | p Value | ICM (T1) n = 27 | ICM (T2) n = 27 | p Value | |
|---|---|---|---|---|---|---|
| CO (L/min) | 6.09 ± 2.58 | 6.82 ± 2.57 | 0.036 | 6.47 ± 2.98 | 6.19 ± 2.43 | 0.829 |
| CI (L/min/m2) | 3.26 ± 1.18 | 3.74 ± 1.27 | 0.013 | 3.28 ± 1.5 | 3.15 ± 1.26 | 0.77 |
| SVR (N*s/m5) | 1241 ± 469 | 1221 ± 595 | 0.859 | 1282 ± 496 | 1269 ± 503 | 0.581 |
| GFR (mL/min) | 35.55 ± 14.16 | 41.55 ± 15.86 | 0.062 | 37.52 ± 21.01 | 43.81 ± 21.18 | 0.082 |
| NTproBNP (pg/mL) | 15616 ± 12611 | 6170 ± 4467 | 0.001 | 15891 ± 12167 | 7677 ± 6700 | 0.001 |
| ST2 (ng/mL) | 103.74 ± 82.67 | 64.19 ± 53.9 | 0.001 | 98.22 ± 74.42 | 72.34 ± 63.67 | 0.042 |
| body weight (kg) | 74.81 ± 12.34 | 72.31 ± 12.04 | <0.001 | 85.26 ± 15.37 | 83.93 ± 15.63 | 0.008 |
| LVEF (%) | 37.48 ± 11.82 | 37.74 ± 10.93 | 0.758 | 24.78 ± 8.08 | 25.3 ± 8.82 | 0.251 |
| Levosimendan (T1) n = 11 | Levosimendan (T2) n = 11 | p Value | Dobutamine (T1) n = 39 | Dobutamine (T2) n = 39 | p Value | |
|---|---|---|---|---|---|---|
| CO (L/min) | 4.55 ± 1.62 | 5.47 ± 2.44 | 0.044 | 6.79 ± 2.86 | 6.77 ± 2.46 | 0.748 |
| CI (L/min/m2) | 2.39 ± 0.76 | 2.87 ± 1.18 | 0.05 | 3.52 ± 1.38 | 3.58 ± 1.29 | 0.562 |
| SVR (N*s/m5) | 1478 ± 410 | 1392 ± 564 | 0.636 | 1203 ± 485 | 1206 ± 536 | 0.596 |
| GFR (mL/min) | 38.8 ± 13.59 | 45.55 ± 14.54 | 0.202 | 36 ± 19.32 | 42 ± 19.99 | 0.029 |
| NT-proBNP (pg/mL) | 16931 ± 11701 | 6337 ± 4653 | 0.004 | 15436 ± 12524 | 7166 ± 6095 | <0.001 |
| ST2 (ng/mL) | 102.23 ± 84.58 | 64.41 ± 56.01 | 0.16 | 100.34 ± 76.63 | 69.77 ± 60.4 | 0.003 |
| body weight (kg) | 78.19 ± 10.62 | 78.51 ± 11.32 | 0.595 | 81.09 ± 15.93 | 78.61 ± 16.6 | <0.001 |
| LVEF (%) | 28.82 ± 9.93 | 30.28 ± 8.63 | 0.317 | 38.05 ± 9.1 | 38.15 ± 9.52 | 0.916 |
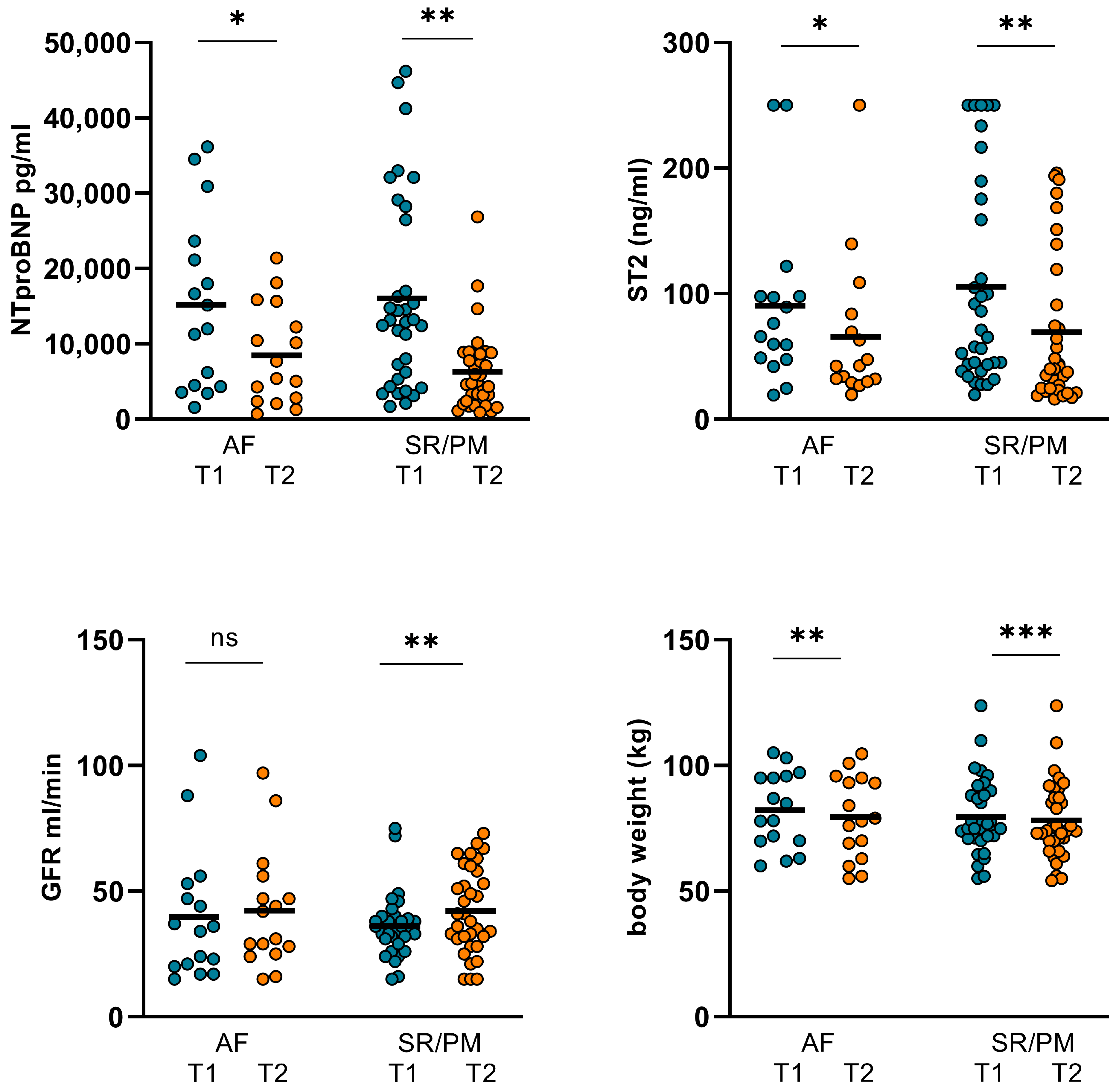
| AF (T1) n = 16 | AF (T2) n = 16 | p Value | SR/PM (T1) n = 34 | SR/PM (T2) n = 34 | p Value | |
|---|---|---|---|---|---|---|
| CO (L/min) | 7.69 ± 3.83 | 6.67 ± 2.58 | 0.352 | 5.64 ± 1.86 | 6.39 ± 2.48 | 0.023 |
| CI (L/min/m2) | 3.93 ± 1.79 | 3.5 ± 1.26 | 0.438 | 2.96 ± 0.97 | 3.38 ± 1.31 | 0.018 |
| SVR (N*s/m5) | 1138 ± 484 | 1173 ± 479 | 0.818 | 1322 ± 473 | 1282 ± 579 | 0.369 |
| GFR (mL/min) | 39.75 ± 25.74 | 42.31 ± 23.44 | 0.665 | 35.12 ± 13.181 | 43.03 ± 16.539 | 0.008 |
| NT-proBNP (pg/mL) | 15184 ± 11474 | 8463 ± 6582 | 0.017 | 16038 ± 12752 | 6288 ± 5316 | <0.001 |
| ST2 (ng/mL) | 90.48 ± 68.34 | 65.74 ± 59.17 | 0.022 | 105.6 ± 82.05 | 69.93 ± 59.68 | 0.004 |
| body weight (kg) | 78.13 ± 14.44 | 75.97 ± 15.27 | 0.014 | 81.45 ± 15.15 | 79.7 ± 15.69 | <0.001 |
| LVEF (%) | 41.19 ± 9.79 | 41.63 ± 9.55 | 0.33 | 33.59 ± 9.21 | 33.97 ± 9.08 | 0.752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheko, J.; Patsalis, N.; Kreutz, J.; Divchev, D.; Chatzis, G.; Schieffer, B.; Markus, B. The Impact of Positive Inotropic Therapy on Hemodynamics and Organ Function in Acute Heart Failure: A Differentiated View. J. Pers. Med. 2024, 14, 17. https://doi.org/10.3390/jpm14010017
Cheko J, Patsalis N, Kreutz J, Divchev D, Chatzis G, Schieffer B, Markus B. The Impact of Positive Inotropic Therapy on Hemodynamics and Organ Function in Acute Heart Failure: A Differentiated View. Journal of Personalized Medicine. 2024; 14(1):17. https://doi.org/10.3390/jpm14010017
Chicago/Turabian StyleCheko, Juan, Nikolaos Patsalis, Julian Kreutz, Dimitar Divchev, Georgios Chatzis, Bernhard Schieffer, and Birgit Markus. 2024. "The Impact of Positive Inotropic Therapy on Hemodynamics and Organ Function in Acute Heart Failure: A Differentiated View" Journal of Personalized Medicine 14, no. 1: 17. https://doi.org/10.3390/jpm14010017
APA StyleCheko, J., Patsalis, N., Kreutz, J., Divchev, D., Chatzis, G., Schieffer, B., & Markus, B. (2024). The Impact of Positive Inotropic Therapy on Hemodynamics and Organ Function in Acute Heart Failure: A Differentiated View. Journal of Personalized Medicine, 14(1), 17. https://doi.org/10.3390/jpm14010017





