Personalized Medicine in Oral Oncology: Imaging Methods and Biological Markers to Support Diagnosis of Oral Squamous Cell Carcinoma (OSCC): A Narrative Literature Review
Abstract
1. Introduction
- -
- Primary prevention (counselling at-risk subjects (stop smoking, stop drinking));
- -
- Secondary prevention (screening and early diagnosis of OPMDs);
- -
- Tertiary prevention (strict follow-up of OSCC survivors to intercept recurrences, metastasis, and/or second primary tumors).
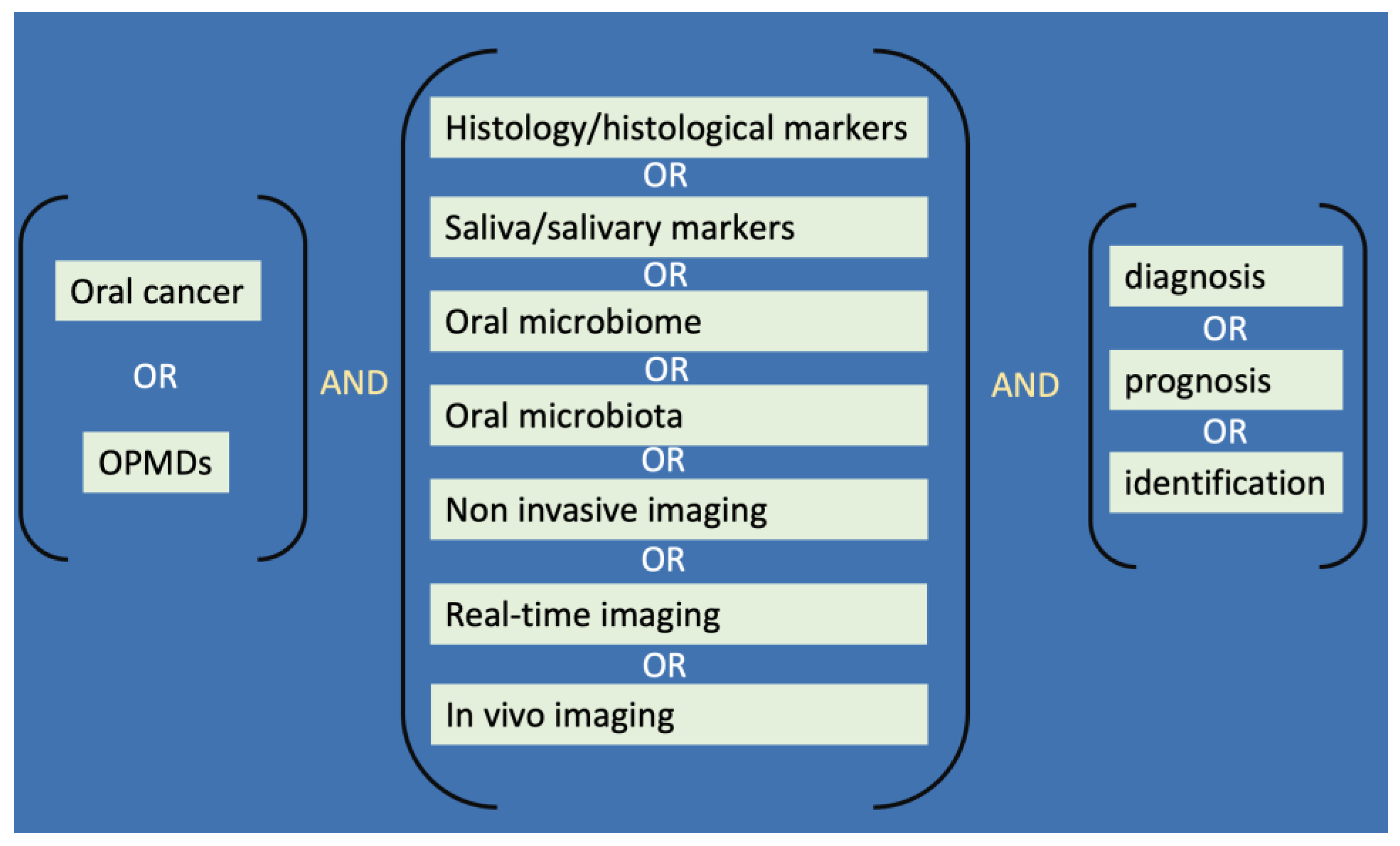
2. Material and Methods
3. Results
3.1. Imaging Techniques
- Two-dimensional imaging (all techniques);
- Microscopic details of the tissues (RCM);
- Depth of invasion measurements (RCM, US, OCT);
- Tumor-associated neoangiogenesis and inflammation detection (NBI, RCM, US);
- Early signs of cancer detection (AF, RCM).
- Conversely, the main disadvantages and limitations can be ascribed to the following:
- Inapplicability to hyperkeratotic or melanocytic lesions (AF);
- Ergonomic limitation of the available devices (RCM);
- Costs and learning curve (RCM, US, OCT).
3.2. Tissue Markers
3.3. Circulating Markers
3.3.1. Salivary Biomarkers
3.3.2. Blood Markers and Circulating Cancer Cells
3.4. Oral Microbiota Changes
- -
- the imbalance of keratinocyte proliferation and death;
- -
- immune dysregulations;
- -
- alterations of metabolisms of food compounds, drugs, and host metabolite.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda-Filho, A.; Bray, F. Global Patterns and Trends in Cancers of the Lip, Tongue and Mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef] [PubMed]
- Jasper, P.; Jungbauer, W.N.; Poupore, N.S.; Nguyen, S.A.; Howell, J.; Neville, B.W.; Day, T.A. Mucosal Melanoma In Situ of the Oral Cavity: A Case Report and Systematic Review of the Literature. Turk. Arch. Otorhinolaryngol. 2022, 60, 161–169. [Google Scholar] [CrossRef]
- Favia, G.; lo Muzio, L.; Serpico, R.; Maiorano, E. Angiosarcoma of the Head and Neck with Intra-Oral Presentation. A Clinico-Pathological Study of Four Cases. Oral Oncol. 2002, 38, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wreesmann, V.B.; Oomen, K.P.; Brennan, P.A. Angiosarcomas of the Head and Neck: Impact of Large Data Analysis on Clinical Management. J. Oral Pathol. Med. 2022, 51, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Yoshifuku, K.; Deguchi, K.; Kurono, Y. Rhabdomyosarcoma of the soft palate: A case report. Pract. Otorhinolaryngol. 2007, 100, 731–736. [Google Scholar] [CrossRef][Green Version]
- Favia, G.; lo Muzio, L.; Serpico, R.; Maiorano, E. Rhabdomyoma of the Head and Neck: Clinicopathologic Features of Two Cases. Head Neck 2003, 25, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Dube, K.; Dube, A.; Jain, P.; Ghosh, S.; Paul, B.; Bhatnagar, N. Maxillary Mucinous Adenocarcinoma Mimicking a Lesion of Endodontic Origin: A Rare Case Report. Eur. Endod. J. 2023, 8, 101–104. [Google Scholar] [CrossRef]
- Cao, C.; Poti, S.M.; Ledgerwood, L.G.; Lai, J. Mixed HPV-related Neuroendocrine Carcinoma and HPV-related Squamous Cell Carcinoma of the Base of Tongue in a Patient with Incidental Identification of Synchronous Metastatic Papillary Thyroid Carcinoma. Anticancer Res. 2021, 41, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Lajolo, C.; Patini, R.; Limongelli, L.; Favia, G.; Tempesta, A.; Contaldo, M.; de Corso, E.; Giuliani, M. Brown Tumors of the Oral Cavity: Presentation of 4 New Cases and a Systematic Literature Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 575–584.e4. [Google Scholar] [CrossRef] [PubMed]
- Moretz-Sohn, P.F.; Dias, F.L.; de Carvalho Marques, C.M. Minor Salivary Gland Cancer of the Head and Neck: A Review of Epidemiologic Aspects, Prognostic Factors, and Outcomes. Curr. Oncol. Rep. 2023, 25, 173–179. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Oral Potentially Malignant Disorders: A Comprehensive Review on Clinical Aspects and Management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Clinical Features and Presentation of Oral Potentially Malignant Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590. [Google Scholar] [CrossRef]
- Boccellino, M.; di Stasio, D.; Romano, A.; Petruzzi, M.; Lucchese, A.; Serpico, R.; Frati, L.; Domenico, M.D.I. Lichen Planus: Molecular Pathway and Clinical Implications in Oral Disorders. J. Biol. Regul. Homeost. Agents 2018, 32, 135–138. [Google Scholar]
- di Spirito, F.; Amato, A.; Romano, A.; Dipalma, G.; Xhajanka, E.; Baroni, A.; Serpico, R.; Inchingolo, F.; Contaldo, M. Analysis of Risk Factors of Oral Cancer and Periodontitis from a Sex- and Gender-Related Perspective: Gender Dentistry. Appl. Sci. 2022, 12, 9135. [Google Scholar] [CrossRef]
- Gönül, M.; Gül, U.; Kaya, I.; Koçak, O.; Çakmak, S.K.; Kiliç, A.; Kiliç, S. Smoking, Alcohol Consumption and Denture Use in Patients with Oral Mucosal Lesions. J. Dermatol. Case Rep. 2011, 5, 64–68. [Google Scholar] [CrossRef]
- Mello, F.W.; Melo, G.; Pasetto, J.J.; Silva, C.A.B.; Warnakulasuriya, S.; Rivero, E.R.C. The Synergistic Effect of Tobacco and Alcohol Consumption on Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2019, 23, 2849–2859. [Google Scholar] [CrossRef]
- Gajendra, S.; McIntosh, S.; Ghosh, S. Effects of tobacco product use on oral health and the role of oral healthcare providers in cessation: A narrative review. Tob. Induc. Dis. 2023, 21, 12. [Google Scholar] [CrossRef]
- Yuk, J.S.; Kim, B.Y. Relationship between Menopausal Hormone Therapy and Oral Cancer: A Cohort Study Based on the Health Insurance Database in South Korea. J. Clin. Med. 2022, 11, 5848. [Google Scholar] [CrossRef]
- Contaldo, M.; Boccellino, M.; Zannini, G.; Romano, A.; Sciarra, A.; Sacco, A.; Settembre, G.; Coppola, M.; di Carlo, A.; D’Angelo, L.; et al. Sex Hormones and Inflammation Role in Oral Cancer Progression: A Molecular and Biological Point of View. J. Oncol. 2020, 2020, 9587971. [Google Scholar] [CrossRef]
- Gilligan, G.; Piemonte, E.; Lazos, J.; Simancas, M.C.; Panico, R.; Warnakulasuriya, S. Oral Squamous Cell Carcinoma Arising from Chronic Traumatic Ulcers. Clin. Oral Investig. 2022, 27, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Vincent-Chong, V.K.; DeJong, H.; Hershberger, P.A.; Seshadri, M. Impact of Dietary Vitamin D on Initiation and Progression of Oral Cancer. J. Steroid Biochem. Mol. Biol. 2020, 199, 105603. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral Cancer: Etiology and Risk Factors: A Review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, I.; Florez, Á.; Paredes-Suárez, C.; Rodríguez-Lojo, R.; González-Vilas, D.; Ramírez-Santos, A.; Paradela, S.; Suárez Conde, I.; Pereiro-Ferreirós, M. Use of Lip Photoprotection in Patients Suffering from Actinic Cheilitis. Eur. J. Dermatol. 2019, 29, 383–386. [Google Scholar] [CrossRef]
- Dancyger, A.; Heard, V.; Huang, B.; Suley, C.; Tang, D.; Ariyawardana, A. Malignant Transformation of Actinic Cheilitis: A Systematic Review of Observational Studies. J. Investig. Clin. Dent. 2018, 9, e12343. [Google Scholar] [CrossRef] [PubMed]
- Louredo, B.V.R.; Prado-Ribeiro, A.C.; Brandão, T.B.; Epstein, J.B.; Migliorati, C.A.; Piña, A.R.; Kowalski, L.P.; Vargas, P.A.; Lopes, M.A.; Santos-Silva, A.R. State-of-the-Science Concepts of HPV-Related Oropharyngeal Squamous Cell Carcinoma: A Comprehensive Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 190–205. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Šimić, I.; Božinović, K.; Milutin Gašperov, N.; Kordić, M.; Pešut, E.; Manojlović, L.; Grce, M.; Dediol, E.; Sabol, I. Head and Neck Cancer Patients’ Survival According to HPV Status, miRNA Profiling, and Tumour Features-A Cohort Study. Int. J. Mol. Sci. 2023, 24, 3344. [Google Scholar] [CrossRef]
- Dhar, L.; Singh, S.; Passey, J.C. Association of human papillomavirus and Epstein-Barr virus with squamous cell carcinoma of upper aerodigestive tract. Natl. J. Maxillofac. Surg. 2022, 13, 367–375. [Google Scholar] [CrossRef]
- Ward, B.J.H.; Schaal, D.L.; Nkadi, E.H.; Scott, R.S. EBV Association with Lymphomas and Carcinomas in the Oral Compartment. Viruses 2022, 14, 2700. [Google Scholar] [CrossRef]
- Law, R.H.; Rizzi, M.D. EBV-Positive Mucocutaneous Ulcer Presenting as a Nasopharyngeal Tumor in an Immune Suppressed Patient. Am. J. Otolaryngol.—Head Neck Med. Surg. 2023, 44, 103685. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Shah, K.V. Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma: Mounting Evidence for an Etiologic Role for Human Papillomavirus in a Subset of Head and Neck Cancers. Curr. Opin. Oncol. 2001, 13, 183–188. [Google Scholar] [CrossRef]
- Katirachi, S.K.; Grønlund, M.P.; Jakobsen, K.K.; Grønhøj, C.; von Buchwald, C. The Prevalence of HPV in Oral Cavity Squamous Cell Carcinoma. Viruses 2023, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Kanduc, D.; Muzio, L.L.; Lucchese, A.; Serpico, R. Possible Association between HPV16 E7 Protein Level and Cytokeratin 19. Int. J. Cancer 2004, 111, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Serpico, R.; Guida, A.; Crincoli, V.; Scully, C.; Kanduc, D. Interkeratin Peptide-Protein Interactions That Promote HPV16 E7 Gene Expression. Int. J. Immunopathol. Pharmacol. 2010, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sypień, P.; Zielonka, T.M. HPV Infections, Related Diseases and Prevention Methods. Fam. Med. Prim. Care Rev. 2022, 24, 88–91. [Google Scholar] [CrossRef]
- Pannone, G.; Santoro, A.; Carinci, F.; Bufo, P.; Papagerakis, S.M.; Rubini, C.; Campisi, G.; Giovannelli, L.; Contaldo, M.; Serpico, R.; et al. Double Demonstration of Oncogenic High Risk Human Papilloma Virus DNA and HPV-E7 Protein in Oral Cancers. Int. J. Immunopathol. Pharmacol. 2011, 24, 95–101. [Google Scholar] [CrossRef]
- della Vella, F.; Lauritano, D.; Pannone, G.; del Prete, R.; di Stasio, D.; Contaldo, M.; Petruzzi, M. Prevalence of HPV in Patients Affected by Oral Lichen Planus: A Prospective Study Using Two Different Chair-Side Sampling Methods. J. Oral Pathol. Med. 2021, 50, 716–722. [Google Scholar] [CrossRef]
- Lucchese, A.; Serpico, R. Effect of SP3 Silencing on Cytokeratin Expression Pattern in HPV-Positive Cells. Int. J. Immunopathol. Pharmacol. 2009, 22, 163–168. [Google Scholar] [CrossRef]
- Rampias, T.; Sasaki, C.; Psyrri, A. Molecular Mechanisms of HPV Induced Carcinogenesis in Head and Neck. Oral Oncol. 2014, 50, 356–363. [Google Scholar] [CrossRef]
- Campisi, G.; Giovannelli, L.; Ammatuna, P.; Capra, G.; Colella, G.; di Liberto, C.; Gandolfo, S.; Pentenero, M.; Carrozzo, M.; Serpico, R.; et al. Proliferative Verrucous vs Conventional Leukoplakia: No Significantly Increased Risk of HPV Infection. Oral Oncol. 2004, 40, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Al-Hebshi, N.N.; Speicher, D.J.; Perera, I.; Johnson, N.W. Emerging Role of Bacteria in Oral Carcinogenesis: A Review with Special Reference to Perio-Pathogenic Bacteria. J. Oral Microbiol. 2016, 8, 32762. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Wilson, M.J.; Crean, S.J. Exploring the Link between Microorganisms and Oral Cancer: A Systematic Review of the Literature. Head Neck 2009, 31, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Kamer, A.R.; Pushalkar, S.; Gulivindala, D.; Butler, T.; Li, Y.; Annam, K.R.C.; Glodzik, L.; Ballman, K.V.; Corby, P.M.; Blennow, K.; et al. Periodontal Dysbiosis Associates with Reduced CSF Aβ42 in Cognitively Normal Elderly. Alzheimers Dement. 2021, 13, e12172. [Google Scholar] [CrossRef]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis from a Microbial and Host Perspective Relative to Oral Health and Disease. Front. Microbiol. 2021, 12, 617485. [Google Scholar] [CrossRef]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Holmstrup, P.; Damgaard, C.; Olsen, I.; Klinge, B.; Flyvbjerg, A.; Nielsen, C.H.; Hansen, P.R. Comorbidity of periodontal disease: Two sides of the same coin? An introduction for the clinician. J. Oral Microbiol. 2017, 9, 1332710. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.; Yin, N. Adaptations and Alterations of Maternal Microbiota: From Physiology to Pathology. Med. Microecol. 2021, 9, 100045. [Google Scholar] [CrossRef]
- de Jesus, V.C.; Khan, M.W.; Mittermuller, B.-A.; Duan, K.; Hu, P.; Schroth, R.J.; Chelikani, P. Characterization of Supragingival Plaque and Oral Swab Microbiomes in Children with Severe Early Childhood Caries. Front. Microbiol. 2021, 12, 683685. [Google Scholar] [CrossRef]
- Willis, J.R.; Saus, E.; Iraola-Guzmán, S.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Blanco, A.; Puig-Sola, A.; et al. Citizen-Science Reveals Changes in the Oral Microbiome in Spain through Age and Lifestyle Factors. NPJ Biofilms Microbiomes 2022, 8, 38. [Google Scholar] [CrossRef]
- Simas, A.M.; Kramer, C.D.; Weinberg, E.O.; Genco, C.A. Oral Infection with a Periodontal Pathogen Alters Oral and Gut Microbiomes. Anaerobe 2021, 71, 102399. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Luo, E.; Wu, B.; Li, Z.; Guo, J.; Xia, Z.; Zheng, C.; Su, Q.; Zeng, Y.; et al. Oral, Nasal, and Gut Microbiota in Parkinson’s Disease. Neuroscience 2022, 480, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Lucchese, A.; Lajolo, C.; Rupe, C.; di Stasio, D.; Romano, A.; Petruzzi, M.; Serpico, R. The Oral Microbiota Changes in Orthodontic Patients and Effects on Oral Health: An Overview. J. Clin. Med. 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Romano, A.; Vella, F.D.; di Stasio, D.; Serpico, R.; Petruzzi, M. Oral Microbiota Features in Subjects with down Syndrome and Periodontal Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9251. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium Nucleatum and Its Associated Systemic Diseases: Epidemiologic Studies and Possible Mechanisms. J. Oral Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef]
- Lucchese, A.; Petruzzi, M.; Lauritano, D. Crossreactivity: The Possible Role of Oral Microbiota in Oral Mucous Membrane Pemphigoid. Autoimmun. Rev. 2021, 20, 102799. [Google Scholar] [CrossRef]
- Rupe, C.; Gioco, G.; Almadori, G.; Galli, J.; Micciché, F.; Olivieri, M.; Cordaro, M.; Lajolo, C. Oral Candida Spp. Colonisation Is a Risk Factor for Severe Oral Mucositis in Patients Undergoing Radiotherapy for Head & Neck Cancer: Results from a Multidisciplinary Mono-Institutional Prospective Observational Study. Cancers 2022, 14, 4746. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; lo Muzio, L.; Pérez-Sayáns, M. Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. J. Fungi 2022, 8, 93. [Google Scholar] [CrossRef]
- dos Santos, E.S.; Pérez-de-Oliveira, M.E.; Normando, A.G.C.; Gueiros, L.A.M.; Rogatto, S.R.; Vargas, P.A.; Lopes, M.A.; da Silva Guerra, E.N.; Leme, A.F.P.; Santos-Silva, A.R. Systemic Conditions Associated with Increased Risk to Develop Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Head Neck 2022, 44, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. BioMed Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, I.; Wongsrita, S.; Sripan, P.; Kongsupapsiri, P.; Phakoetsuk, P.; Chachvarat, S.; Kittidachanan, K. An underestimated pitfall of oral candidiasis in head and neck cancer patients undergoing radiotherapy: An observation study. BMC Oral Health 2021, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Debta, P.; Swain, S.K.; Sahu, M.C.; Abuderman, A.A.; Alzahrani, K.J.; Banjer, H.J.; Qureshi, A.A.; Bakri, M.M.H.; Sarode, G.S.; Patro, S.; et al. Evaluation of Candidiasis in Upper-Aerodigestive Squamous Cell Carcinoma Patients-A Clinico-Mycological Aspect. Int. J. Environ. Res. Public Health 2022, 19, 8510. [Google Scholar] [CrossRef]
- Paoletti, I.; Fusco, A.; Grimaldi, E.; Perillo, L.; Coretti, L.; di Domenico, M.; Cozza, V.; Lucchese, A.; Contaldo, M.; Serpico, R.; et al. Assessment of Host Defence Mechanisms Induced by Candida Species. Int. J. Immunopathol. Pharmacol. 2013, 26, 663–672. [Google Scholar] [CrossRef]
- Sufiawati, I.; Piliang, A.; Ramamoorthy, V.R. Oral Microbiota in Oral Cancer Patients and Healthy Individuals: A Scoping Review. Dent. J. 2022, 55, 186–193. [Google Scholar] [CrossRef]
- Liao, C.-T.; Huang, S.-F.; Chen, I.-H.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Cheng, A.-J.; et al. Risk Stratification of Patients with Oral Cavity Squamous Cell Carcinoma and Contralateral Neck Recurrence Following Radical Surgery. Ann. Surg. Oncol. 2009, 16, 159–170. [Google Scholar] [CrossRef]
- Kjeldsted, E.; Dalton, S.O.; Frederiksen, K.; Andersen, E.; Nielsen, A.L.; Stafström, M.; Kjaer, T.K. Association between Human Papillomavirus Status and Health-Related Quality of Life in Oropharyngeal and Oral Cavity Cancer Survivors. Oral Oncol. 2020, 109, 104918. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Sarode, S.C.; Dasgupta, S.; Sharma, B.; Hedaoo, A.; Sharma, A.; Sarode, G.S.; Yuwanati, M.; Gondivkar, R.S.; et al. Prevalence of Trismus and Its Impact on Oral Health-Related Quality of Life in Patients Treated for Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 2437–2444. [Google Scholar] [CrossRef]
- Petersen, L.Ø.; Jensen, J.S.; Jakobsen, K.K.; Grønhøj, C.; Wessel, I.; von Buchwald, C. Second Primary Cancer Following Primary Oral Squamous Cell Carcinoma: A Population-Based, Retrospective Study. Acta Oncol. 2022, 61, 916–921. [Google Scholar] [CrossRef]
- Wang, W.; Adeoye, J.; Thomson, P.; Choi, S.-W. Multiple Tumour Recurrence in Oral, Head and Neck Cancer: Characterising the Patient Journey. J. Oral Pathol. Med. 2021, 50, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Mamani, M.; Terrero-Pérez, Á.; Tucunduva, R.M.A.; Rubira, C.M.F.; Santos, P.S.D.S.; Honório, H.M.; Rubira-Bullen, I.R.F. Occurrence of Field Cancerization in Clinically Normal Oral Mucosa: A Systematic Review and Meta-Analysis. Arch. Oral Biol. 2022, 143, 105544. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Bizzoca, M.E.; Contaldo, M.; Serpico, R.; lo Muzio, L.; Santarelli, A. Lymphovascular Invasion as a Prognostic Tool for Oral Squamous Cell Carcinoma: A Comprehensive Review. Int. J. Oral Maxillofac. Surg. 2022, 51, 1–9. [Google Scholar] [CrossRef]
- Contaldo, M.; di Napoli, A.; Pannone, G.; Franco, R.; Ionna, F.; Feola, A.; de Rosa, A.; Santoro, A.; Sbordone, C.; Longo, F.; et al. Prognostic Implications of Node Metastatic Features in OSCC: A Retrospective Study on 121 Neck Dissections. Oncol. Rep. 2013, 30, 2697–2704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spoerl, S.; Spoerl, S.; Reil, S.; Gerken, M.; Ludwig, N.; Taxis, J.; Fischer, R.; Ettl, T.; Reichert, T.E.; Spanier, G. Prognostic Value of Perineural Invasion on Survival and Recurrence in Oral Squamous Cell Carcinoma. Diagnostics 2022, 12, 1062. [Google Scholar] [CrossRef]
- Brennan, P.A.; Dylgjeri, F.; Coletta, R.D.; Arakeri, G.; Goodson, A.M. Surgical Tumour Margins and Their Significance in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2022, 51, 311–314. [Google Scholar] [CrossRef]
- Ghantous, Y.; Nashef, A.; Sidransky, D.; Abdelraziq, M.; Alkeesh, K.; Araidy, S.; Koch, W.; Brait, M.; Abu El-Naaj, I. Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System. Cancers 2022, 14, 4632. [Google Scholar] [CrossRef]
- Mishra, A.; Das, A.; Dhal, I.; Shankar, R.; Bhavya, B.M.; Singh, N.; Tripathi, P.; Daga, D.; Rai, A.; Gupta, M.; et al. Worst Pattern of Invasion in Oral Squamous Cell Carcinoma Is an Independent Prognostic Factor. J. Oral Biol. Craniofac. Res. 2022, 12, 771–776. [Google Scholar] [CrossRef]
- Kahn, M.A. Screening for Oral Cancer--a Matter of Life or Death. J. Mass. Dent. Soc. 2006, 54, 24–27. [Google Scholar]
- Rosenheck, A.H. Oral Cancer Detection/Prevention: Oral Cancer, The Silent Killer. Dent. Today 2015, 34, 106. [Google Scholar]
- della Vella, F.; Lauritano, D.; Lajolo, C.; Lucchese, A.; di Stasio, D.; Contaldo, M.; Serpico, R.; Petruzzi, M. The Pseudolesions of the Oral Mucosa: Differential Diagnosis and Related Systemic Conditions. Appl. Sci. 2019, 9, 2412. [Google Scholar] [CrossRef]
- Romano, A.; di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.; Palla, B.; Callahan, N.; Pyka, T.; Wolff, C.; von Schacky, C.E.; Schmid, A.; Froelich, M.F.; Rübenthaler, J.; Makowski, M.R.; et al. Comparison of CT, MRI, and F-18 FDG PET/CT for Initial N-Staging of Oral Squamous Cell Carcinoma: A Cost-Effectiveness Analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Maio, C.; Romano, A.; Laino, L.; Lucchese, A. The Potential Role of in Vivo Optical Coherence Tomography for Evaluating Oral Soft Tissue: A Systematic Review. J. Oral Pathol. Med. 2017, 46, 864–876. [Google Scholar] [CrossRef]
- Panzarella, V.; Bartolone, A.; Coniglio, R.; Rodolico, V.; Maniscalco, L.; Capocasale, G.; Carbone, M.I.; Campisi, G. Diagnostic Concordance between Optical Coherence Tomography and Histological Investigations for Immune-Mediated Desquamative Gingivitis: Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 9095. [Google Scholar] [CrossRef]
- Panzarella, V.; Bartolone, A.; Rodolico, V.; Capocasale, G.; Maniscalco, L.; Matranga, D.; di Fede, O.; Campisi, G. Immune-Mediated Desquamative Gingivitis and Optical Coherence Tomography Diagnostic Patterns: Clinical Implication from a Systematic Review. Diagnostics 2021, 11, 1453. [Google Scholar] [CrossRef]
- Lucchese, A.; Gentile, E.; Romano, A.; Maio, C.; Laino, L.; Serpico, R. The Potential Role of in Vivo Reflectance Confocal Microscopy for Evaluating Oral Cavity Lesions: A Systematic Review. J. Oral Pathol. Med. 2016, 45, 723–729. [Google Scholar] [CrossRef]
- Walther, J.; Golde, J.; Kirsten, L.; Tetschke, F.; Hempel, F.; Rosenauer, T.; Hannig, C.; Koch, E. In vivo imaging of human oral hard and soft tissues by polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2017, 22, 1–17. [Google Scholar] [CrossRef]
- Contaldo, M.; Agozzino, M.; Moscarella, E.; Esposito, S.; Serpico, R.; Ardigò, M. In Vivo Characterization of Healthy Oral Mucosa by Reflectance Confocal Microscopy: A Translational Research for Optical Biopsy. Ultrastruct. Pathol. 2013, 37, 151–158. [Google Scholar] [CrossRef]
- Contaldo, M.; Poh, C.F.; Guillaud, M.; Lucchese, A.; Rullo, R.; Lam, S.; Serpico, R.; Macaulay, C.E.; Lane, P.M. Oral Mucosa Optical Biopsy by a Novel Handheld Fluorescent Confocal Microscope Specifically Developed: Technologic Improvements and Future Prospects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 752–758. [Google Scholar] [CrossRef]
- Contaldo, M.; Agozzino, M.; Ardigò, M. In Vivo Reflectance Confocal Microscopy for Oral Mucosa Assessment. In Non Invasive Diagnostic Techniques in Clinical Dermatology; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642321092. [Google Scholar]
- Crincoli, V.; Scivetti, M.; di Risceglie, M.B.; Lucchese, A.; Favia, G. Complex Odontoma: Confocal Laser Scanning Microscopy Analysis of a Case. Minerva Stomatol. 2006, 55, 315–319. [Google Scholar] [PubMed]
- Scivetti, M.; Lucchese, A.; Ficarra, G.; Giuliani, M.; Lajolo, C.; Maiorano, E.; Favia, G. Oral Pulse Granuloma: Histological Findings by Confocal Laser Scanning Microscopy. Ultrastruct. Pathol. 2009, 33, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Scivetti, M.; di Bisceglie, M.B.; Lucchese, A.; Favia, G. Odontoma: Retrospective Study and Confocal Laser Scanning Microscope Analysis of 52 Cases. Minerva Stomatol. 2007, 56, 611–620. [Google Scholar]
- Laino, L.; Favia, G.; Menditti, D.; de Francesco, F.; Salerno, C.; Scivetti, M.; Serpico, R.; Lucchese, A. Confocal Laser Scanning Microscopy Analysis of 10 Cases of Craniofacial Fibrous Dysplasia. Ultrastruct. Pathol. 2015, 39, 231–234. [Google Scholar] [CrossRef]
- Lucchese, A.; Scivetti, M.; Pilolli, G.P.; Favia, G. Analysis of Ghost Cells in Calcifying Cystic Odontogenic Tumors by Confocal Laser Scanning Microscopy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Scivetti, M.; Pilolli, G.P.; Corsalini, M.; Lucchese, A.; Favia, G. Confocal Laser Scanning Microscopy of Human Cementocytes: Analysis of Three-Dimensional Image Reconstruction. Ann. Anat. 2007, 189, 169–174. [Google Scholar] [CrossRef]
- Ardigo, M.; Donadio, C.; Franceschini, C.; Catricalà, C.; Agozzino, M. Interest of Reflectance Confocal Microscopy for Inflammatory Oral Mucosal Diseases. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1850–1853. [Google Scholar] [CrossRef]
- Romano, A.; Santarelli, A.; Lajolo, C.; della Vella, F.; Mascitti, M.; Serpico, R.; Contaldo, M. Analysis of Oral Mucosa Erosive-Ulcerative Lesions by Reflectance Confocal Microscopy. J. Biol. Regul. Homeost. Agents 2019, 33, 11–18. [Google Scholar]
- Contaldo, M.; di Stasio, D.; Petruzzi, M.; Serpico, R.; Lucchese, A. In Vivo Reflectance Confocal Microscopy of Oral Lichen Planus. Int. J. Dermatol. 2019, 58, 940–945. [Google Scholar] [CrossRef]
- Alessi, S.S.; Nico, M.M.S.; Fernandes, J.D.; Lourenço, S.V. Reflectance Confocal Microscopy as a New Tool in the in Vivo Evaluation of Desquamative Gingivitis: Patterns in Mucous Membrane Pemphigoid, Pemphigus Vulgaris and Oral Lichen Planus. Br. J. Dermatol. 2013, 168, 257–264. [Google Scholar] [CrossRef]
- Contaldo, M.; Lajolo, C.; di Petrillo, M.; Ballini, A.; Inchingolo, F.; Serpico, R.; Romano, A. Analysis of Lip Pigmentations by Reflectance Confocal Microscopy: Report of Two Cases. J. Biol. Regul. Homeost. Agents 2019, 33, 19–26. [Google Scholar] [PubMed]
- Maher, N.G.; Solinas, A.; Scolyer, R.A.; Guitera, P. In Vivo Reflectance Confocal Microscopy for Evaluating Melanoma of the Lip and Its Differential Diagnoses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Collgros, H.; Scolyer, R.A.; Menzies, S.W.; Guitera, P. In Vivo Reflectance Confocal Microscopy for the Diagnosis of Melanoma and Melanotic Macules of the Lip. JAMA Dermatol. 2017, 153, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Grassia, V.; Gentile, E.; di Stasio, D.; Jamilian, A.; Matarese, G.; D’Apuzzo, F.; Santoro, R.; Perillo, L.; Serpico, R.; Lucchese, A. In Vivo Confocal Microscopy Analysis of Enamel Defects after Orthodontic Treatment: A Preliminary Study. Ultrastruct. Pathol. 2016, 40, 317–323. [Google Scholar] [CrossRef]
- Contaldo, M.; di Stasio, D.; Santoro, R.; Laino, L.; Perillo, L.; Petruzzi, M.; Lauritano, D.; Serpico, R.; Lucchese, A. Non-Invasive In Vivo Visualization of Enamel Defects by Reflectance Confocal Microscopy (RCM). Odontology 2015, 103, 177–184. [Google Scholar] [CrossRef]
- Contaldo, M.; Serpico, R.; Lucchese, A. In Vivo Imaging of Enamel by Reflectance Confocal Microscopy (RCM): Non-Invasive Analysis of Dental Surface. Odontology 2014, 102, 325–329. [Google Scholar] [CrossRef]
- de Rosa, A.; di Stasio, D.; Lauritano, D.; Santoro, R.; Marotta, A.; Itro, A.; Lucchese, A. Non-Invasive Analysis of Bleaching Effect of Hydrogen Peroxide on Enamel by Reflectance Confocal Microscopy (RCM): Study of Series of Cases. Odontology 2019, 107, 285–290. [Google Scholar] [CrossRef]
- Contaldo, M.; di Stasio, D.; Vella, F.D.; Lauritano, D.; Serpico, R.; Santoro, R.; Lucchese, A. Real Time in Vivo Confocal Microscopic Analysis of the Enamel Remineralization by Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP): A Clinical Proof-of-Concept Study. Appl. Sci. 2020, 10, 4155. [Google Scholar] [CrossRef]
- Gentile, E.; di Stasio, D.; Santoro, R.; Contaldo, M.; Salerno, C.; Serpico, R.; Lucchese, A. In Vivo Microstructural Analysis of Enamel in Permanent and Deciduous Teeth. Ultrastruct. Pathol. 2015, 39, 131–134. [Google Scholar] [CrossRef]
- Romano, A.; di Spirito, F.; Amato, A.; Ferraro, G.A.; Dipalma, G.; Xhajanka, E.; Serpico, R.; Inchingolo, F.; Contaldo, M. Dental Microstructural Imaging: From Conventional Radiology to In Vivo Confocal Microscopy. Appl. Sci. 2022, 12, 10654. [Google Scholar] [CrossRef]
- Contaldo, M.; Lauritano, D.; Carinci, F.; Romano, A.; di Stasio, D.; Lajolo, C.; della Vella, F.; Serpico, R.; Lucchese, A. Intraoral Confocal Microscopy of Suspicious Oral Lesions: A Prospective Case Series. Int. J. Dermatol. 2020, 59, 82–90. [Google Scholar] [CrossRef]
- Ramani, R.S.; Tan, I.; Bussau, L.; Angel, C.M.; McCullough, M.; Yap, T. Confocal Microscopy in Oral Cancer and Oral Potentially Malignant Disorders: A Systematic Review. Oral Dis. 2022; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; Casiraghi, O.; Vergez, S.; Maillard, A.; Lakhdar, A.B.; de Leeuw, F.; Crestani, S.; Ngo, C.; Koscielny, S.; Ferchiou, M.; et al. Diagnostic Accuracy of in Vivo Early Tumor Imaging from Probe-Based Confocal Laser Endomicroscopy versus Histologic Examination in Head and Neck Squamous Cell Carcinoma. Clin. Oral Investig. 2022, 26, 1823–1833. [Google Scholar] [CrossRef]
- Sievert, M.; Oetter, N.; Mantsopoulos, K.; Gostian, A.-O.; Mueller, S.K.; Koch, M.; Balk, M.; Thimsen, V.; Stelzle, F.; Eckstein, M.; et al. Systematic Classification of Confocal Laser Endomicroscopy for the Diagnosis of Oral Cavity Carcinoma. Oral Oncol. 2022, 132, 105978. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.; Zanoni, D.K.; Ardigo, M.; Migliacci, J.C.; Patel, S.G.; Rajadhyaksha, M. Feasibility of a Video-Mosaicking Approach to Extend the Field-of-View For Reflectance Confocal Microscopy in the Oral Cavity In Vivo. Lasers Surg. Med. 2019, 51, 439–451. [Google Scholar] [CrossRef] [PubMed]
- di Stasio, D.; Lauritano, D.; Iquebal, H.; Romano, A.; Gentile, E.; Lucchese, A. Measurement of Oral Epithelial Thickness by Optical Coherence Tomography. Diagnostics 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In Vivo Characterization of Oral Pemphigus Vulgaris by Optical Coherence Tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar]
- Stasio, D.D.; Lauritano, D.; Loffredo, F.; Gentile, E.; Vella, F.D.; Petruzzi, M.; Lucchese, A. Optical Coherence Tomography Imaging of Oral Mucosa Bullous Diseases: A Preliminary Study. Dentomaxillofac. Radiol. 2019, 48, 20190071. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Li, S.; Zhong, J.; Huang, X. Noninvasive Oral Cancer Screening Based on Local Residual Adaptation Network Using Optical Coherence Tomography. Med. Biol. Eng. Comput. 2022, 60, 1363–1375. [Google Scholar] [CrossRef]
- di Stasio, D.; Lauritano, D.; Paparella, R.; Franco, R.; Montella, M.; Serpico, R.; Lucchese, A. Ultrasound Imaging of Oral Fibroma: A Case Report. J. Biol. Regul. Homeost. Agents 2017, 31, 23–26. [Google Scholar]
- di Stasio, D.; Romano, A.; Montella, M.; Contaldo, M.; Petruzzi, M.; Hasan, I.; Serpico, R.; Lucchese, A. Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study. Appl. Sci. 2022, 12, 6829. [Google Scholar] [CrossRef]
- di Stasio, D.; Montella, M.; Romano, A.; Colella, G.; Serpico, R.; Lucchese, A. High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers 2022, 14, 564. [Google Scholar] [CrossRef]
- Romano, A.; Montella, M.; Ronchi, A.; Borgia, R.; Fiori, F.; Contaldo, M.; Serpico, R. An Overview of High-Definition Ultrasounds Applied in Oral Diseases. J. Biol. Regul. Homeost. Agents 2022, 36, 77–86. [Google Scholar] [CrossRef]
- de Koning, K.J.; van Es, R.J.J.; Klijn, R.J.; Breimer, G.E.; Willem Dankbaar, J.; Braunius, W.W.; van Cann, E.M.; Dieleman, F.J.; Rijken, J.A.; Tijink, B.M.; et al. Application and Accuracy of Ultrasound-Guided Resections of Tongue Cancer. Oral Oncol. 2022, 133, 106023. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Knutsson, J.; Landström, F.J.; Magnuson, A.; von Beckerath, M. Ultrasound Accurately Assesses Depth of Invasion in T1–T2 Oral Tongue Cancer. Laryngoscope Investig. Otolaryngol. 2022, 7, 1448–1455. [Google Scholar] [CrossRef]
- Caprioli, S.; Casaleggio, A.; Tagliafico, A.S.; Conforti, C.; Borda, F.; Fiannacca, M.; Filauro, M.; Iandelli, A.; Marchi, F.; Parrinello, G.; et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations. Int. J. Environ. Res. Public Health 2022, 19, 4900. [Google Scholar] [CrossRef] [PubMed]
- Nogami, S.; Yamauchi, K.; Kitamura, J.; Miyashita, H.; Kojima, I.; Kouketsu, A.; Furuuchi, T.; Iikubo, M.; Kumamoto, H.; Takahashi, T. The Accuracy of Ultrasound and Magnetic Resonance Imaging for Estimating Thickness of Oral Tongue Squamous Cell Carcinoma and Influence of Biopsy on Those Findings. Oral Sci. Int. 2022, 19, 24–30. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.W.P.; de Bree, R.; van Es, R.J.J. Intraoral ultrasonography to measure tumor thickness of oral cancer: A systematic review and meta-analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review. Laryngoscope. 2019, 129, 662–670. [Google Scholar] [CrossRef]
- Yesuratnam, A.; Wiesenfeld, D.; Tsui, A.; Iseli, T.A.; Hoorn, S.V.; Ang, M.T.; Guiney, A.; Phal, P.M. Preoperative evaluation of oral tongue squamous cell carcinoma with intraoral ultrasound and magnetic resonance imaging-comparison with histopathological tumour thickness and accuracy in guiding patient management. Int. J. Oral Maxillofac. Surg. 2014, 43, 787–794. [Google Scholar] [CrossRef]
- Lo, W.C.; Chang, C.M.; Cheng, P.C.; Wen, M.H.; Wang, C.T.; Cheng, P.W.; Liao, L.J. The Applications and Potential Developments of Ultrasound in Oral Cancer Management. Technol. Cancer Res. Treat. 2022, 21, 15330338221133216. [Google Scholar] [CrossRef]
- Baba, A.; Hashimoto, K.; Kayama, R.; Yamauchi, H.; Ikeda, K.; Ojiri, H. Correction to: Radiological approach for the newly incorporated T staging factor, depth of invasion (DOI), of the oral tongue cancer in the 8th edition of American Joint Committee on Cancer (AJCC) staging manual: Assessment of the necessity for elective neck dissection. Jpn. J. Radiol. 2021, 39, 100. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.H.; Patil, S. Efficacy of Autofluorescence Imaging as an Adjunctive Technique for Examination and Detection of Oral Potentially Malignant Disorders: A Systematic Review. J. Contemp. Dent. Pract. 2015, 16, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Warnakulasuriya, S. The Use of Light-Based (Optical) Detection Systems as Adjuncts in the Detection of Oral Cancer and Oral Potentially Malignant Disorders: A Systematic Review. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2015, 44, 307–328. [Google Scholar] [CrossRef]
- Coll, Y.; Geddes, A.; Thomson, E. The Light at the End of the Tunnel? Can Light-Based Tests Increase the Accuracy of Our Diagnoses of Pre-Cancerous/Cancerous Lesions? Evid. Based Dent. 2022, 23, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Flores dos Santos, L.C.; Fernandes, J.R.; Lima, I.F.P.; Bittencourt, L.D.S.; Martins, M.D.; Lamers, M.L. Applicability of Autofluorescence and Fluorescent Probes in Early Detection of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Data Analysis. Photodiagn. Photodyn. Ther. 2022, 38, 102764. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Gentile, E.; Zulli, C.; Petruzzi, M.; Lauritano, D.; Amato, M.R.; Esposito, P.; Riegler, G.; Serpico, R. Evaluation of the Intraepithelial Papillary Capillary Loops in Benign and Malignant Oral Lesions by in Vivo Virtual Chromoendoscopic Magnification: A Preliminary Study. J. Biol. Regul. Homeost. Agents 2017, 31, 11–22. [Google Scholar]
- Saraniti, C.; Greco, G.; Verro, B.; Lazim, N.-M.; Chianetta, E. Impact of Narrow Band Imaging in Pre-Operative Assessment of Suspicious Oral Cavity Lesions: A Systematic Review. Iran. J. Otorhinolaryngol. 2021, 33, 127–135. [Google Scholar] [CrossRef]
- Nair, D.; Qayyumi, B.; Sharin, F.; Mair, M.; Bal, M.; Pimple, S.; Mishra, G.; Nair, S.; Chaturvedi, P. Narrow Band Imaging Observed Oral Mucosa Microvasculature as a Tool to Detect Early Oral Cancer: An Indian Experience. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3965–3971. [Google Scholar] [CrossRef]
- Ota, A.; Miyamoto, I.; Ohashi, Y.; Chiba, T.; Takeda, Y.; Yamada, H. Diagnostic Accuracy of High-Grade Intraepithelial Papillary Capillary Loops by Narrow Band Imaging for Early Detection of Oral Malignancy: A Cross-Sectional Clinicopathological Imaging Study. Cancers 2022, 14, 2415. [Google Scholar] [CrossRef]
- Sachdeva, K.; Saji, T.A.; Sachdeva, N.; Karun, H. A Prospective Study to Evaluate the Role of Narrow Band Imaging and Toludine Blue in the Screening of Premalignant and Malignant Lesions of the Oral Cavity in a Tertiary Referral Centre. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 2177–2183. [Google Scholar] [CrossRef]
- de Wit, J.G.; van Schaik, J.E.; Voskuil, F.J.; Vonk, J.; de Visscher, S.A.H.J.; Schepman, K.-P.; van der Laan, B.F.A.M.; Doff, J.J.; van der Vegt, B.; Plaat, B.E.C.; et al. Comparison of Narrow Band and Fluorescence Molecular Imaging to Improve Intraoperative Tumour Margin Assessment in Oral Cancer Surgery. Oral Oncol. 2022, 134, 106099. [Google Scholar] [CrossRef] [PubMed]
- Puneeta, N.; Santosh, T.; Mishra, I.; Gaikwad, P.; Sahu, A. Evaluation of E-Cadherin and Vimentin Expression for Different Grades of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma—An Immunohistochemical Study. J. Oral Maxillofac. Pathol. 2022, 26, 285. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Patil, B.; Gangane, N. E-Cadherin as a Prognostic Biomarker in Oral Squamous Cell Carcinoma: A Pilot Study at Tertiary Care Hospital. Med. J. Dr. D.Y. Patil Vidyapeeth 2022, 15, 501–506. [Google Scholar] [CrossRef]
- lo Muzio, L.; Campisi, G.; Farina, A.; Rubini, C.; Pannone, G.; Serpico, R.; Laino, G.; de Lillo, A.; Carinci, F. P-Cadherin Expression and Survival Rate in Oral Squamous Cell Carcinoma: An Immunohistochemical Study. BMC Cancer 2005, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Milillo, L.; Marinosci, F.; lo Muzio, L.; Serpico, R.; Antonaci, S. Altered Expression of Integrins and Basement Membrane Proteins in Malignant and Pre-Malignant Lesions of Oral Mucosa. J. Biol. Regul. Homeost. Agents 2001, 15, 375–380. [Google Scholar] [PubMed]
- Pannone, G.; Santoro, A.; Feola, A.; Bufo, P.; Papagerakis, P.; lo Muzio, L.; Staibano, S.; Ionna, F.; Longo, F.; Franco, R.; et al. The Role of E-Cadherin down-Regulation in Oral Cancer: CDH1 Gene Expression and Epigenetic Blockage. Curr. Cancer Drug Targets 2014, 14, 115–127. [Google Scholar] [CrossRef]
- Chung, C.H.; Ely, K.; McGavran, L.; Varella-Garcia, M.; Parker, J.; Parker, N.; Jarrett, C.; Carter, J.; Murphy, B.A.; Netterville, J.; et al. Increased Epidermal Growth Factor Receptor Gene Copy Number Is Associated with Poor Prognosis in Head and Neck Squamous Cell Carcinomas. J. Clin. Oncol. 2006, 24, 4170–4176. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Zhang, W.; He, J.; Zhao, Y.; Chen, X. Prognostic Role of Epidermal Growth Factor Receptor in Head and Neck Cancer: A Meta-Analysis. J. Surg. Oncol. 2013, 108, 387–397. [Google Scholar] [CrossRef]
- Aquino, G.; Pannone, G.; Santoro, A.; Liguori, G.; Franco, R.; Serpico, R.; Florio, G.; de Rosa, A.; Mattoni, M.; Cozza, V.; et al. PEGFR-Tyr 845 Expression as Prognostic Factors in Oral Squamous Cell Carcinoma: A Tissue-Microarray Study with Clinic-Pathological Correlations. Cancer Biol. Ther. 2012, 13, 967–977. [Google Scholar] [CrossRef]
- de Kort, W.W.B.; Spelier, S.; Devriese, L.A.; van Es, R.J.J.; Willems, S.M. Predictive Value of EGFR-PI3K-AKT-MTOR-Pathway Inhibitor Biomarkers for Head and Neck Squamous Cell Carcinoma: A Systematic Review. Mol. Diagn. Ther. 2021, 25, 123–136. [Google Scholar] [CrossRef]
- Nie, D.; Wang, X.; Sun, M.; Feng, Z.; Pei, F.; Liu, W.; Wang, Z.; Han, F. The Primary Site of Head and Neck Squamous Cell Carcinoma Predicts Survival Benefits of EGFR Inhibitors: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2021, 158, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Pannone, G.; Hindi, S.A.H.; Santoro, A.; Sanguedolce, F.; Rubini, C.; Cincione, R.I.; de Maria, S.; Tortorella, S.; Rocchetti, R.; Cagiano, S.; et al. Aurora B Expression as a Prognostic Indicator and Possibile Therapeutic Target in Oral Squamous Cell Carcinoma. Int. J. Immunopathol. Pharmacol. 2011, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pannone, G.; Bufo, P.; Santoro, A.; Franco, R.; Aquino, G.; Longo, F.; Botti, G.; Serpico, R.; Cafarelli, B.; Abbruzzese, A.; et al. WNT Pathway in Oral Cancer: Epigenetic Inactivation of WNT-Inhibitors. Oncol. Rep. 2010, 24, 1035–1041. [Google Scholar] [CrossRef][Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt Signaling in Cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Arvanitidis, E.; Andreadis, P.; Andreadis, D.; Belazi, M.; Epivatianos, A. Reviewing the Oral Carcinogenic Process: Key Genetic Events, Growth Factors and Molecular Signaling Pathways. J. Biol. Res. 2011, 16, 313–336. [Google Scholar]
- Chandler, K.B.; Alamoud, K.A.; Stahl, V.L.; Nguyen, B.-C.; Kartha, V.K.; Bais, M.V.; Nomoto, K.; Owa, T.; Monti, S.; Kukuruzinska, M.A.; et al. SS-Catenin/CBP Inhibition Alters Epidermal Growth Factor Receptor Fucosylation Status in Oral Squamous Cell Carcinoma. Mol. Omics 2020, 16, 195–209. [Google Scholar] [CrossRef]
- lo Muzio, L.; Leonardi, R.; Mignogna, M.D.; Pannone, G.; Rubini, C.; Pieramici, T.; Trevisiol, L.; Ferrari, F.; Serpico, R.; Testa, N.; et al. Scatter Factor Receptor (c-Met) as Possible Prognostic Factor in Patients with Oral Squamous Cell Carcinoma. Anticancer Res. 2004, 24, 1063–1069. [Google Scholar]
- Pallavi, N.; Nalabolu, G.R.K.; Hiremath, S.K.S. Bcl-2 and c-Myc Expression in Oral Dysplasia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study to Assess Tumor Progression. J. Oral Maxillofac. Pathol. 2018, 22, 325–331. [Google Scholar] [CrossRef]
- lo Muzio, L.; Mignogna, M.D.; Pannone, G.; Rubini, C.; Grassi, R.; Nocini, P.F.; Ferrari, F.; Serpico, R.; Favia, G.; de Rosa, G.; et al. Expression of Bcl-2 in Oral Squamous Cell Carcinoma: An Immunohistochemical Study of 90 Cases with Clinico-Pathological Correlations. Oncol. Rep. 2003, 10, 285–291. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Shigematsu, H.; Nakajima, T.; Motohashi, S.; Sekine, Y.; Shibuya, K.; Iizasa, T.; Hiroshima, K.; Nakatani, Y.; Fujisawa, T.; et al. Synchronous Alterations of Wnt and EGFR Signaling Pathways through Aberrant Methylation and Mutation in Non-Small Cell Lung Cancer. Jpn. J. Lung Cancer 2009, 49, 416–421. [Google Scholar] [CrossRef][Green Version]
- Pannone, G.; Bufo, P.; Caiaffa, M.F.; Serpico, R.; Lanza, A.; lo Muzio, L.; Rubini, C.; Staibano, S.; Petruzzi, M.; de Benedictis, M.; et al. Cyclooxygenase-2 Expression in Oral Squamous Cell Carcinoma. Int. J. Immunopathol. Pharmacol. 2004, 17, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pannone, G.; Sanguedolce, F.; de Maria, S.; Farina, E.; lo Muzio, L.; Serpico, R.; Emanuelli, M.; Rubini, C.; de Rosa, G.; Staibano, S.; et al. Cyclooxygenase Isozymes in Oral Squamous Cell Carcinoma: A Real-Time RT-PCR Study with Clinic Pathological Correlations. Int. J. Immunopathol. Pharmacol. 2007, 20, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, A.; Ferri, C.; Mezzetti, A.; Cipollone, F. COX-2: Friend or Foe? Curr. Pharm. Des. 2007, 13, 1715–1721. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What Are Omics Sciences? In Periparturient Diseases of Dairy Cows; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–7. [Google Scholar]
- Shaw, A.K.; Garcha, V.; Shetty, V.; Vinay, V.; Bhor, K.; Ambildhok, K.; Karande, P. Diagnostic Accuracy of Salivary Biomarkers in Detecting Early Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2022, 23, 1483–1495. [Google Scholar] [CrossRef]
- Walsh, T.; Macey, R.; Kerr, A.R.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S. Diagnostic Tests for Oral Cancer and Potentially Malignant Disorders in Patients Presenting with Clinically Evident Lesions. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Hema Shree, K.; Ramani, P.; Sherlin, H.; Sukumaran, G.; Jeyaraj, G.; Don, K.R.; Santhanam, A.; Ramasubramanian, A.; Sundar, R. Saliva as a Diagnostic Tool in Oral Squamous Cell Carcinoma—A Systematic Review with Meta Analysis. Pathol. Oncol. Res. 2019, 25, 447–453. [Google Scholar] [CrossRef]
- Riccardi, G.; Bellizzi, M.G.; Fatuzzo, I.; Zoccali, F.; Cavalcanti, L.; Greco, A.; de Vincentiis, M.; Ralli, M.; Fiore, M.; Petrella, C.; et al. Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview. Proteomes 2022, 10, 37. [Google Scholar] [CrossRef]
- Arroyo, E.; Donís, S.P.; Petronacci, C.M.C.; Alves, M.G.O.; Mendía, X.M.; Fernandes, D.; Pouso, A.I.L.; Bufalino, A.; Bravo López, S.; Sayáns, M.P. Usefulness of Protein-Based Salivary Markers in the Diagnosis of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Cancer Biomark. 2021, 32, 411–424. [Google Scholar] [CrossRef]
- Airoldi, M.; Piantino, P.; Pacchioni, D.; Mastromatteo, V.; Pedani, F.; Gandolfo, S. Gastrointestinal Cancer-Associated Antigen (GICA) in Oral Carcinoma. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 263–267. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, L.; Yuan, W.; Xu, J.; Yu, X.; Wang, F.; Sun, L.; Zeng, Y. Clinical Value of Naa10p and CEA Levels in Saliva and Serum for Diagnosis of Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2018, 47, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, K.; Ramya, R.; Nandhini, G.; Rajashree, P.; Ramesh Kumar, A.; Nirmala Anandan, S. Salivary and Serum Level of CYFRA 21-1 in Oral Precancer and Oral Squamous Cell Carcinoma. Oral Dis. 2015, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N. Role of Salivary Biomarkers in Early Detection of Oral Squamous Cell Carcinoma. Indian J. Pathol. Microbiol. 2017, 60, 464–468. [Google Scholar] [CrossRef]
- Lucchese, A.; Mittelman, A.; Tessitore, L.; Serpico, R.; Sinha, A.A.; Kanduc, D. Proteomic Definition of a Desmoglein Linear Determinant Common to Pemphigus Vulgaris and Pemphigus Foliaceous. J. Transl. Med. 2006, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pandey, R.; Anthony, E.R.; Chandra, S.; Mehrotra, D. Expression and bioinformatics analyses show HSP70 complements BCL2 action in oral carcinogenesis. J. Oral Biol. Craniofacial Res. 2022, 12, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Viveka, T.S.; Arvind, K.; Shyamsundar, V.; Kanchan, M.; Alex, S.A.; Chandrasekaran, N.; Vijayalakshmi, R.; Mukherjee, A. A facile gold nanoparticle-based ELISA system for detection of osteopontin in saliva: Towards oral cancer diagnostics. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 477, 166–172. [Google Scholar] [CrossRef] [PubMed]
- AlAli, A.M.; Walsh, T.; Maranzano, M. CYFRA 21-1 and MMP-9 as Salivary Biomarkers for the Detection of Oral Squamous Cell Carcinoma: A Systematic Review of Diagnostic Test Accuracy. Int. J. Oral Maxillofac. Surg. 2020, 49, 973–983. [Google Scholar] [CrossRef]
- Elmahgoub, F. Could Salivary Biomarkers Be Useful in the Early Detection of Oral Cancer and Oral Potentially Malignant Disorders, and Is There a Relationship between These Biomarkers and Risk Factors? Evid. Based Dent. 2022, 23, 30–31. [Google Scholar] [CrossRef]
- Korostoff, A.; Reder, L.; Masood, R.; Sinha, U.K. The Role of Salivary Cytokine Biomarkers in Tongue Cancer Invasion and Mortality. Oral Oncol. 2011, 47, 282–287. [Google Scholar] [CrossRef]
- Stott-Miller, M.; Houck, J.R.; Lohavanichbutr, P.; Méndez, E.; Upton, M.P.; Futran, N.D.; Schwartz, S.M.; Chen, C. Tumor and Salivary Matrix Metalloproteinase Levels Are Strong Diagnostic Markers of Oral Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2628–2636. [Google Scholar] [CrossRef]
- Smriti, K.; Ray, M.; Chatterjee, T.; Shenoy, R.-P.; Gadicherla, S.; Pentapati, K.-C.; Rustaqi, N. Salivary MMP-9 as a Biomarker for the Diagnosis of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Thiruvalluvan, A.; Reddy, J.R.C.; Sekizhar, V.; Subramanyam, V.; Sivasankari, T.; Varsha, V. Estimation of Salivary Matrix Metalloproteinase-9 in Oral Leukoplakia, Oral Submucous Fibrosis, and Healthy Individuals: A Comparative Observational Study. J. Stomatol. 2021, 74, 221–226. [Google Scholar] [CrossRef]
- Rakesh, N.; Nagesh, K.S.; Iyengar, A.R.; Patil, D.B. Inflammation in Oral Cancer and Role of COX-2: A Review. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 319–329. [Google Scholar]
- Yang, W.-H.; Wang, S.-J.; Chang, Y.-S.; Su, C.-M.; Yang, S.-F.; Tang, C.-H. Association of Resistin Gene Polymorphisms with Oral Squamous Cell Carcinoma Progression and Development. Biomed Res. Int. 2018, 2018, 9531315. [Google Scholar] [CrossRef] [PubMed]
- Sodnom-Ish, B.; Eo, M.Y.; Myoung, H.; Lee, J.H.; Kim, S.M. Next Generation Sequencing-Based Salivary Biomarkers in Oral Squamous Cell Carcinoma. J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 3–12. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, W.; Ye, P.; Wu, M. Preoperative circulating fibrinogen to albumin ratio in predicting 5-year prognosis of oral cancer radical surgery. Neoplasma 2022, 69, 1246–1252. [Google Scholar] [CrossRef]
- Barone, B.; Napolitano, L.; Reccia, P.; De Luca, L.; Morra, S.; Turco, C.; Melchionna, A.; Caputo, V.F.; Cirillo, L.; Fusco, G.M.; et al. Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A MonocentricRetrospective Study. Medicina 2022, 58, 1490. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Simeone, P.; Bologna, G.; Pieragostino, D.; Cufaro, M.C.; Graziano, V.; Peri, M.; Di Marino, P.; De Tursi, M.; et al. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J. Oncol. 2019, 18, 5879616, Erratum in J. Oncol. 2020, 31, 8947367. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Ding, Y.; Xiao, B.; Wang, X.; Ali, M.M.; Ma, L.; Xie, Z.; Gu, Z.; Chen, G.; et al. Proteomic and phosphoproteomic landscape of salivary extracellular vesicles to assess OSCC therapeutical outcomes. Proteomics 2022, 27, e2200319. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Costela-Ruiz, V.J.; García-Recio, E.; Olmedo-Gaya, M.V.; Ruiz, C.; Reyes-Botella, C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 12215. [Google Scholar] [CrossRef]
- de Paula Silva, E.; Marti, L.C.; Andreghetto, F.M.; de Sales, R.O.; Hoberman, M.; Dos Santos Dias, B.; Diniz, L.F.A.; Dos Santos, A.M.; Moyses, R.A.; Curioni, O.A.; et al. Extracellular vesicles cargo from head and neck cancer cell lines disrupt dendritic cells function and match plasma microRNAs. Sci. Rep. 2021, 11, 18534. [Google Scholar] [CrossRef] [PubMed]
- Victoria Martinez, B.; Dhahbi, J.M.; Nunez Lopez, Y.O.; Lamperska, K.; Golusinski, P.; Luczewski, L.; Kolenda, T.; Atamna, H.; Spindler, S.R.; Golusinski, W.; et al. Circulating small non-coding RNA signature in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 19246–19263. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A. Plasma circulating tumor DNA as a molecular marker for oral cancer. Oral Oncol. 2022, 130, 105926. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Chen, S.; Liu, J.; Cao, W.; Zhang, D.; Feng, C. Circulating tumor cells in blood as a prognostic biomarker in tongue squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 213–219. [Google Scholar] [CrossRef]
- Qayyumi, B.; Bharde, A.; Aland, G.; D’Souza, A.; Jayant, S.; Singh, N.; Tripathi, S.; Badave, R.; Kale, N.; Singh, B.; et al. Circulating tumor cells as a predictor for poor prognostic factors and overall survival in treatment naïve oral squamous cell carcinoma patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 73–83. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The Role of Oral Microbiome in Respiratory Health and Diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Pandey, D.; Szczesniak, M.; Maclean, J.; Yim, H.C.H.; Zhang, F.; Graham, P.; El-Omar, E.M.; Wu, P. Dysbiosis in Head and Neck Cancer: Determining Optimal Sampling Site for Oral Microbiome Collection. Pathogens 2022, 11, 1550. [Google Scholar] [CrossRef]
- Kamer, A.R.; Fortea, J.O.; Videla, S.; Mayoral, A.; Janal, M.; Carmona-Iragui, M.; Benejam, B.; Craig, R.G.; Saxena, D.; Corby, P.; et al. Periodontal Disease’s Contribution to Alzheimer’s Disease Progression in Down Syndrome. Alzheimers Dement. 2016, 2, 49–57. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Contaldo, M.; di Stasio, D.; Romano, A.; Fiori, F.; della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr. Drug Deliv. 2022, 19, 441–456. [Google Scholar] [CrossRef]
- Pinke, K.H.; Freitas, P.; Viera, N.A.; Honório, H.M.; Porto, V.C.; Lara, V.S. Decreased production of proinflammatory cytokines by monocytes from individuals presenting Candida-associated denture stomatitis. Cytokine 2016, 77, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rossano, F.; Gombos, F.; Liguori, G.; Gaeta, G.M.; Esposito, C.; Serpico, R.; di Nunzio, C.; Marinelli, A.; Marinelli, P. Malignant Transformation of Oral Lichen Planus by Candida Albicans. Int. J. Immunopathol. Pharmacol. 1993, 6, 125–134. [Google Scholar]
- Guo, Z.C.; Jing, S.L.; Jumatai, S.; Gong, Z.C. Porphyromonas gingivalis promotes the progression of oral squamous cell carcinoma by activating the neutrophil chemotaxis in the tumour microenvironment. Cancer Immunol. Immunother. 2022, 72, 1523–1539. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Su, Y.; Gong, Z.C.; Liu, H. Porphyromonas gingivalis Activation of Tumor-Associated Macrophages via DOK3 Promotes Recurrence of Oral Squamous Cell Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e937126. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhang, L. Role of the Microbiome in Oral Cancer Occurrence, Progression and Therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Pignatelli, P.; Romei, F.M.; Bondi, D.; Giuliani, M.; Piattelli, A.; Curia, M.C. Microbiota and Oral Cancer as a Complex and Dynamic Microenvironment: A Narrative Review from Etiology to Prognosis. Int. J. Mol. Sci. 2022, 23, 8323. [Google Scholar] [CrossRef]
- Nie, F.; Wang, L.; Huang, Y.; Yang, P.; Gong, P.; Feng, Q.; Yang, C. Characteristics of Microbial Distribution in Different Oral Niches of Oral Squamous Cell Carcinoma. Front. Cell Infect. Microbiol. 2022, 12, 905653. [Google Scholar] [CrossRef]
- Yaegaki, K. Oral Malodorous Compounds Are Periodontally Pathogenic and Carcinogenic. Jpn. Dent. Sci. Rev. 2008, 44, 100–108. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shao, Z.; Liu, K.; Zhou, X.; Wang, L.; Jiang, E.; Luo, T.; Shang, Z. Salivary Porphyromonas gingivalis Predicts Outcome in Oral Squamous Cell Carcinomas: A Cohort Study. BMC Oral Health 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging Roles of Lactic Acid Bacteria in Protection against Colorectal Cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Muto, M.; Hitomi, Y.; Ohtsu, A.; Shimada, H.; Kashiwase, Y.; Sasaki, H.; Yoshida, S.; Esumi, H. Acetaldehyde Production by Non-Pathogenic Neisseria in Human Oral Microflora: Implications for Carcinogenesis in Upper Aerodigestive Tract. Int. J. Cancer 2000, 88, 342–350. [Google Scholar] [CrossRef]
- Csősz, É.; Lábiscsák, P.; Kalló, G.; Márkus, B.; Emri, M.; Szabó, A.; Tar, I.; Tőzsér, J.; Kiss, C.; Márton, I. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE 2017, 12, e0177282. [Google Scholar] [CrossRef]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The pivotal role of oral microbiota in health and disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Nastri, L.; Donnarumma, G.; Porzio, C.; de Gregorio, V.; Tufano, M.A.; Caruso, F.; Mazza, C.; Serpico, R. Effects of Toluidine Blue-Mediated Photodynamic Therapy on Periopathogens and Periodontal Biofilm: In Vitro Evaluation. Int. J. Immunopathol. Pharmacol. 2010, 23, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- di Stasio, D.; Romano, A.; Gentile, C.; Maio, C.; Lucchese, A.; Serpico, R.; Paparella, R.; Minervini, G.; Candotto, V.; Laino, L. Systemic and Topical Photodynamic Therapy (PDT) on Oral Mucosa Lesions: An Overview. J. Biol. Regul. Homeost. Agents 2018, 32, 123–126. [Google Scholar] [PubMed]
- Romano, A.; di Stasio, D.; Gentile, E.; Petruzzi, M.; Serpico, R.; Lucchese, A. The Potential Role of Photodynamic Therapy in Oral Premalignant and Malignant Lesions: A Systematic Review. J. Oral Pathol. Med. 2021, 50, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; di Stasio, D.; Lauritano, D.; Lajolo, C.; Fiori, F.; Gentile, E.; Lucchese, A. Topical Photodynamic Therapy in the Treatment of Benign Oral Mucosal Lesions: A Systematic Review. J. Oral Pathol. Med. 2021, 50, 639–648. [Google Scholar] [CrossRef] [PubMed]
- di Stasio, D.; Romano, A.; Russo, D.; Fiori, F.; Laino, L.; Caponio, V.C.A.; Troiano, G.; Muzio, L.L.; Serpico, R.; Lucchese, A. Photodynamic Therapy Using Topical Toluidine Blue for the Treatment of Oral Leukoplakia: A Prospective Case Series. Photodiagn. Photodyn. Ther. 2020, 31, 101888. [Google Scholar] [CrossRef]
- Romano, A.; Contaldo, M.; della Vella, F.; Russo, D.; Lajolo, C.; Serpico, R.; di Stasio, D. Topical Toluidine Blue-Mediated Photodynamic Therapy for the Treatment of Oral Lichen Planus. J. Biol. Regul. Homeost. Agents 2019, 33, 27–34. [Google Scholar]
- Mittelman, A.; Lucchese, A.; Sinha, A.A.; Kanduc, D. Monoclonal and Polyclonal Humoral Immune Response to EC HER-2/Neu Peptides with Low Similarity to the Host’s Proteome. Int. J. Cancer 2002, 98, 741–747. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Barati, M.; Jabbari, M.; Abdi Ghavidel, A.; Nikmehr, P.; Arzhang, P.; Aynehchi, A.; Babashahi, M.; Mosharkesh, E.; Roshanravan, N.; Shabani, M.; et al. The Engineered Probiotics for the Treatment of Chronic Diseases: A Systematic Review. J. Food Biochem. 2022, 46, e14343. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Gaur, S. Probiotics as Multifaceted Oral Vaccines against Colon Cancer: A Review. Front. Immunol. 2022, 13, 1002674. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-Y.; Chen, Q.-W.; Fu, Z.-J.; Cheng, S.-X.; Zhang, X.-Z. Probiotic Spore-Based Oral Drug Delivery System for Enhancing Pancreatic Cancer Chemotherapy by Gut-Pancreas-Axis-Guided Delivery. Nano Lett. 2022, 22, 8608–8617. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, S.; Konishi, H.; Tanaka, H.; Yamamura, C.; Moriichi, K.; Ogawa, N.; Fujiya, M. Probiotic-Derived Heptelidic Acid Exerts Antitumor Effects on Extraintestinal Melanoma through Glyceraldehyde-3-Phosphate Dehydrogenase Activity Control. BMC Microbiol. 2022, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Papale, F.; Santonocito, S.; Polizzi, A.; Giudice, A.L.; Capodiferro, S.; Favia, G.; Isola, G. The New Era of Salivaomics in Dentistry: Frontiers and Facts in the Early Diagnosis and Prevention of Oral Diseases and Cancer. Metabolites 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Laronde, D.; Palcic, B.; Guillaud, M. The role of DNA image cytometry in screening oral potentially malignant lesions using brushings: A systematic review. Oral Oncol. 2019, 96, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Teh, M.-T.; Ma, H.; Liang, Y.-Y.; Solomon, M.C.; Chaurasia, A.; Patil, R.; Tekade, S.A.; Mishra, D.; Qadir, F.; Yeung, J.-Y.S.; et al. Molecular Signatures of Tumour and Its Microenvironment for Precise Quantitative Diagnosis of Oral Squamous Cell Carcinoma: An International Multi-Cohort Diagnostic Validation Study. Cancers 2022, 14, 1389. [Google Scholar] [CrossRef]

| Procedure | First Author (Year). Title. Country | Aims of the Study | Patients | Main Findings |
|---|---|---|---|---|
| Noninvasive imaging, reflectance confocal microscopy (RCM) | Contaldo et al. (2020). Intraoral Confocal Microscopy of Suspicious Oral Lesions: A Prospective Case Series. Italy [112] | To describe RCM cytoarchitectural findings in oral mucosae affected by OSCC and its precursors | Thirty oral sites in 21 patients with suspicious lesions; RCM vs. conventional histology | In vivo RCM was able to detect the main cytological and histological signs of oral malignancies
|
| Noninvasive imaging, reflectance confocal microscopy (RCM) | Peterson et al. (2019). Feasibility of a Video-Mosaicking Approach to Extend the Field-of-View For Reflectance Confocal Microscopy in the Oral Cavity In Vivo. USA [116] | To test a video-mosaicking approach to extend the field of view for intraoral RCM imaging | Oral sites from four healthy volunteers (normal oral mucosa), one patient (with an amalgam tattoo), and twenty OSCC patients were video-recorded by RCM to extend the field of view, compared with single-frame images | The video-mosaicking allowed appreciation of the whole lesions and their boundaries with a wider field of view than the classical single frames |
| Noninvasive imaging, fluorescent confocal endomicroscopy (FEM) | Abbaci et al. (2022). Diagnostic Accuracy of in Vivo Early Tumor Imaging from Probe-Based Confocal Laser Endomicroscopy versus Histologic Examination in Head and Neck Squamous Cell Carcinoma. France [114] | To assess the diagnostic performance of in vivo FEM in improving the management of early HNSCC | Forty-four patients with early head and neck lesions. FEM vs. conventional histology |
|
| Noninvasive imaging, optical coherence tomography (OCT) | Yuan et al. (2022). Noninvasive Oral Cancer Screening Based on Local Residual Adaptation Network Using Optical Coherence Tomography. China [120] | To test a novel deep learning method for noninvasive oral cancer screening on OCT images | Twenty noncancerous OCT images and one hundred and forty-four cancerous images were considered from 25 patients |
|
| Noninvasive imaging, intraoral ultrasonography (ioUS) | Di Stasio et al. (2022). High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Italy [123] | To describe the qualitative characteristics of tongue squamous cell carcinoma images obtained with high-definition intraoral ultrasound by comparing them with the corresponding histopathological sample | Twenty patients with tongue SCC were imaged by ioUS | Io-US was able to distinguish the tumor from the homogenous composition of the tongue tissues and the tumor margins |
| Noninvasive imaging, intraoral ultrasonography (ioUS) | De Koning et al. Application and Accuracy of Ultrasound-Guided Resections of Tongue Cancer (2022). The Netherlands. [125] | To test ultrasound (US)-guided surgical removal of squamous cell carcinoma of the tongue (SCCT) | Forty patients with SCCT underwent US-guided SCCT during surgery and the results were compared with ninety-six tongue cancer patients who had undergone conventional surgery | US-guided SCCT resections improve margin status and reduce the frequency of adjuvant radiotherapy. In the US cohort, the frequency of free margin status was significantly higher than in the conventional cohort, and the frequency of positive margin status was significantly lower |
| Noninvasive imaging, intraoral ultrasonography (ioUS) | Nilsson et al. Ultrasound Accurately Assesses Depth of Invasion in T1–T2 Oral Tongue Cancer (2022). Sweden [126] | To investigate the assessment of DOI using ultrasounds (US-DOI) | The DOI was assessed in 40 patients with T1–T3 SCCT by ultrasound, palpation, computed tomography, (CT) and magnetic resonance imaging (MRI). Histological DOI (pDOI) was gold standard | Ultrasound seems to be the most accurate method to assess DOI in T1–T2 SCCT. MRI overestimates DOI and cannot assess a substantial proportion of the tumors |
| Noninvasive imaging, intraoral ultrasonography (ioUS) | Caprioli et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations (2022). Italy [127] | To investigate the accuracy of ioUS in the assessment of the DOI in early OSCC (CIS, pT1, and pT2) compared with conventional histological DOI | Forty-one patients with tongue SCCs (CIS-T2) underwent a preoperative high-frequency US and the US-DOI was compared with the histological DOI (pDOI) and MRI-DOI | The ioUS was significantly accurate at determining the T stage
|
| Noninvasive imaging, intraoral ultrasonography (ioUS) | Nogami et al. (2022). The Accuracy of Ultrasound and Magnetic Resonance Imaging for Estimating Thickness of Oral Tongue Squamous Cell Carcinoma and Influence of Biopsy on Those Findings. The Netherlands [128] | To compare the accuracy of MRI and ioUS to estimate DOI compared with their histological DOI (pDOI) | Eighty-three patients with tongue cancer underwent MRI and one hundred and seven ioUS. All MRI-DOIs and ioUS-DOIs were compared with their pDOI | ioUS-DOI is close to the pDOI in tongue cancers with DOI up to 10 mm. ioUS tends to underestimate DOI in tumors > 10 mm DOI. MRI tends to overestimate DOI in both thin and thick tumors |
| Noninvasive imaging, intraoral ultrasonography (ioUS) | Yesuratnam et al. Preoperative evaluation of oral tongue squamous cell carcinoma with intraoral ultrasound and magnetic resonance imaging-comparison with histopathological tumour thickness and accuracy in guiding patient management (2013). Australia [131] | To compare the accuracy of MRI and ioUS to estimate tumor thickness (TT) compared with their histological TT (pTT) | Eighty-eight patients with the presumptive diagnosis of invasive tongue SCC were analyzed. Seventy-nine patients had preoperative US and eighty-one had MRI | ioUS-TT demonstrated high correlation and MRI-TT moderate correlation with pTT |
| Noninvasive imaging, narrow-band imaging (NBI) | Contaldo et al. (2017). Evaluation of the Intraepithelial Papillary Capillary Loops in Benign & Malignant Oral Lesions by in Vivo Virtual Chromoendoscopic Magnification: A Preliminary Study. Italy [138] | To establish NBI’s feasibility to visualize and distinguish the intraepithelial papillary capillary loop (IPCL) patterns of benign oral pathologies from malignant ones | Benign lesions or OSCC from thirty-one patients were imaged by NBI before surgery and the IPCL classified according to their features into a four-class system | IPCL type IV was found only in malignancies
|
| Noninvasive imaging, narrow-band imaging (NBI) | Nair et al. Narrow Band Imaging Observed Oral Mucosa Microvasculature as a Tool to Detect Early Oral Cancer: An Indian Experience. India [140] | To compare the capability of NBI and white light in defining the nature of clinically suspicious lesions | Fifty patients with suspicious malignant/premalignant lesions underwent white light imaging (WLI) and NBI to assess their IPCLs and were then compared with the standard histopathology |
|
| Noninvasive imaging, narrow-band imaging (NBI) | Ota et al. Diagnostic Accuracy of High-Grade Intraepithelial Papillary Capillary Loops by Narrow Band Imaging for Early Detection of Oral Malignancy: A Cross-Sectional Clinicopathological Imaging Study. Japan [141] | To clarify the advantages and disadvantages of conventional visual inspection (CVI), endoscopic white light imaging (WLI), and narrow-band imaging (NBI) and to examine the diagnostic accuracy of intraepithelial papillary capillary loops (IPCLs) for the detection of OSCC | Sixty participants with oral mucosal diseases suspected of having oral potentially malignant disorders (OPMDs) or OSCC underwent CVI, WLI, NBI, and incisional biopsy. Images were evaluated to assess the lesion size, color, texture, and IPCLs |
|
| Noninvasive imaging, narrow-band imaging (NBI) | Sachdeva et al. (2022). A Prospective Study to Evaluate the Role of Narrow Band Imaging and Toludine Blue in the Screening of Premalignant and Malignant Lesions of the Oral Cavity in a Tertiary Referral Centre. India [142] | To compare NBI and toluidine blue (TB) for OSCC screening | Forty-four patients with suspicious oral cavity lesions (premalignant and malignant) underwent NBI and TB screening |
|
| Noninvasive imaging, narrow-band imaging (NBI) | De Wit et al. (2022). Comparison of Narrow Band and Fluorescence Molecular Imaging to Improve Intraoperative Tumour Margin Assessment in Oral Cancer Surgery. the Netherlands [143] | To compare NBI with fluorescence molecular imaging (FMI), to study which intraoperative technique best assesses the mucosal tumor margins | Sixteen patients were halved into a group receiving NBI and another FMI. FMI was an ex vivo procedure, after patients intravenously received cetuximab, two days before surgery | Ex vivo FMI performed more accurately than in vivo NBI in mucosal margin assessment, mainly because NBI cannot detect submucosal extension. NBI adequately identified the mucosal margin especially in early-stage and not previously irradiated tumors |
| Marker, Clinical Relevance | First Author (Year). Title. Country | Aims of the Study | Methods | Main Findings | Conclusions |
|---|---|---|---|---|---|
| E-cadherin, vimentin prognostic relevance | Puneeta et al. (2022). Evaluation of E-Cadherin and Vimentin Expression for Different Grades of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma—An Immunohistochemical Study. India [144] | To evaluate the expression of vimentin and E-cadherin in different grades of oral epithelial dysplasias (OEDs) and oral squamous cell carcinoma (OSCC) | Immunoistochemical (IHC) analysis of E-cadherin and vimentin expression in H&E-stained specimens from 5 normal oral mucosa, 60 oral epithelial dysplasias (OEDs), and 60 different grades of OSCC |
| The E-cadherin downregulation and vimentin neoexpression in epithelial cells are indicators of progression from OED to OSCC and OSCC’s potential to metastatize |
| E-cadherin diagnostic relevance | Khan et al. (2022). E-Cadherin as a Prognostic Biomarker in Oral Squamous Cell Carcinoma: A Pilot Study at Tertiary Care Hospital. India [145] | To investigate the expression of E-cadherin in different grades and stages of OSCC and to elucidate its role as a reliable and potential marker | Immunoistochemical (IHC) analysis of E-cadherin and vimentin expression in H&E-stained specimens from 50 specimens of OSCC |
| OSCC shows E-cadherin delocalized in cytoplasm. E-cadherin expression depended on histological grading of OSCC. It was also found that as the tumor grade increased, there was decrease in membranous positivity. However, there was no correlation with the stage of disease |
| P-cadherin prognostic relevance | Lo Muzio et al. (2005). P-Cadherin Expression and Survival Rate in Oral Squamous Cell Carcinoma: An Immunohistochemical Study. Italy [146] | To assess the prevalence of P-cad expression in oral squamous cell carcinoma (OSCC) and to verify whether P-cad can be considered a marker of prognosis in patients with OSCC | Immunoistochemical (IHC) analysis of E-cadherin and vimentin expression in H&E-stained specimens from 67 specimens of OSCC |
| It is possible to suggest P-cad as an early marker of poor prognosis. The abnormal or lack of P-cad expression could constitute a hallmark of aggressive biological behavior in OSCC |
| E-cadherin prognostic relevance | Pannone et al. (2014). The Role of E-Cadherin down-Regulation in Oral Cancer: CDH1 Gene Expression and Epigenetic Blockage. Italy [148] | To investigate E-cadherin gene (CDH1) promoter methylation status in OSCC and its correlation with E-cadherin protein expression, clinicopathological characteristics, and patient outcome | Histologically proven OSCC and paired normal mucosa were analyzed for CDH1 promoter methylation status and E-cadherin protein expression by methylation-specific polymerase chain reaction and immunohistochemistry. Co-localization of E-cadherin with epidermal growth factor (EGF) receptor (EGFR) was evidenced by confocal microscopy and by immunoprecipitation analyses | E-cadherin downregulation and delocalization from the membrane to the cytoplasm in cancer cells is correlated to aggressive, poorly differentiated, high-grade OSCC and lower patient survival. Protein downregulation appeared to be due to E-cadherin mRNA downregulation and CDH1 promoter hypermethylation | Low E-cadherin expression is a negative prognostic factor of OSCC and is likely due to the hypermethylation of CDH1 promoter. The delocalization of E-cadherin from membrane to cytoplasm could be also due to the increased expression |
| Integrins and ECM components | Giannelli et al. (2001). Altered Expression of Integrins and Basement Membrane Proteins in Malignant and Pre-Malignant Lesions of Oral Mucosa. Italy [147] | To study the expression of integrins and ECM components (laminin-1, laminin-5, and collagen IV) of the BM in OSCC and OPMDs | IHC analysis on frozen specimens of OSCC, leucoplakia, and oral lichen planus |
In OSCC, integrins’ polarity and distribution were altered. Ln-1, Ln-5, and Coll IV were discontinuous and interrupted in invasive SCC, whereas they were normal in the in situ carcinoma. In both premalignant lesions and lichen planus specimens, integrins were expressed in a polarized manner in the presence of a normal BM, whereas they were abnormally distributed in those tissues with altered staining patterns of the ECM components | Abnormal redistribution of integrins and expression of ECM components such as Ln-5 could play an important role in SCC invasion and metastasis |
| EGFR | Chung et al. (2006). Increased Epidermal Growth Factor Receptor Gene Copy Number Is Associated with Poor Prognosis in Head and Neck Squamous Cell Carcinomas. USA [149] | To establish if high epidermal growth factor receptor (EGFR) gene copy number is associated with poor prognosis in HNSCC | GFR status was analyzed in 86 tumor samples from 82 HNSCC patients by fluorescent in situ hybridization (FISH) to determine EGFR gene copy number, by polymerase chain reaction and direct sequencing for activating mutations, and by DNA microarray and immunohistochemistry for RNA and protein expression |
| High EGFR gene copy number by FISH is frequent in HNSCC and is a poor prognostic indicator. Additional investigation is indicated to determine the biologic significance and implications for EGFR inhibitor therapies in HNSCC |
| WNT pathway | Pannone et al. (2010). WNT Pathway in Oral Cancer: Epigenetic Inactivation of WNT-Inhibitors. Italy. [156] | To demonstrate the role of WNT pathway activation irrespective of beta-catenin mutation in oral cancerogenesis | Methylation-specific PCR (MSP) was used to study the methylation status of a complete panel of genes (SFRP-1, SFRP-2, SFRP-4, SFRP-5, WIF-1, DKK-3) involved in WNT pathway in thirty-seven cases of formalin-fixed, paraffin-embedded OSCC with relative controls of normal oral epithelium |
| Epigenetic activation of some genes may activate WNT pathway in oral cancer |
| c-Met | Lo Muzio et al. (2004). Scatter Factor Receptor (c-Met) as Possible Prognostic Factor in Patients with Oral Squamous Cell Carcinoma. Italy [160] | To study the biological role of c-Met in oral tumorigenesis | IHC analysis on seventy-three cases of OSCC and ten of normal mucosa for c-Met expression |
| c-Met expression may be useful to identify cases of oral squamous cell carcinoma with a more aggressive and invasive phenotype |
| Bcl-2, c-Myc | Pallavi et al. (2018). Bcl-2 and c-Myc Expression in Oral Dysplasia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study to Assess Tumor Progression. India [161] | To assess the expression of Bcl-2 and c-Myc in OED and OSCC | Thirty OEDs, thirty OSCCs, and ten normal gingiva were immunohistochemically assessed for Bcl-2 and c-Myc distribution, intensity, percentage of positive cells, localization, and immunoreactive scores |
| Variable expression of c-Myc and Bcl-2 reveals that these proteins act in synergism in early phases of carcinogenesis, whereas in later stages, due to the diminished activity of Bcl-2, c-Myc interacts in coordination with other oncogenes contributing to tumor progression |
| Bcl-2 | Lo Muzio et al. (2003). Expression of Bcl-2 in Oral Squamous Cell Carcinoma: An Immunohistochemical Study of 90 Cases with Clinico-Pathological Correlations. Italy [162] | To explore Bcl-2 immunoreactivity in oral cancers and to assess its potential clinicopathological implications | Ninety OSCC and ten normal mucosal formalin-fixed, paraffin-embedded samples were analyzed for Bcl-2 expression by immunohistochemistry |
| Patients with absent or low (scores 0 and 1) Bcl-2 in immunoreactive tumors manifested poorer overall survival rates |
| Cyclooxygenase type 2 (COX-2) | Pannone et al. (2004). Cyclooxygenase-2 Expression in Oral Squamous Cell Carcinoma. Italy [164] | To analyze the expression of COX-2, at the protein level, in OSCC | IHC analysis on 45 OSCC specimens to define the COX-2 expression |
| COX-2 is overexpressed in OSCC |
| Cyclooxygenase type 2 (COX-2) | Pannone et al. (2007). Cyclooxygenase Isozymes in Oral Squamous Cell Carcinoma: A Real-Time RT-PCR Study with Clinic Pathological Correlations. Italy [165] | To measure COX-1 and COX-2 mRNA expression in samples | COX-1 and COX-2 mRNA expression was measured by RT-PCR in 22 patients (OSCC and contralateral healthy mucosa) |
| COX-1 may play a role in oral carcinogenesis and could be regarded as a potential therapeutic target by chemopreventive drugs; moreover, COX-2 expression might be addressed as a new prognostic tool in the clinical management of OSCC |
| Screening/Diagnosis | Prognosis | Follow Up | |
|---|---|---|---|
| Noninvasive imaging | Real-time, surgery-free identification of histological and cellular abnormalities; tumoral neoangiogenesis evaluation; tumor thickness and behavior (infiltrating vs. growing) | Some parameters (cellular pleomorphisms, haploidies, depth of invasion, tumoral vascularization) identified through these methods can correlate with prognostic indicators of OSCC (metastases, survival) | Real-time, surgery-free, multiple control of healing or recurrences |
| Tissue markers | Invasive (requires biopsy) identification of molecules over- or hypoexpressed in OSCC. Not affordable for follow-up purposes (it requires further biopsies) | ||
| Circulating markers (salivary and plasma) and liquid biopsy | Noninvasive (from saliva) or less invasive (from blood) identification and measurement of molecules and circulating tumor cells and tumor DNA predictive of OSCC | Noninvasive (from saliva) or less invasive (from blood) identification and measurements of molecules and circulating tumor cells and tumor DNA associated with prognostic indicators of OSCC (metastases, responsiveness to treatments, survival) | Noninvasive (from saliva) or less invasive (from blood) identification and measurements of molecules and circulating tumor cells and tumor DNA associated with OSCC recurrence or occult metastases |
| Oral microbiota | Identification, characterization, and quantification of microbiota composition peculiar to the tumor microenvironment before OSCC diagnosis, associated with prognostic indicators and for follow-up purposes | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menditti, D.; Santagata, M.; Imola, G.; Staglianò, S.; Vitagliano, R.; Boschetti, C.E.; Inchingolo, A.M. Personalized Medicine in Oral Oncology: Imaging Methods and Biological Markers to Support Diagnosis of Oral Squamous Cell Carcinoma (OSCC): A Narrative Literature Review. J. Pers. Med. 2023, 13, 1397. https://doi.org/10.3390/jpm13091397
Menditti D, Santagata M, Imola G, Staglianò S, Vitagliano R, Boschetti CE, Inchingolo AM. Personalized Medicine in Oral Oncology: Imaging Methods and Biological Markers to Support Diagnosis of Oral Squamous Cell Carcinoma (OSCC): A Narrative Literature Review. Journal of Personalized Medicine. 2023; 13(9):1397. https://doi.org/10.3390/jpm13091397
Chicago/Turabian StyleMenditti, Dardo, Mario Santagata, Gianmaria Imola, Samuel Staglianò, Rita Vitagliano, Ciro Emiliano Boschetti, and Angelo Michele Inchingolo. 2023. "Personalized Medicine in Oral Oncology: Imaging Methods and Biological Markers to Support Diagnosis of Oral Squamous Cell Carcinoma (OSCC): A Narrative Literature Review" Journal of Personalized Medicine 13, no. 9: 1397. https://doi.org/10.3390/jpm13091397
APA StyleMenditti, D., Santagata, M., Imola, G., Staglianò, S., Vitagliano, R., Boschetti, C. E., & Inchingolo, A. M. (2023). Personalized Medicine in Oral Oncology: Imaging Methods and Biological Markers to Support Diagnosis of Oral Squamous Cell Carcinoma (OSCC): A Narrative Literature Review. Journal of Personalized Medicine, 13(9), 1397. https://doi.org/10.3390/jpm13091397








