From Admission to Discharge: Predicting National Institutes of Health Stroke Scale Progression in Stroke Patients Using Biomarkers and Explainable Machine Learning
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Data Description
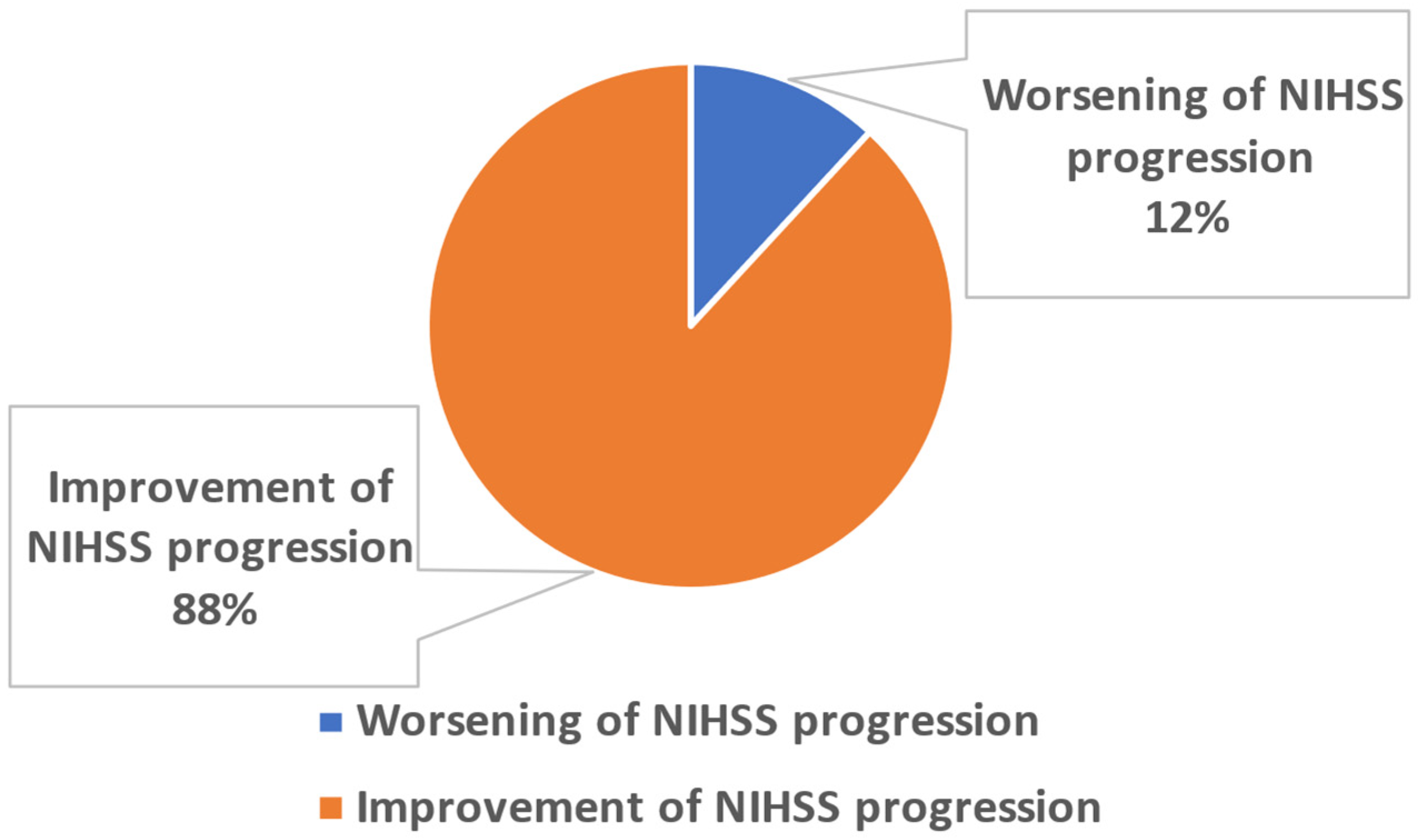
2.3. Problem Definition
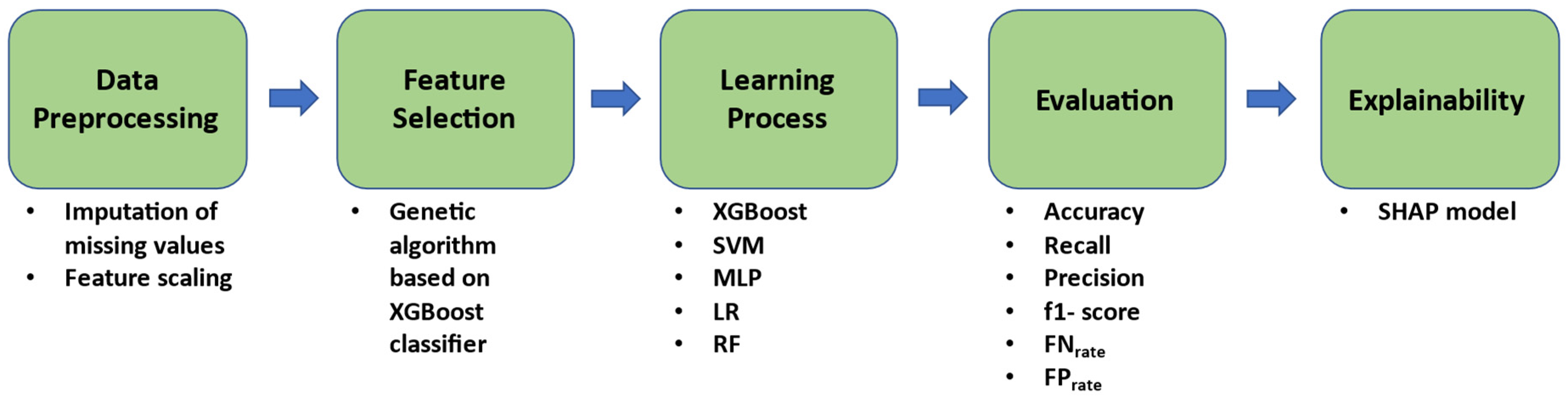
2.4. Proposed Methodology
2.4.1. Pre-Processing
2.4.2. Feature Selection
2.4.3. Learning and Validation Strategy
2.4.4. Explainability
3. Results
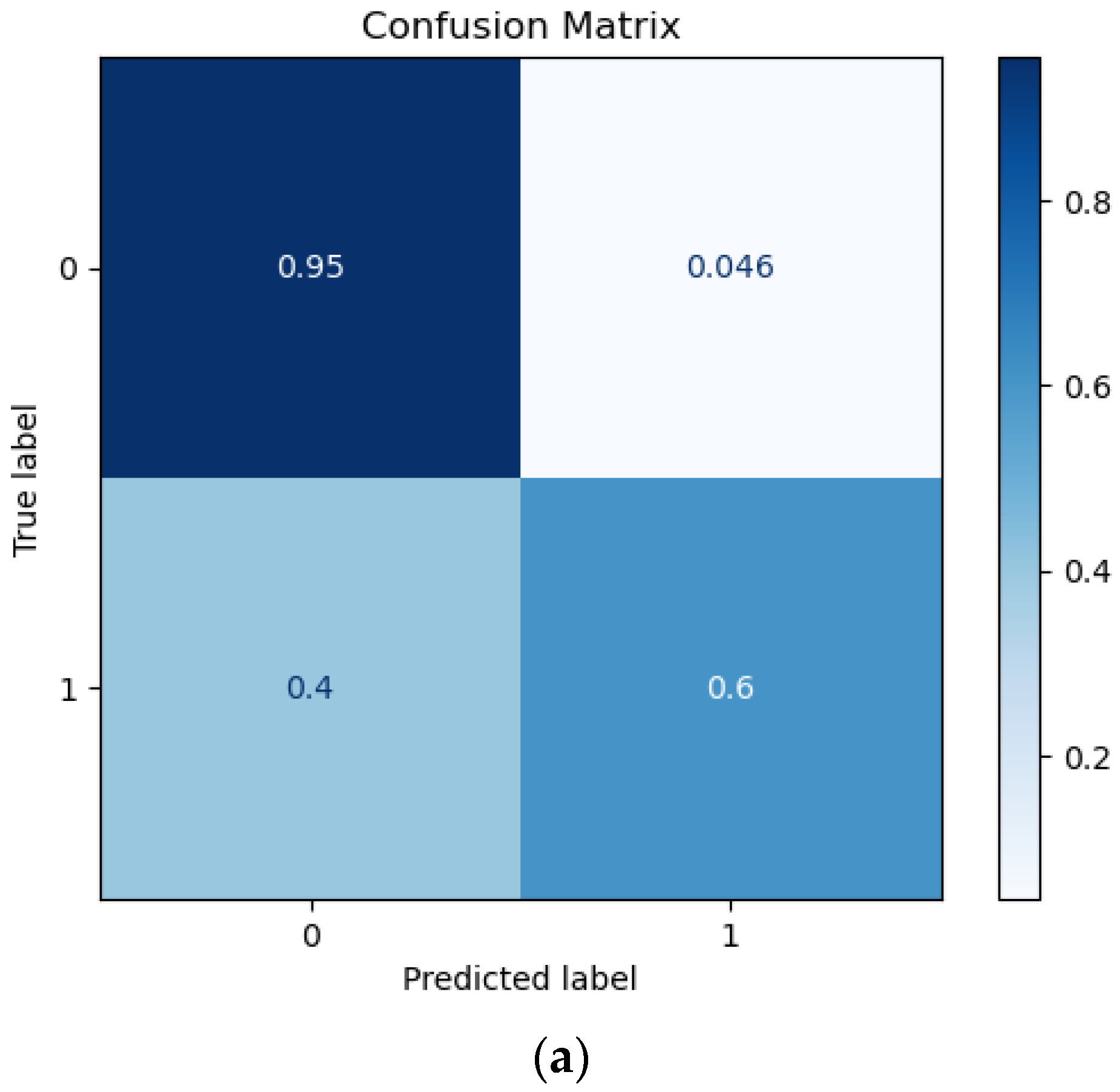
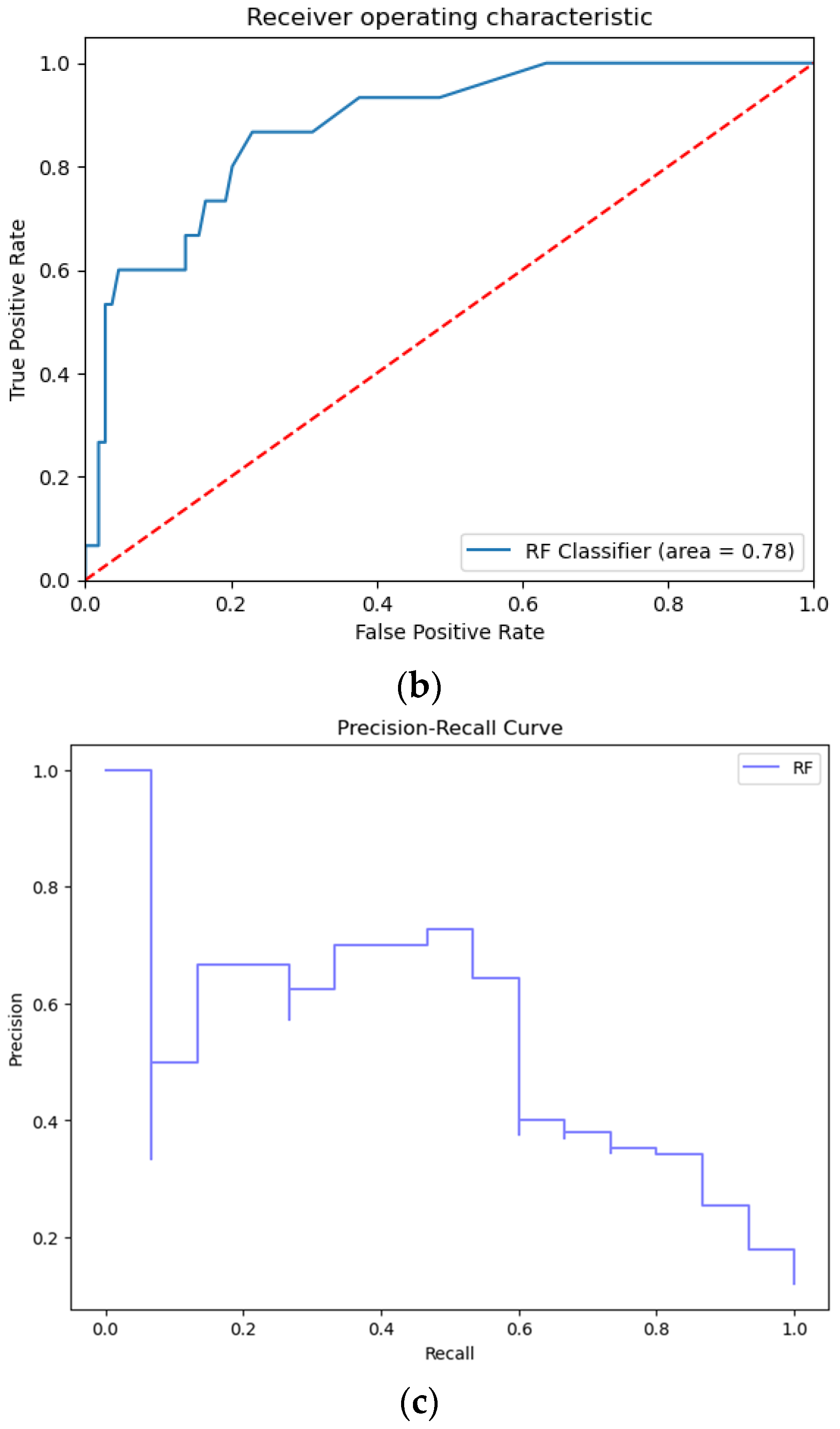
3.1. Prediction Performance
3.2. Selected Features
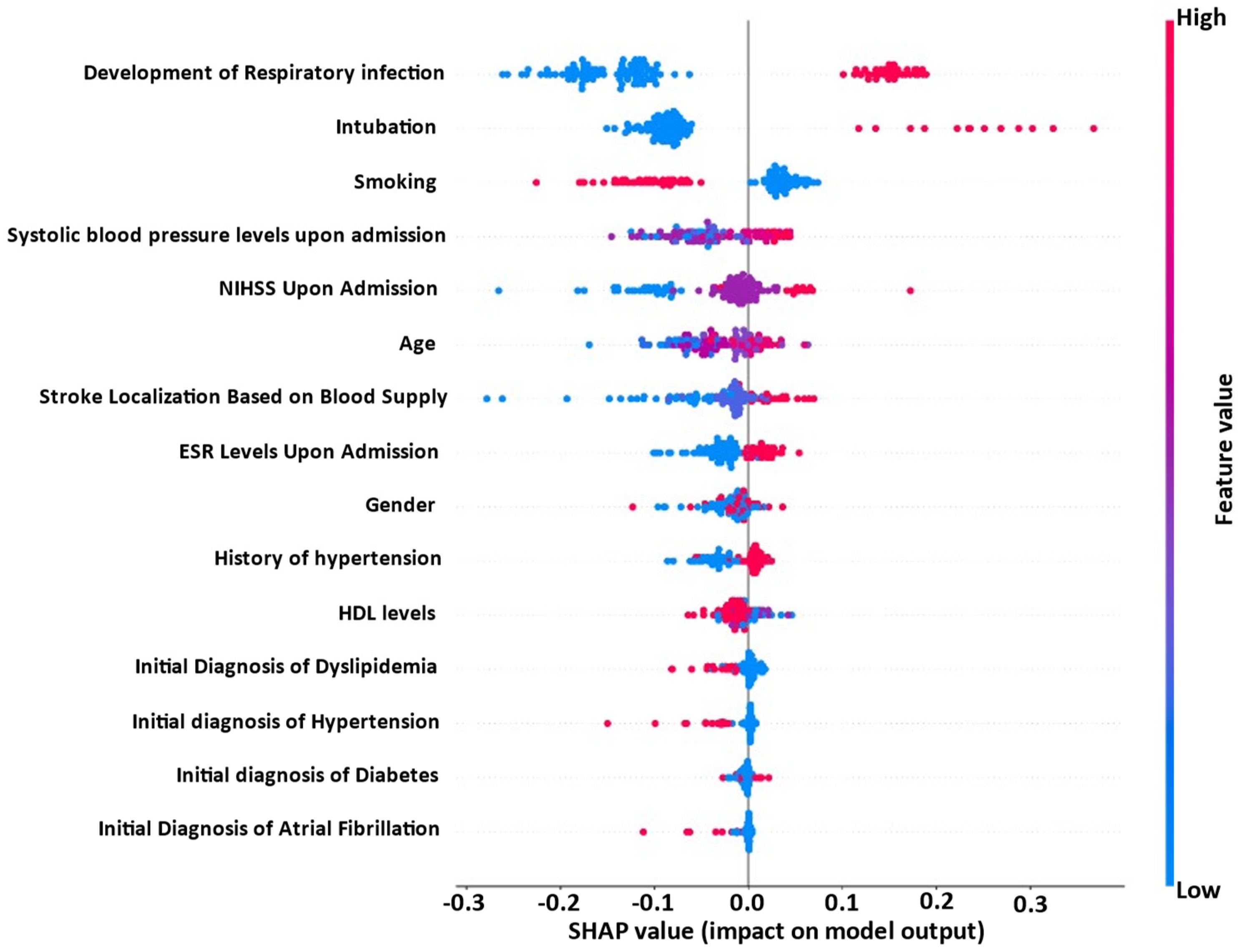
3.3. Explainability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Writing Group Members; Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; et al. Executive Summary: Heart Disease and Stroke Statistics—2010 Update. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Claus, J.J.; Berghout, B.B.; Ikram, M.K.; Wolters, F.J. Validity of stroke severity assessment using medical records in a population-based cohort. J. Stroke Cerebrovasc. Dis. 2023, 32, 106992. [Google Scholar] [CrossRef] [PubMed]
- Appelros, P.; Nydevik, I.; Viitanen, M. Poor Outcome After First-Ever Stroke. Stroke 2003, 34, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-J.; Li, Q.-X.; Liu, T.-J.; Wang, D.-L.; An, Y.-C.; Zhang, J.; Peng, Y.-B.; Chen, R.-Y.; Chang, L.-S.; Wang, Y.; et al. Predictive values of CSS and NIHSS in the prognosis of patients with acute cerebral infarction. Medicine 2018, 97, e12419. [Google Scholar] [CrossRef]
- Rost, N.S.; Bottle, A.; Lee, J.; Randall, M.; Middleton, S.; Shaw, L.; Thijs, V.; Rinkel, G.J.E.; Hemmen, T.M.; the Global Comparators Stroke GOAL Collaborators; et al. Stroke Severity Is a Crucial Predictor of Outcome: An International Prospective Validation Study. J. Am. Heart Assoc. 2016, 5, e002433. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Reeves, M.J.; Smith, E.E.; Saver, J.L.; Zhao, X.; Olson, D.W.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Characteristics, Performance Measures, and In-Hospital Outcomes of the First One Million Stroke and Transient Ischemic Attack Admissions in Get With The Guidelines-Stroke. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 291–302. [Google Scholar] [CrossRef]
- Reeves, M.J.; Grau-Sepulveda, M.V.; Fonarow, G.C.; Olson, D.M.; Smith, E.E.; Schwamm, L.H. Are Quality Improvements in the Get With The Guidelines-Stroke Program Related to Better Care or Better Data Documentation? Circ. Cardiovasc. Qual. Outcomes 2011, 4, 503–511. [Google Scholar] [CrossRef]
- Gkantzios, A.; Kokkotis, C.; Tsiptsios, D.; Moustakidis, S.; Gkartzonika, E.; Avramidis, T.; Aggelousis, N.; Vadikolias, K. Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning. Diagnostics 2023, 13, 532. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Campagnini, S.; Liuzzi, P.; Mannini, A.; Basagni, B.; Macchi, C.; Carrozza, M.C.; Cecchi, F. Cross-validation of predictive models for functional recovery after post-stroke rehabilitation. J. Neuroeng. Rehabil. 2022, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Gkantzios, A.; Tsiptsios, D.; Karatzetzou, S.; Kitmeridou, S.; Karapepera, V.; Giannakou, E.; Vlotinou, P.; Aggelousis, N.; Vadikolias, K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022, 14, 784–803. [Google Scholar] [CrossRef] [PubMed]
- The GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Drozdowska, B.A.; Singh, S.; Quinn, T.J. Thinking About the Future: A Review of Prognostic Scales Used in Acute Stroke. Front. Neurol. 2019, 10, 274. [Google Scholar] [CrossRef]
- Sung, S.; Chen, C.; Pan, R.; Hu, Y.; Jeng, J. Natural Language Processing Enhances Prediction of Functional Outcome After Acute Ischemic Stroke. J. Am. Heart Assoc. 2021, 10, e023486. [Google Scholar] [CrossRef]
- Winters, C.; Kwakkel, G.; van Wegen, E.E.; Nijland, R.H.; Veerbeek, J.M.; Meskers, C.G. Moving stroke rehabilitation forward: The need to change research. NeuroRehabilitation 2018, 43, 19–30. [Google Scholar] [CrossRef]
- Campagnini, S.; Arienti, C.; Patrini, M.; Liuzzi, P.; Mannini, A.; Carrozza, M.C. Machine learning methods for functional recovery prediction and prognosis in post-stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2022, 19, 54. [Google Scholar] [CrossRef]
- Cho, J.S.; Hu, Z.; Fell, N.; Heath, G.W.; Qayyum, R.; Sartipi, M. Hospital Discharge Disposition of Stroke Patients in Tennessee. South. Med. J. 2017, 110, 594–600. [Google Scholar] [CrossRef]
- Luker, J.A.; Bernhardt, J.; Grimmer, K.A.; Edwards, I. A qualitative exploration of discharge destination as an outcome or a driver of acute stroke care. BMC Health Serv. Res. 2014, 14, 193. [Google Scholar] [CrossRef]
- Bacchi, S.; Oakden-Rayner, L.; Menon, D.K.; Jannes, J.; Kleinig, T.; Koblar, S. Stroke prognostication for discharge planning with machine learning: A derivation study. J. Clin. Neurosci. 2020, 79, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Gkantzios, A.; Tsiptsios, D.; Karapepera, V.; Karatzetzou, S.; Kiamelidis, S.; Vlotinou, P.; Giannakou, E.; Karampina, E.; Paschalidou, K.; Kourkoutsakis, N.; et al. Monocyte to HDL and Neutrophil to HDL Ratios as Potential Ischemic Stroke Prognostic Biomarkers. Neurol. Int. 2023, 15, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Chen, C.-H.; Tseng, Y.-J.; Tsai, Y.-T.; Chang, C.-Y.; Wang, H.-Y.; Chen, C.-K. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef] [PubMed]
- van Os, H.J.A.; Ramos, L.A.; Hilbert, A.; van Leeuwen, M.; van Walderveen, M.A.A.; Kruyt, N.D.; Dippel, D.W.J.; Steyerberg, E.W.; van der Schaaf, I.C.; Lingsma, H.F.; et al. Predicting Outcome of Endovascular Treatment for Acute Ischemic Stroke: Potential Value of Machine Learning Algorithms. Front. Neurol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Debs, N.; Rasti, P.; Victor, L.; Cho, T.-H.; Frindel, C.; Rousseau, D. Simulated perfusion MRI data to boost training of convolutional neural networks for lesion fate prediction in acute stroke. Comput. Biol. Med. 2020, 116, 103579. [Google Scholar] [CrossRef]
- Fang, G.; Liu, W.; Wang, L. A machine learning approach to select features important to stroke prognosis. Comput. Biol. Chem. 2020, 88, 107316. [Google Scholar] [CrossRef]
- Fang, G.; Huang, Z.; Wang, Z. Predicting Ischemic Stroke Outcome Using Deep Learning Approaches. Front. Genet. 2022, 12, 827522. [Google Scholar] [CrossRef]
- Hofer, I.S.; Burns, M.; Kendale, S.; Wanderer, J.P. Realistically Integrating Machine Learning Into Clinical Practice: A Road Map of Opportunities, Challenges, and a Potential Future. Obstet. Anesthesia Dig. 2020, 130, 1115–1118. [Google Scholar] [CrossRef]
- Verma, A.A.; Murray, J.; Greiner, R.; Cohen, J.P.; Shojania, K.G.; Ghassemi, M.; Straus, S.E.; Pou-Prom, C.; Mamdani, M. Implementing machine learning in medicine. Can. Med. Assoc. J. 2021, 193, E1351–E1357. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Papageorgiou, E.; Giakas, G.; Tsaopoulos, D. Machine learning in knee osteoarthritis: A review. Osteoarthr. Cartil. Open 2020, 2, 100069. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hügle, M.; Omoumi, P.; van Laar, J.M.; Boedecker, J.; Hügle, T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol. Adv. Pract. 2020, 4, rkaa005. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning–Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-K.; Chang, J.Y.; Lee, J.S.; Lee, E.-J.; Kim, Y.-H.; Han, J.H.; Chang, D.-I.; Cho, H.J.; Cha, J.-K.; Yu, K.H.; et al. Reliability and Clinical Utility of Machine Learning to Predict Stroke Prognosis: Comparison with Logistic Regression. J. Stroke 2020, 22, 403–406. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsu, K.-C.; Johnson, K.R.; Fann, Y.C.; Tsai, C.-H.; Sun, Y.; Lien, L.-M.; Chang, W.-L.; Chen, P.-L.; Hsu, C.Y. Evaluation of machine learning methods to stroke outcome prediction using a nationwide disease registry. Comput. Methods Programs Biomed. 2020, 190, 105381. [Google Scholar] [CrossRef]
- Ding, L.; Liu, C.; Li, Z.; Wang, Y. Incorporating Artificial Intelligence Into Stroke Care and Research. Stroke 2020, 51, e351–e354. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nohara, Y.; Soejima, H.; Yonehara, T.; Nakashima, N.; Kamouchi, M. Stroke Prognostic Scores and Data-Driven Prediction of Clinical Outcomes After Acute Ischemic Stroke. Stroke 2020, 51, 1477–1483. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. Health Professionals. 2023. Available online: https://www.ninds.nih.gov/health-information/public-education/know-stroke/health-professionals (accessed on 10 March 2023).
- Spilker, J.; Kongable, G.; Barch, C.; Braimah, J.; Brattina, P.; Daley, S.; Donnarumma, R.; Rapp, K.; Sailor, S. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 1997, 29, 384–392. [Google Scholar] [CrossRef]
- Adams, H.P.; Davis, P.H.; Leira, E.C.; Chang, K.-C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.D. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999, 53, 126. [Google Scholar] [CrossRef]
- Runde, D. Calculated Decisions: NIH stroke scale/score (NIHSS). Emerg. Med. Pract. 2020, 22, CD6–CD7. [Google Scholar]
- Goldstein, L.B.; Samsa, G.P. Reliability of the National Institutes of Health Stroke Scale. Stroke 1997, 28, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Alfian, G.; Fitriyani, N.L.; Anshari, M.; Hadibarata, T.; Fatwanto, A.; Rhee, J. A Self-Care Prediction Model for Children with Disability Based on Genetic Algorithm and Extreme Gradient Boosting. Mathematics 2020, 8, 1590. [Google Scholar] [CrossRef]
- Ali, A.A. Stroke Prediction using Distributed Machine Learning Based on Apache Spark. Stroke 2019, 28, 89–97. [Google Scholar]
- Yu, D.; Liu, Z.; Su, C.; Han, Y.; Duan, X.; Zhang, R.; Liu, X.; Yang, Y.; Xu, S. Copy number variation in plasma as a tool for lung cancer prediction using Extreme Gradient Boosting (XGBoost) classifier. Thorac. Cancer 2019, 11, 95–102. [Google Scholar] [CrossRef]
- Fernandez-Lozano, C.; Hervella, P.; Mato-Abad, V.; Rodríguez-Yáñez, M.; Suárez-Garaboa, S.; López-Dequidt, I.; Estany-Gestal, A.; Sobrino, T.; Campos, F.; Castillo, J.; et al. Random forest-based prediction of stroke outcome. Sci. Rep. 2021, 11, 10071. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Li, H.; Chan, P.; Wen, C. A machine learning-based approach to decipher multi-etiology of knee osteoarthritis onset and deterioration. Osteoarthr. Cartil. Open 2021, 3, 100135. [Google Scholar] [CrossRef]
- Mohr, M.; von Tscharner, V.; Emery, C.A.; Nigg, B.M. Classification of gait muscle activation patterns according to knee injury history using a support vector machine approach. Hum. Mov. Sci. 2019, 66, 335–346. [Google Scholar] [CrossRef]
- Kokkotis, C.; Giarmatzis, G.; Giannakou, E.; Moustakidis, S.; Tsatalas, T.; Tsiptsios, D.; Vadikolias, K.; Aggelousis, N. An Explainable Machine Learning Pipeline for Stroke Prediction on Imbalanced Data. Diagnostics 2022, 12, 2392. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Janzing, D.; Minorics, L.; Blöbaum, P. Feature relevance quantification in explainable AI: A causal problem. In Proceedings of the 23th International Conference on Artificial Intelligence and Statistics, PMLR, Online, 26–28 August 2020; Volume 108, pp. 2907–2916. [Google Scholar]
- Lai, Y.-L.; Wu, Y.-D.; Yeh, H.-J.; Wu, Y.-T.; Tsai, H.-Y.; Chen, J.-C. Using convolutional neural network to analyze brain MRI images for predicting functional outcomes of stroke. Med. Biol. Eng. Comput. 2022, 60, 2841–2849. [Google Scholar] [CrossRef]
- Rajashekar, D.; Hill, M.D.; Demchuk, A.M.; Goyal, M.; Fiehler, J.; Forkert, N.D. Prediction of Clinical Outcomes in Acute Ischaemic Stroke Patients: A Comparative Study. Front. Neurol. 2021, 12, 663899. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Goff, D.C. Population shifts and the future of stroke: Forecasts of the future burden of stroke. Ann. N. Y. Acad. Sci. 2012, 1268, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.K.; Nguyen, M.H. Elderly Stroke Rehabilitation: Overcoming the Complications and Its Associated Challenges. Curr. Gerontol. Geriatr. Res. 2018, 2018, 9853837. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.A.; Poupore, N.; Nathaniel, T.I. Age Stratification and Stroke Severity in the Telestroke Network. J. Clin. Med. 2023, 12, 1519. [Google Scholar] [CrossRef]
- Ansari, A.K.; Akhund, I.A.; Shaikh, A.Q. Stroke in elderly; identification of risk factors. J. Ayub Med. Coll. Abbottabad JAMC 2002, 13, 11–13. [Google Scholar]
- Murakami, K.; Asayama, K.; Satoh, M.; Inoue, R.; Tsubota-Utsugi, M.; Hosaka, M.; Matsuda, A.; Nomura, K.; Murakami, T.; Kikuya, M.; et al. Risk Factors for Stroke among Young-Old and Old-Old Community-Dwelling Adults in Japan: The Ohasama Study. J. Atheroscler. Thromb. 2017, 24, 290–300. [Google Scholar] [CrossRef]
- Engstad, T.; Engstad, T.T.; Viitanen, M.; Ellekjær, H. Epidemiology of stroke in the elderly in the Nordic countries. Incidence, survival, prevalence and risk factors. Nor. Epidemiol. 2012, 22, 1557. [Google Scholar] [CrossRef]
- Long, X.; Lou, Y.; Gu, H.; Guo, X.; Wang, T.; Zhu, Y.; Zhao, W.; Ning, X.; Li, B.; Wang, J.; et al. Mortality, Recurrence, and Dependency Rates Are Higher after Acute Ischemic Stroke in Elderly Patients with Diabetes Compared to Younger Patients. Front. Aging Neurosci. 2016, 8, 142. [Google Scholar] [CrossRef]
- Kammersgaard, L.P.; Jørgensen, H.S.; Reith, J.; Nakayama, H.; Pedersen, P.M.; Olsen, T.S. Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing 2004, 33, 149–154. [Google Scholar] [CrossRef]
- Samuthpongtorn, C.; Jereerat, T.; Suwanwela, N.C. Stroke risk factors, subtypes and outcome in elderly Thai patients. BMC Neurol. 2021, 21, 322. [Google Scholar] [CrossRef]
- Saposnik, G.; Guzik, A.K.; Reeves, M.; Ovbiagele, B.; Johnston, S.C. Stroke Prognostication using Age and NIH Stroke Scale: SPAN-100. Neurology 2012, 80, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Flores, S.; Rabinstein, A.; Biller, J.; Elkind, M.S.; Griffith, P.; Gorelick, P.B.; Howard, G.; Leira, E.C.; Morgenstern, L.B.; Ovbiagele, B.; et al. Racial-Ethnic Disparities in Stroke Care: The American Experience. Stroke 2011, 42, 2091–2116. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.K.; Fang, J.; Hill, M.D.; Silver, F.; Richards, J.; Jaigobin, C.; Cheung, A.M. Sex Differences in Stroke Care and Outcomes. Stroke 2005, 36, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Dougu, N.; Takashima, S.; Sasahara, E.; Taguchi, Y.; Toyoda, S.; Hirai, T.; Nozawa, T.; Tanaka, K.; Inoue, H. Predictors of Poor Outcome in Patients with Acute Cerebral Infarction. J. Clin. Neurol. 2011, 7, 197–202. [Google Scholar] [CrossRef]
- Di Carlo, A.; Lamassa, M.; Baldereschi, M.; Pracucci, G.; Basile, A.M.; Wolfe, C.D.; Giroud, M.; Rudd, A.; Ghetti, A.; Inzitari, D.; et al. Sex Differences in the Clinical Presentation, Resource Use, and 3-Month Outcome of Acute Stroke in Europe. Stroke 2003, 34, 1114–1119. [Google Scholar] [CrossRef]
- Boehme, A.K.; Siegler, J.E.; Mullen, M.T.; Albright, K.C.; Lyerly, M.J.; Monlezun, D.J.; Jones, E.M.; Tanner, R.; Gonzales, N.R.; Beasley, T.M.; et al. Racial and Gender Differences in Stroke Severity, Outcomes, and Treatment in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, e255–e261. [Google Scholar] [CrossRef]
- Caso, V.; Paciaroni, M.; Agnelli, G.; Corea, F.; Ageno, W.; Alberti, A.; Lanari, A.; Micheli, S.; Bertolani, L.; Venti, M.; et al. Gender Differences in Patients with Acute Ischemic Stroke. Women’s Health 2010, 6, 51–57. [Google Scholar] [CrossRef]
- Santalucia, P.; Pezzella, F.; Sessa, M.; Monaco, S.; Torgano, G.; Anticoli, S.; Zanoli, E.; Baronello, M.M.; Paciaroni, M.; Caso, V. Sex differences in clinical presentation, severity and outcome of stroke: Results from a hospital-based registry. Eur. J. Intern. Med. 2012, 24, 167–171. [Google Scholar] [CrossRef]
- Jamrozik, K. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Vemmos, K.N.; Tsivgoulis, G.; Spengos, K.; Zakopoulos, N.; Synetos, A.; Manios, E.; Konstantopoulou, P.; Mavrikakis, M. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J. Intern. Med. 2004, 255, 257–265. [Google Scholar] [CrossRef]
- Pezzini, A.; Grassi, M.; Del Zotto, E.; Volonghi, I.; Giossi, A.; Costa, P.; Cappellari, M.; Magoni, M.; Padovani, A. Influence of acute blood pressure on short- and mid-term outcome of ischemic and hemorrhagic stroke. J. Neurol. 2010, 258, 634–640. [Google Scholar] [CrossRef]
- Ohwaki, K.; Yano, E.; Nagashima, H.; Hirata, M.; Nakagomi, T.; Tamura, A. Blood Pressure Management in Acute Intracerebral Hemorrhage. Stroke 2004, 35, 1364–1367. [Google Scholar] [CrossRef]
- Liu, C.-H.; the Stroke Registry in Chang Gung Healthcare System (SRICHS) Investigators; Wei, Y.-C.; Lin, J.-R.; Chang, C.-H.; Chang, T.-Y.; Huang, K.-L.; Chang, Y.-J.; Ryu, S.-J.; Lin, L.-C.; et al. Initial blood pressure is associated with stroke severity and is predictive of admission cost and one-year outcome in different stroke subtypes: A SRICHS registry study. BMC Neurol. 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Maïer, B.; Kubis, N. Hypertension and Its Impact on Stroke Recovery: From a Vascular to a Parenchymal Overview. Neural Plast. 2019, 2019, 6843895. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.A.; Spring, K.J.; Beran, R.G.; Chatzis, D.; Killingsworth, M.C.; Bhaskar, S.M.M. Role of diabetes in stroke: Recent advances in pathophysiology and clinical management. Diabetes/Metab. Res. Rev. 2021, 38, e3495. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Daidone, M.; Pacinella, G.; Norrito, R.L.; Pinto, A.; Tuttolomondo, A. Diabetes and Ischemic Stroke: An Old and New Relationship an Overview of the Close Interaction between These Diseases. Int. J. Mol. Sci. 2022, 23, 2397. [Google Scholar] [CrossRef]
- Ntaios, G.; Egli, M.; Faouzi, M.; Michel, P. J-Shaped Association Between Serum Glucose and Functional Outcome in Acute Ischemic Stroke. Stroke 2010, 41, 2366–2370. [Google Scholar] [CrossRef]
- Shah, R.S.; Cole, J.W. Smoking and stroke: The more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010, 8, 917–932. [Google Scholar] [CrossRef]
- Rotimi, O.R.; Ajani, I.F.; Penwell, A.; Lari, S.; Walker, B.; Nathaniel, T.I. In acute ischemic stroke patients with smoking incidence, are more women than men more likely to be included or excluded from thrombolysis therapy? Women’s Health 2020, 16, 1745506520922760. [Google Scholar] [CrossRef]
- Tong, X.; Wang, C.; Liao, X.; Pan, Y.; Yan, H.; Cao, Y.; Liu, L.; Zheng, H.; Zhao, X.; Wang, C.; et al. Smoking–Thrombolysis Relationship Depends on Ischemic Stroke Subtype. Stroke 2016, 47, 1811–1816. [Google Scholar] [CrossRef]
- Ali, S.F.; Smith, E.E.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H. Paradoxical Association of Smoking With In-Hospital Mortality Among Patients Admitted With Acute Ischemic Stroke. J. Am. Heart Assoc. 2013, 2, e000171. [Google Scholar] [CrossRef]
- Wang, H.-K.; Huang, C.-Y.; Sun, Y.-T.; Li, J.-Y.; Chen, C.-H.; Sun, Y.; Liu, C.-H.; Lin, C.-H.; Chang, W.-L.; Lee, J.-T.; et al. Smoking Paradox in Stroke Survivors? Stroke 2020, 51, 1248–1256. [Google Scholar] [CrossRef]
- Kurth, T.; Everett, B.M.; Buring, J.E.; Kase, C.S.; Ridker, P.M.; Gaziano, J.M. Lipid levels and the risk of ischemic stroke in women. Neurology 2007, 68, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Elwood, P.C.; Nikitin, Y.; Salonen, J.T.; de Concalves, A.F.; Inzitari, D.; Sivenius, J.; Benetou, V.; Tuomilehto, J.; Koudstaal, P.J.; et al. Total and HDL cholesterol and risk of stroke. EUROSTROKE 2002, 56, i19–i24. [Google Scholar]
- Bowman, T.S.; Sesso, H.D.; Ma, J.; Kurth, T.; Kase, C.S.; Stampfer, M.J.; Gaziano, J.M. Cholesterol and the Risk of Ischemic Stroke. Stroke 2003, 34, 2930–2934. [Google Scholar] [CrossRef]
- Suh, I.; Jee, S.H.; Kim, H.C.; Nam, C.M.; Kim, I.S.; Appel, L.J. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet 2001, 357, 922–925. [Google Scholar] [CrossRef]
- Sturgeon, J.D.; Folsom, A.R.; Longstreth, W.; Shahar, E.; Rosamond, W.D.; Cushman, M. Risk Factors for Intracerebral Hemorrhage in a Pooled Prospective Study. Stroke 2007, 38, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, A.; Kurth, T.; Pico, F.; Barberger-Gateau, P.; Ritchie, K.; Stapf, C.; Tzourio, C. Triglycerides and risk of hemorrhagic stroke vs. ischemic vascular events: The Three-City Study. Atherosclerosis 2010, 210, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wieberdink, R.G.; Poels, M.M.; Vernooij, M.W.; Koudstaal, P.J.; Hofman, A.; van der Lugt, A.; Breteler, M.M.; Ikram, M.A. Serum Lipid Levels and the Risk of Intracerebral Hemorrhage: The Rotterdam Study. Arter. Thromb. Vasc. Biol. 2011, 31, 2982–2989. [Google Scholar] [CrossRef]
- Amarenco, P.; Labreuche, J.; Touboul, P.-J. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: A systematic review. Atherosclerosis 2008, 196, 489–496. [Google Scholar] [CrossRef]
- Bots, M.L.; Visseren, F.L.; Evans, G.W.; Riley, W.A.; Revkin, J.H.; Tegeler, C.H.; Shear, C.L.; Duggan, W.T.; Vicari, R.M.; Grobbee, D.E.; et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): A randomised, double-blind trial. Lancet 2007, 370, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo, E.; Gardener, H.; Hudson, B.I.; Dong, C.; Della-Morte, D.; Crisby, M.; Goldberg, R.B.; Elkind, M.S.; Cheung, Y.K.; Wright, C.B.; et al. High-density lipoprotein subfractions and carotid plaque: The Northern Manhattan Study. Atherosclerosis 2014, 237, 163–168. [Google Scholar] [CrossRef][Green Version]
- Shahar, E.; Chambless, L.E.; Rosamond, W.D.; Boland, L.L.; Ballantyne, C.M.; McGovern, P.G.; Sharrett, A.R. Plasma Lipid Profile and Incident Ischemic Stroke. Stroke 2003, 34, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Benson, R.T.; Kargman, D.E.; Boden-Albala, B.; Tuck, C.; Lin, I.-F.; Cheng, J.F.; Paik, M.C.; Shea, S.; Berglund, L. High-Density Lipoprotein Cholesterol and Ischemic Stroke in the Elderly. JAMA 2001, 285, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Elkind, M.S. Lipids and Cerebrovascular Disease. Stroke 2015, 46, 3322–3328. [Google Scholar] [CrossRef]
- Borowsky, L.H.; Regan, S.; Chang, Y.; Ayres, A.; Greenberg, S.M.; Singer, D.E. First Diagnosis of Atrial Fibrillation at the Time of Stroke. Cerebrovasc. Dis. 2017, 43, 192–199. [Google Scholar] [CrossRef]
- Vinding, N.E.; Kristensen, S.L.; Rørth, R.; Butt, J.H.; Østergaard, L.; Olesen, J.B.; Torp-Pedersen, C.; Gislason, G.H.; Køber, L.; Kruuse, C.; et al. Ischemic Stroke Severity and Mortality in Patients With and Without Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e022638. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, Y.D.; Kim, J.; Han, S.W.; Oh, M.S.; Lee, J.S.; Lee, K.-Y. Initial Stroke Severity in Patients With Atrial Fibrillation According to Antithrombotic Therapy Before Ischemic Stroke. Stroke 2020, 51, 2733–2741. [Google Scholar] [CrossRef]
- Watanabe, K.; Okazaki, S.; Kitano, T.; Sugiyama, S.; Ohara, M.; Kanki, H.; Sasaki, T.; Sakaguchi, M.; Mochizuki, H.; Todo, K. Stroke Severity and Outcomes in Patients With Newly Diagnosed Atrial Fibrillation. Front. Neurol. 2021, 12, 666491. [Google Scholar] [CrossRef]
- Kimura, K.; Minematsu, K.; Yamaguchi, T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15 831 patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 679–683. [Google Scholar] [CrossRef]
- Esato, M.; Chun, Y.-H.; An, Y.; Ogawa, H.; Wada, H.; Hasegawa, K.; Tsuji, H.; Abe, M.; Lip, G.Y.; Akao, M. Clinical Impact of Asymptomatic Presentation Status in Patients With Paroxysmal and Sustained Atrial Fibrillation. Chest 2017, 152, 1266–1275. [Google Scholar] [CrossRef]
- Rizos, T.; Horstmann, S.; Dittgen, F.; Täger, T.; Jenetzky, E.; Heuschmann, P.; Veltkamp, R. Preexisting Heart Disease Underlies Newly Diagnosed Atrial Fibrillation After Acute Ischemic Stroke. Stroke 2016, 47, 336–341. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cerasuolo, J.O.; Cipriano, L.E.; Fang, J.; Fridman, S.; Paquet, M.; Saposnik, G.; on behalf of the PARADISE Study Group. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology 2018, 90, e924–e931. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-M.; Rao, Z.-Z.; Gu, H.-Q.; Zhao, X.-Q.; Wang, C.-J.; Liu, L.-P.; Liu, C.; Wang, Y.-L.; Li, Z.-X.; Xiao, R.-P.; et al. Atrial Fibrillation Known Before or Detected After Stroke Share Similar Risk of Ischemic Stroke Recurrence and Death. Stroke 2019, 50, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Atam, V.; Yathish, B.E.; Das, L.; Koonwar, S. Role of erythrocyte sedimentation rate in ischemic stroke as an inflammatory marker of carotid atherosclerosis. J. Neurosci. Rural. Pract. 2014, 5, 40–45. [Google Scholar] [CrossRef]
- Chamorro, Á. Role of Inflammation in Stroke and Atherothrombosis. Cerebrovasc. Dis. 2004, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kisialiou, A.; Pelone, G.; Carrizzo, A.; Grillea, G.; Trimarco, V.; Marino, M.; Bartolo, M.; De Nunzio, A.M.; Grella, R.; Landolfi, A.; et al. Blood biomarkers role in acute ischemic stroke patients: Higher is worse or better? Immun. Ageing 2012, 9, 22. [Google Scholar] [CrossRef]
- Anuk, T.; Assayag, E.B.; Rotstein, R.; Fusman, R.; Zeltser, D.; Berliner, S.; Avitzour, D.; Shapira, I.; Arber, N.; Bornstein, N.M. Prognostic implications of admission inflammatory profile in acute ischemic neurological events. Acta Neurol. Scand. 2002, 106, 196–199. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, A.; Kaur, A. Erythrocyte Sedimentation Rate: Its Determinants and Relationship with Risk Factors Involved in Ischemic Stroke. Korean J. Clin. Lab. Sci. 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.-D.; Dijkgraaf, M.G.; van de Beek, D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Armstrong, J.R.; Mosher, B.D. Aspiration Pneumonia After Stroke: Intervention and prevention. Neurohospitalist 2011, 1, 85–93. [Google Scholar] [CrossRef]
- Grossmann, I.; Rodriguez, K.; Soni, M.; Joshi, P.K.; Patel, S.C.; Shreya, D.; I Zamora, D.; Patel, G.S.; Sange, I. Stroke and Pneumonia: Mechanisms, Risk Factors, Management, and Prevention. Cureus 2021, 13, e19912. [Google Scholar] [CrossRef]
- de Jonge, J.C.; van de Beek, D.; Lyden, P.; Brady, M.C.; Bath, P.M.; van der Worp, H.B.; Lees, K.; Alexandrov, A.; Berge, E.; Bluhmki, E.; et al. Temporal Profile of Pneumonia After Stroke. Stroke 2022, 53, 53–60. [Google Scholar] [CrossRef]
- Vermeij, F.H.; Reimer, W.J.S.O.; de Man, P.; van Oostenbrugge, R.J.; Franke, C.L.; de Jong, G.; de Kort, P.L.; Dippel, D.W. Stroke-Associated Infection Is an Independent Risk Factor for Poor Outcome after Acute Ischemic Stroke: Data from the Netherlands Stroke Survey. Cerebrovasc. Dis. 2009, 27, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, O.; Kapral, M.; Hall, R.; Asllani, E.; Selchen, D.; Saposnik, G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 2011, 77, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Ingeman, A.; Andersen, G.; Hundborg, H.H.; Svendsen, M.L.; Johnsen, S.P. In-Hospital Medical Complications, Length of Stay, and Mortality Among Stroke Unit Patients. Stroke 2011, 42, 3214–3218. [Google Scholar] [CrossRef] [PubMed]
- Hilker, R.; Poetter, C.; Findeisen, N.; Sobesky, J.; Jacobs, A.; Neveling, M.; Heiss, W.-D. Nosocomial Pneumonia After Acute Stroke. Stroke 2003, 34, 975–981. [Google Scholar] [CrossRef]
- Warusevitane, A.; Karunatilake, D.; Sim, J.; Smith, C.; Roffe, C. Early Diagnosis of Pneumonia in Severe Stroke: Clinical Features and the Diagnostic Role of C-Reactive Protein. PLoS ONE 2016, 11, e0150269. [Google Scholar] [CrossRef]
- Learoyd, A.E.; on behalf of the ENOS Trial Investigators; Woodhouse, L.; Shaw, L.; Sprigg, N.; Bereczki, D.; Berge, E.; Caso, V.; Christensen, H.; Collins, R.; et al. Infections Up to 76 Days After Stroke Increase Disability and Death. Transl. Stroke Res. 2017, 8, 541–548. [Google Scholar] [CrossRef]
- Bösel, J. Use and Timing of Tracheostomy After Severe Stroke. Stroke 2017, 48, 2638–2643. [Google Scholar] [CrossRef]
- Lahiri, S.; Mayer, S.A.; Fink, M.E.; Lord, A.S.; Rosengart, A.; Mangat, H.S.; Segal, A.Z.; Claassen, J.; Kamel, H. Mechanical Ventilation for Acute Stroke: A Multi-state Population-Based Study. Neurocrit. Care 2014, 23, 28–32. [Google Scholar] [CrossRef]
- de Montmollin, E.; Terzi, N.; Dupuis, C.; Garrouste-Orgeas, M.; da Silva, D.; Darmon, M.; Laurent, V.; Thiéry, G.; Oziel, J.; Marcotte, G.; et al. One-year survival in acute stroke patients requiring mechanical ventilation: A multicenter cohort study. Ann. Intensive Care 2020, 10, 53. [Google Scholar] [CrossRef]
- Hannawi, Y.; Hannawi, B.; Rao, C.P.V.; Suarez, J.I.; Bershad, E.M. Stroke-Associated Pneumonia: Major Advances and Obstacles. Cerebrovasc. Dis. 2013, 35, 430–443. [Google Scholar] [CrossRef]
- Smith, C.J.; Bray, B.D.; Hoffman, A.; Meisel, A.; Heuschmann, P.U.; Wolfe, C.D.A.; Tyrrell, P.J.; Rudd, A.G.; the Intercollegiate Stroke Working Party Group. Can a Novel Clinical Risk Score Improve Pneumonia Prediction in Acute Stroke Care? A UK Multicenter Cohort Study. J. Am. Heart Assoc. 2015, 4, e001307. [Google Scholar] [CrossRef]
- Robba, C.; Bonatti, G.; Battaglini, D.; Rocco, P.R.M.; Pelosi, P. Mechanical ventilation in patients with acute ischaemic stroke: From pathophysiology to clinical practice. Crit. Care 2019, 23, 388. [Google Scholar] [CrossRef]
- Meyfroidt, G.; Bollaert, P.-E.; Marik, P.E. Acute ischemic stroke in the ICU: To admit or not to admit? Intensive Care Med. 2014, 40, 749–751. [Google Scholar] [CrossRef]
- Cheng, B.; Forkert, N.D.; Zavaglia, M.; Hilgetag, C.C.; Golsari, A.; Siemonsen, S.; Fiehler, J.; Pedraza, S.; Puig, J.; Cho, T.-H.; et al. Influence of Stroke Infarct Location on Functional Outcome Measured by the Modified Rankin Scale. Stroke 2014, 45, 1695–1702. [Google Scholar] [CrossRef]
- Laufer, Y.; Sivan, D.; Schwarzmann, R.; Sprecher, E. Standing Balance and Functional Recovery of Patients with Right and Left Hemiparesis in the Early Stages of Rehabilitation. Neurorehabil. Neural Repair 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Rangaraju, S.; Streib, C.; Aghaebrahim, A.; Jadhav, A.; Frankel, M.; Jovin, T.G. Relationship Between Lesion Topology and Clinical Outcome in Anterior Circulation Large Vessel Occlusions. Stroke 2015, 46, 1787–1792. [Google Scholar] [CrossRef]
- Königsberg, A.; DeMarco, A.T.; Mayer, C.; Wouters, A.; Schlemm, E.; Ebinger, M.; Cho, T.-H.; Endres, M.; Fiebach, J.B.; Fiehler, J.; et al. Influence of stroke infarct location on quality of life assessed in a multivariate lesion-symptom mapping study. Sci. Rep. 2021, 11, 13490. [Google Scholar] [CrossRef]
- Ernst, M.; Boers, A.M.M.; Forkert, N.D.; Berkhemer, O.A.; Roos, Y.B.; Dippel, D.W.J.; van der Lugt, A.; Van Oostenbrugge, R.J.; Van Zwam, W.H.; Vettorazzi, E.; et al. Impact of Ischemic Lesion Location on the mRS Score in Patients with Ischemic Stroke: A Voxel-Based Approach. AJNR Am. J. Neuroradiol. 2018, 39, 1989–1994. [Google Scholar] [CrossRef]
| Classifier | Hyperparameters |
|---|---|
| LR | penalty = l1, l2 C = 0, 1, 2, 4, 10 |
| XGBoost | maximum depth: 1, 2, 3, 4, 5, 6, 7, 8, minimum child weight: 1, 3, 4, 5, 6, 8, gamma: 0, 0.4, 0.5, 0.6 |
| RF | criterion: Gini, entropy, n estimators: 10, 15, 20, 25, 30, minimum_samples leaf: 1, 2, 3, minimum samples split: 3, 4, 5, 6, 7 |
| MLP | hidden_layer_sizes: (2, 5, 10), (5, 10, 20), (10, 20, 50), activation: tanh, ReLU, solver: SGD, Adam, alpha: 0.0001, 0.05, learning rate: constant, adaptive |
| SVM | C: 0.001, 0.01, 0.1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, kernel: linear, sigmoid, RBF, poly gamma: scale |
| Classifier | Accuracy (%) | Recall (%) | Precision (%) | f1-Score (%) | FNrate (%) | FPrate (%) |
|---|---|---|---|---|---|---|
| LR | 84.68 | 84.68 | 92.24 | 86.81 | 6.67 | 16.51 |
| XGBoost | 86.29 | 86.29 | 87.48 | 86.82 | 13.71 | 9.17 |
| RF | 91.13 | 91.13 | 90.89 | 91.00 | 8.87 | 4.59 |
| MLP | 87.90 | 87.90 | 88.25 | 88.07 | 12.10 | 7.34 |
| SVM | 83.07 | 83.07 | 85.38 | 84.08 | 16.94 | 11.92 |
| Features | Type of Data |
|---|---|
| Age | Categorical |
| Gender | Categorical |
| NIHSS upon admission | Categorical |
| Intubation | Categorical |
| History of hypertension | Categorical |
| Smoking | Categorical |
| Initial diagnosis of hypertension | Categorical |
| Initial diagnosis of diabetes | Categorical |
| Initial diagnosis of dyslipidemia | Categorical |
| HDL levels | Categorical |
| Initial diagnosis of atrial fibrillation | Categorical |
| Stroke localization based on blood supply | Categorical |
| Systolic blood pressure levels upon admission | Categorical |
| ESR levels upon admission | Categorical |
| Development of respiratory infection | Categorical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantzios, A.; Kokkotis, C.; Tsiptsios, D.; Moustakidis, S.; Gkartzonika, E.; Avramidis, T.; Tripsianis, G.; Iliopoulos, I.; Aggelousis, N.; Vadikolias, K. From Admission to Discharge: Predicting National Institutes of Health Stroke Scale Progression in Stroke Patients Using Biomarkers and Explainable Machine Learning. J. Pers. Med. 2023, 13, 1375. https://doi.org/10.3390/jpm13091375
Gkantzios A, Kokkotis C, Tsiptsios D, Moustakidis S, Gkartzonika E, Avramidis T, Tripsianis G, Iliopoulos I, Aggelousis N, Vadikolias K. From Admission to Discharge: Predicting National Institutes of Health Stroke Scale Progression in Stroke Patients Using Biomarkers and Explainable Machine Learning. Journal of Personalized Medicine. 2023; 13(9):1375. https://doi.org/10.3390/jpm13091375
Chicago/Turabian StyleGkantzios, Aimilios, Christos Kokkotis, Dimitrios Tsiptsios, Serafeim Moustakidis, Elena Gkartzonika, Theodoros Avramidis, Gregory Tripsianis, Ioannis Iliopoulos, Nikolaos Aggelousis, and Konstantinos Vadikolias. 2023. "From Admission to Discharge: Predicting National Institutes of Health Stroke Scale Progression in Stroke Patients Using Biomarkers and Explainable Machine Learning" Journal of Personalized Medicine 13, no. 9: 1375. https://doi.org/10.3390/jpm13091375
APA StyleGkantzios, A., Kokkotis, C., Tsiptsios, D., Moustakidis, S., Gkartzonika, E., Avramidis, T., Tripsianis, G., Iliopoulos, I., Aggelousis, N., & Vadikolias, K. (2023). From Admission to Discharge: Predicting National Institutes of Health Stroke Scale Progression in Stroke Patients Using Biomarkers and Explainable Machine Learning. Journal of Personalized Medicine, 13(9), 1375. https://doi.org/10.3390/jpm13091375









