Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors
Abstract
:1. Introduction
2. Rare and Exclusive Events
2.1. Carpal Tunnel Syndrome Associated with Atezolizumab in a Male with Non-Small-Cell Lung Cancer
2.2. Carpal Tunnel Syndrome Associated with Nivolumab in a Female with Renal Cell Carcinoma
2.3. Carpal Tunnel Syndrome Associated with Nivolumab in a Male with Melanoma
2.4. Carpal Tunnel Syndrome Associated with Ipilimumab plus Nivolumab in a Male with Melanoma
3. The ICI Mechanisms of Action
4. Immune Checkpoint Inhibitors Therapies
4.1. PDL-1 Inhibitors
4.1.1. Atezolizumab
4.1.2. Durvalumab
4.1.3. Avelumab
4.2. PD-1 Inhibitors
4.2.1. Pembrolizumab
4.2.2. Nivolumab
4.2.3. Cemiplimab
4.3. CTLA-4 Inhibitors
Ipilimumab
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Jama, A.A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.H.; Shalata, W.; Yakobson, A.; Agbarya, A. Neoadjuvant and Adjuvant Immunotherapy in Early-Stage Non-Small-Cell Lung Cancer, Past, Present, and Future. J. Clin. Med. 2021, 10, 5614. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Shalata, W.; Massalha, I.; Ishay, S.Y.; Chernomordikova, E.; Jama, A.A.; Rouvinov, K.; Dudnik, Y.; Yakobson, A. Radiotherapy-Induced Atrial Myxoma: A Case Report and Literature Review. Life 2023, 13, 1585. [Google Scholar] [CrossRef]
- Cha, J.H.; Chan, L.C.; Song, M.S.; Hung, M.C. New Approaches on Cancer Immunotherapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036863. [Google Scholar] [CrossRef]
- Gandini, A.; Puglisi, S.; Pirrone, C.; Martelli, V.; Catalano, F.; Nardin, S.; Seeber, A.; Puccini, A.; Sciallero, S. The role of immunotherapy in microsatellites stable metastatic colorectal cancer: State of the art and future perspectives. Front. Oncol. 2023, 13, 1161048. [Google Scholar] [CrossRef]
- Grau, J.F.; Farinas-Madrid, L.; Garcia-Duran, C.; Garcia-Illescas, D.; Oaknin, A. Advances in immunotherapy in cervical cancer. Int. J. Gynecol. Cancer 2023, 33, 403–413. [Google Scholar] [CrossRef]
- Desai, A.; Peters, S. Immunotherapy-based combinations in metastatic NSCLC. Cancer Treat. Rev. 2023, 116, 102545. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Motzer, R.J.; Choueiri, T.K.; McDermott, D.F.; Powles, T.; Vano, Y.A.; Gupta, S.; Yao, J.; Han, C.; Ammar, R.; Papillon-Cavanagh, S.; et al. Biomarker analysis from CheckMate 214: Nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J. Immunother. Cancer 2022, 10, e004316. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Weissmann, S.; Itzhaki Gabay, S.; Sheva, K.; Abu Saleh, O.; Jama, A.A.; Yakobson, A.; Rouvinov, K. A Retrospective, Single-Institution Experience of Bullous Pemphigoid as an Adverse Effect of Immune Checkpoint Inhibitors. Cancers 2022, 14, 5451. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Triggianese, P.; Novelli, L.; Galdiero, M.R.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; Gerli, R. Immune Checkpoint Inhibitors-Induced Autoimmunity: The Impact of Gender. Autoimmun. Rev. 2020, 19, 102590. [Google Scholar] [CrossRef]
- Lal, J.C.; Brown, S.-A.; Collier, P.; Cheng, F. A Retrospective Analysis of Cardiovascular Adverse Events Associated with Immune Checkpoint Inhibitors. Cardiooncology 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and Interleukin-6 Are Prognostic Factors for Autoimmune Toxicity Following Treatment With Anti-CTLA4 Blockade. J. Trans. Med. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-Checkpoint Inhibitors Associated With Interstitial Lung Disease in Cancer Patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef]
- Gesiotto, Q.J.; Swoboda, D.M.; Shallis, R.M.; Al Ali, N.; Padron, E.; Kuykendall, A.T.; Song, J.; Talati, C.; Sweet, K.; Lancet, J.E.; et al. Evaluating Predictors of Immune-Related Adverse Events and Response to Checkpoint Inhibitors in Myeloid Malignancies. Clin. Lymphoma Myeloma Leuk. 2021, 21, 421–424. [Google Scholar] [CrossRef]
- Sher, A.F.; Golshani, G.M.; Wu, S. Fatal Adverse Events Associated with Pembrolizumab in Cancer Patients: A Meta-Analysis. Cancer Investig. 2020, 38, 130–138. [Google Scholar] [CrossRef]
- Kalinich, M.; Murphy, W.; Wongvibulsin, S.; Pahalyants, V.; Yu, K.H.; Lu, C.; Wang, F.; Zubiri, L.; Naranbhai, V.; Gusev, A.; et al. Prediction of Severe Immune-Related Adverse Events Requiring Hospital Admission in Patients on Immune Checkpoint Inhibitors: Study of a Population Level Insurance Claims Database From the USA. J. Immunother. Cancer 2021, 9, e001935. [Google Scholar] [CrossRef] [PubMed]
- Guidon, A.C.; Burton, L.B.; Chwalisz, B.K.; Hillis, J.; Schaller, T.H.; Amato, A.A.; Warner, A.B.; Brastianos, P.K.; Cho, T.A.; Clardy, S.L.; et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J. Immunother. Cancer. 2021, 9, e002890, published correction appears in J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Osiak, K.; Elnazir, P.; Walocha, J.A.; Pasternak, A. Carpal tunnel syndrome: State-of-the-art review. Folia Morphol. 2022, 81, 851–862. [Google Scholar] [CrossRef]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef]
- Trinh, S.; Le, A.; Gowani, S.; La-Beck, N.M. Management of Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy: A Minireview of Current Clinical Guidelines. Asia Pac. J. Oncol. Nurs. 2019, 6, 154–160. [Google Scholar] [CrossRef]
- Huse, M. The T-cell-receptor signaling network. J. Cell Sci. 2009, 122, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242, Erratum in Nat. Rev. Immunol. 2013, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Boussiotis, V.A.; Lorsbach, R.B.; Abbas, A.K.; Sharpe, A.H. CTLA-4 regulates induction of anergy in vivo. Immunity 2001, 14, 145–155. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Yakobson, A.; Abu Jama, A.; Abu Saleh, O.; Michlin, R.; Shalata, W. PD-1 Inhibitors in Elderly and Immunocompromised Patients with Advanced or Metastatic Cutaneous Squamous Cell Carcinoma. Cancers 2023, 15, 4041. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- FDA. Highlights of Prescribing Information: Atezolizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761034s033s034s035s036s037s038lbl.pdf (accessed on 6 June 2023).
- FDA. Highlights of Prescribing Information: Durvalumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf (accessed on 6 June 2023).
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012; Avelumab: 23 June 2022.
- FDA. Highlights of Prescribing Information: Avelumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s096lbl.pdf (accessed on 3 June 2023).
- Lechevalier, D.; Denis, D.; Le Corre, Y.; Heidelberger, V.; Brunet-Possenti, F.; Longvert, C.; Piot, J.M.; Maillard, H.; Beneton, N.; French Skin Cancer Group. Carpal Tunnel Syndrome: A New Adverse Effect of Immune Checkpoint Inhibitors, 11 Cases. J. Immunother. 2021, 44, 122–126. [Google Scholar] [CrossRef] [PubMed]
- FDA. Highlights of Prescribing Information: Pembrolizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf (accessed on 3 June 2023).
- FDA. Highlights of Prescribing Information: Nivolumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125554s090lbl.pdf (accessed on 3 June 2023).
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef]
- FDA. Highlights of Prescribing Information: Cemiplimab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf (accessed on 16 March 2023).
- FDA. Highlights of Prescribing Information: Ipilimumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s110lbl.pdf (accessed on 3 June 2023).
- Georgeto, S.M.; Andraus, R.A.C.; de Oliveira Júnior, E.; da Silva, R.A.; Ngomo, S.; Fernandes, K.B.P. Bilateral Idiopathic Carpal Tunnel Syndrome: Clinical-Functional Characterization and Efficacy of Two Combined Postoperative Physiotherapeutic Treatments. Orthop. Surg. 2023, 15, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.L.; Chen, Y.; Chesterton, L.S.; van der Windt, D.A. Trends in the prevalence, incidence and surgical management of carpal tunnel syndrome between 1993 and 2013: An observational analysis of UK primary care records. BMJ Open 2018, 8, e020166. [Google Scholar] [CrossRef] [PubMed]
- Genova, A.; Dix, O.; Saefan, A.; Thakur, M.; Hassan, A. Carpal Tunnel Syndrome: A Review of Literature. Cureus 2020, 12, e7333. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Yakobson, A.; Cohen, A.Y.; Goldstein, I.; Saleh, O.A.; Dudnik, Y.; Rouvinov, K. Unexpected Adverse Events of Immune Checkpoint Inhibitors. Life 2023, 13, 1657. [Google Scholar] [CrossRef]
- Day, D.; Hansen, A.R. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. BioDrugs 2016, 30, 571–584. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Electronic address: Clinicalguidelines@esmo.org. Management of toxici-ties from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Jackaman, C.; Bundell, C.S.; Kinnear, B.F.; Smith, A.M.; Filion, P.; van Hagen, D.; Robinson, B.W.; Nelson, D.J. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: A novel mechanism for IL-2. J. Immunol. 2003, 171, 5051–5063. [Google Scholar] [CrossRef]
- Falvo, E.; Diviccaro, S.; Melcangi, R.C.; Giatti, S. Physiopathological Role of Neuroactive Steroids in the Peripheral Nervous System. Int. J. Mol. Sci. 2020, 21, 9000. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Magnaghi, V.; Galbiati, M.; Ghelarducci, B.; Sebastiani, L.; Martini, L. The action of steroid hormones on peripheral myelin proteins: A possible new tool for the rebuilding of myelin? J. Neurocytol. 2000, 29, 327–339. [Google Scholar] [CrossRef]
- Jarahi, M.; Sheibani, V.; Safakhah, H.A.; Torkmandi, H.; Rashidy-Pour, A. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: A behavioral and electrophysiological study. Neuroscience 2014, 256, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. New steps forward in the neuroactive steroid field. J. Steroid Biochem. Mol. Biol. 2015, 153, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Roglio, I.; Bianchi, R.; Gotti, S.; Scurati, S.; Giatti, S.; Pesaresi, M.; Caruso, D.; Panzica, G.C.; Melcangi, R.C. Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience 2008, 155, 673–685. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; De Nicola, A.F.; González, S.L. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur. J. Pain 2014, 18, 348–359. [Google Scholar] [CrossRef]

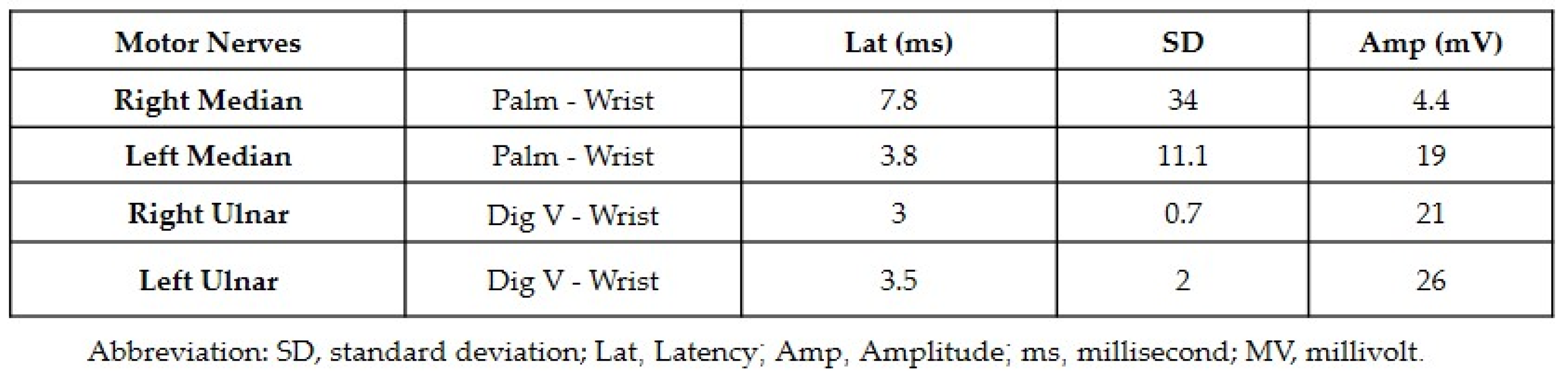
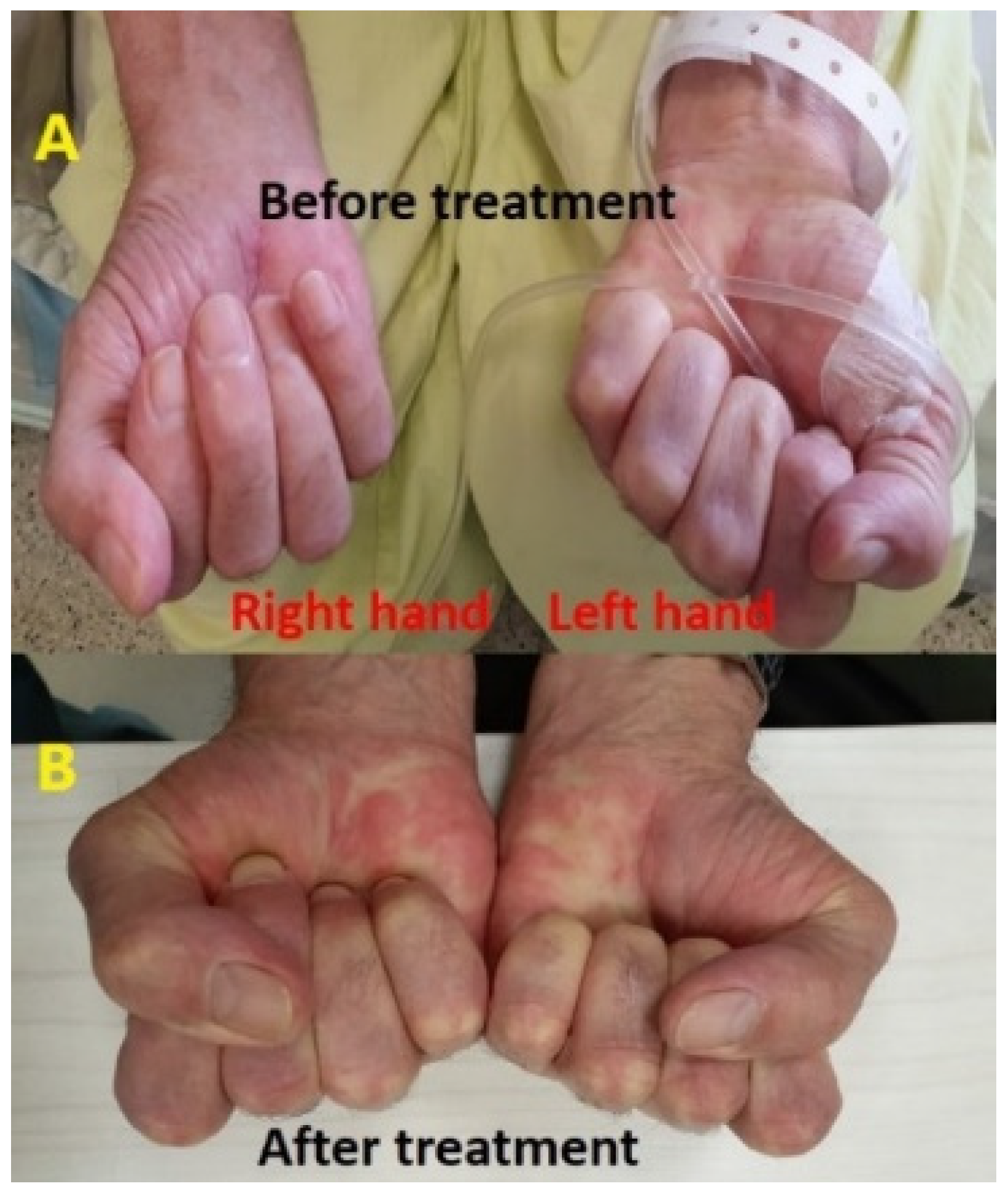

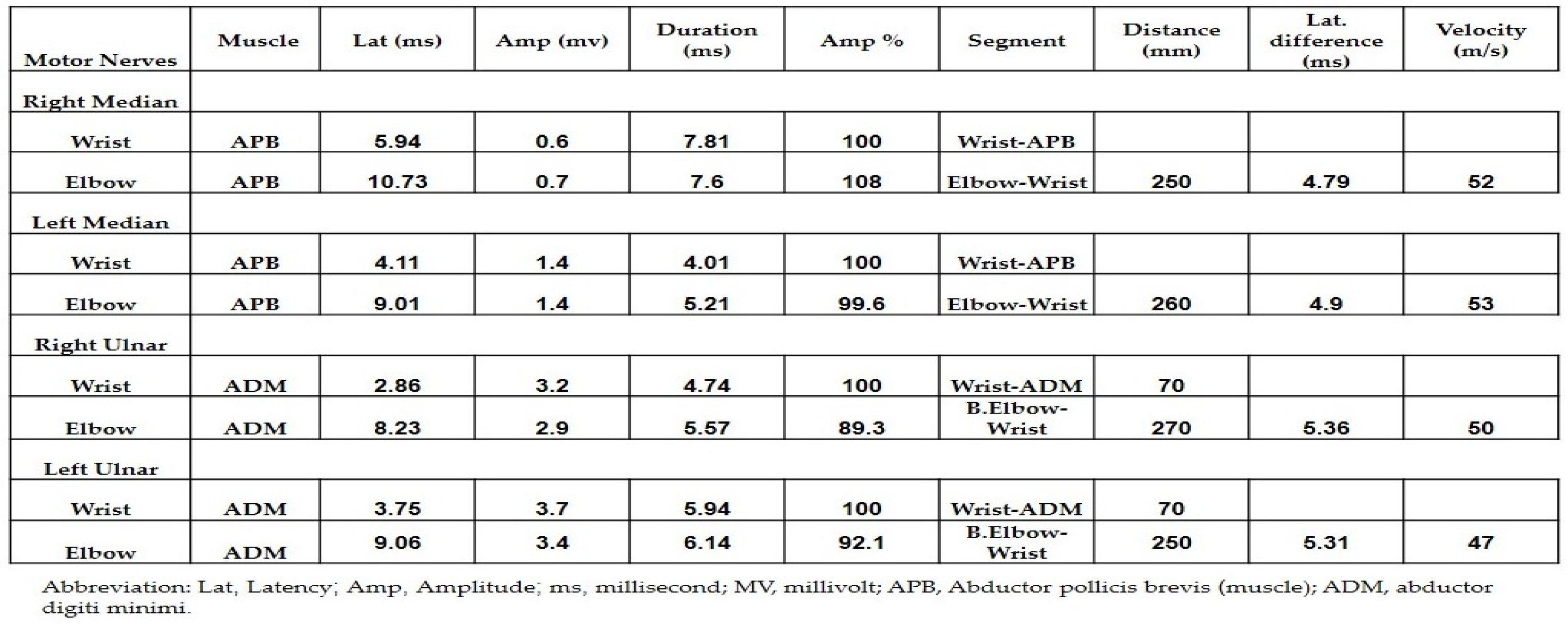
| Therapy | ICI Therapies | Approval | Cancer Diagnoses | Predominant Adverse Events |
|---|---|---|---|---|
| Ipilimumab | CTLA-4 inhibitor | 2011 | CRC, Melanoma, NSCLC, HCC, RCC, and SCLC | Pruritis, fatigue, rash, diarrhea, transaminitis, and colitis |
| Pembrolizumab | PD-1 inhibitor | 2014 | Melanoma, SCC, lung cancer, lymphomas, cancers high in MSI, urothelial carcinoma, MMR-deficient cancers, gastric cancers, cervical cancers, HCC, Merkel cell carcinoma, esophageal cancers, RCC, endometrial carcinoma, tumor mutational burden-high cancer, and triple-negative breast cancer | Myalgia, fatigue, decreased appetite, diarrhea, constipation, rash, fever, cough, pruritis, and nausea |
| Nivolumab | PD-1 inhibitor | 2014 | NSCLC, Melanoma, urothelial carcinoma, RCC, malignant pleural mesothelioma, HCC, classic Hodgkin lymphoma, HNSCC, CRC, and SCC of the esophagus | Fatigue, rash, diarrhea, and pruritis |
| Cemiplimab | PD-1 inhibitor | 2018 | Cutaneous SCC, basal cell carcinoma, and NSCLC | Nephritis, pneumonitis, hepatitis, rash, hypothyroidism or hyperthyroidism, pruritus, and colitis |
| Atezolizumab | PDL-1 inhibitor | 2016 | NSCLC, Urothelial carcinoma, SCLC, HCC, melanoma, and triple-negative breast cancer | Fatigue, nausea, vomiting, cough, dyspnea, constipation, decreased appetite, diarrhea, alopecia, or headache and rash |
| Durvalumab | PDL-1 inhibitor | 2017 | NSCLC and Urothelial carcinoma | Fatigue, constipation, UTIs, edema, pneumonitis, dyspnea, rash, cough, and nausea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakobson, A.; Rouvinov, K.; Cohen, A.Y.; Goldstein, I.; Abu Saleh, O.; Solomon, A.; Dudnik, Y.; Shalata, W. Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors. J. Pers. Med. 2023, 13, 1340. https://doi.org/10.3390/jpm13091340
Yakobson A, Rouvinov K, Cohen AY, Goldstein I, Abu Saleh O, Solomon A, Dudnik Y, Shalata W. Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors. Journal of Personalized Medicine. 2023; 13(9):1340. https://doi.org/10.3390/jpm13091340
Chicago/Turabian StyleYakobson, Alexander, Keren Rouvinov, Aharon Y. Cohen, Iris Goldstein, Omar Abu Saleh, Adam Solomon, Yulia Dudnik, and Walid Shalata. 2023. "Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors" Journal of Personalized Medicine 13, no. 9: 1340. https://doi.org/10.3390/jpm13091340
APA StyleYakobson, A., Rouvinov, K., Cohen, A. Y., Goldstein, I., Abu Saleh, O., Solomon, A., Dudnik, Y., & Shalata, W. (2023). Carpal Tunnel Syndrome Associated with Immune Checkpoint Inhibitors. Journal of Personalized Medicine, 13(9), 1340. https://doi.org/10.3390/jpm13091340







