Copy Number Variations and Gene Mutations Identified by Multiplex Ligation-Dependent Probe Amplification in Romanian Chronic Lymphocytic Leukemia Patients
Abstract
:1. Introduction
1.1. Copy Number Variations
1.2. Somatic Mutations
1.2.1. SF3B1 and NOTCH1
1.2.2. Guidelines
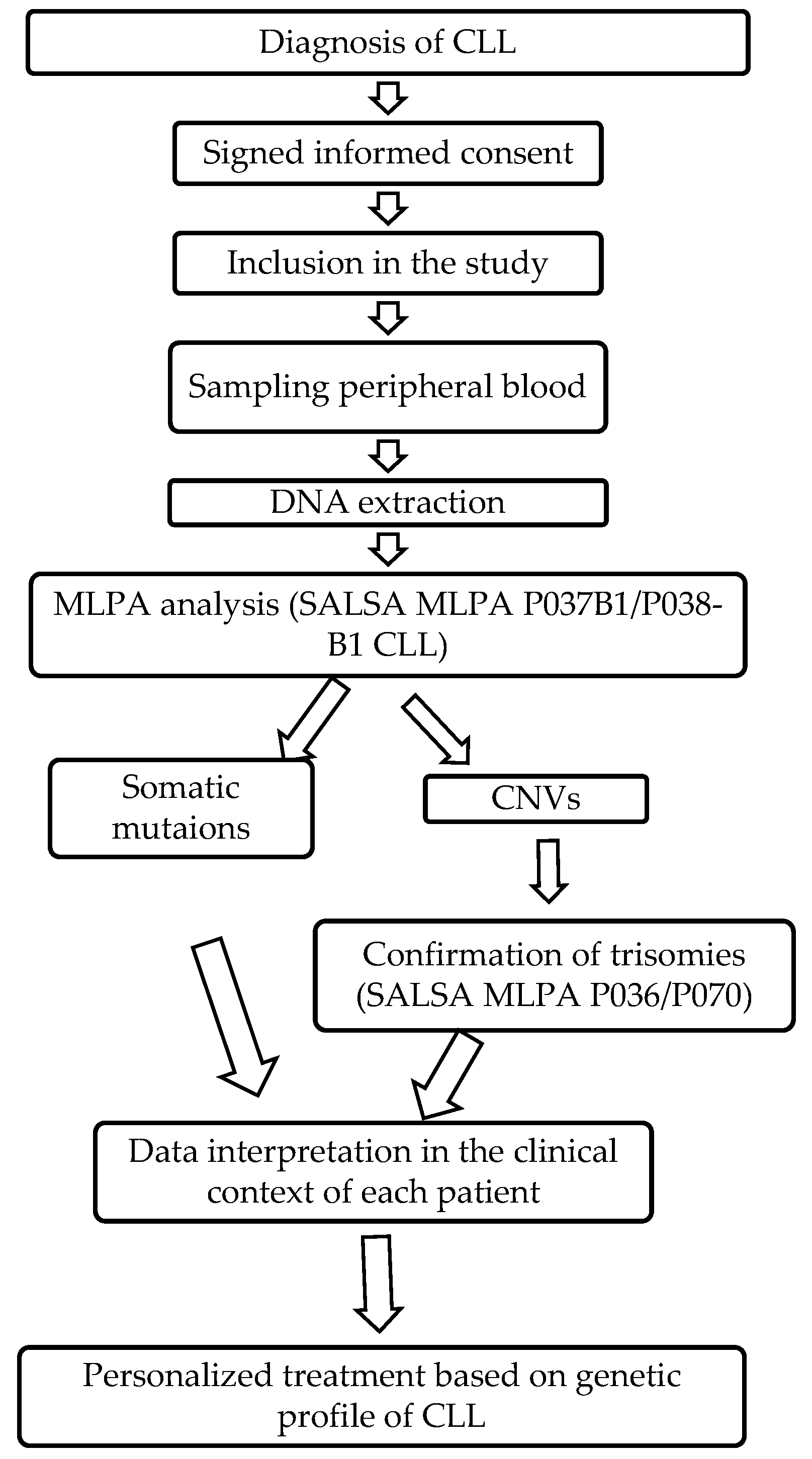
1.2.3. MLPA Analysis
2. Results
2.1. Clinical Characteristics of Patients
2.2. Results of MLPA Analysis
2.3. Survival of Patients
3. Discussion
3.1. Clinical Characteristics of CLL Patients
3.2. CNVs in CLL Patients
3.3. Somatic Mutations in CLL Patients
3.4. Co-Occurrence of CNVs and Somatic Mutations in CLL Patients
3.5. Techniques for CNV Investigation: Benefits, Challenges, and Limitations
3.5.1. MLPA and Conventional Cytogenetics
3.5.2. MLPA and FISH
3.5.3. FISH and aCGH
3.5.4. MLPA and aCGH
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanafelt, T.D.; Geyer, S.M.; Kay, N.E. Prognosis at Diagnosis: Integrating Molecular Biologic Insights into Clinical Practice for Patients with CLL. Blood 2004, 103, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primer. 2017, 3, 16096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campo, E.; Cymbalista, F.; Ghia, P.; Jäger, U.; Pospisilova, S.; Rosenquist, R.; Schuh, A.; Stilgenbauer, S. TP53 Aberrations in Chronic Lymphocytic Leukemia: An Overview of the Clinical Implications of Improved Diagnostics. Haematologica 2018, 103, 1956–1968. [Google Scholar] [CrossRef] [Green Version]
- Puiggros, A.; Ramos-Campoy, S.; Kamaso, J.; de la Rosa, M.; Salido, M.; Melero, C.; Rodríguez-Rivera, M.; Bougeon, S.; Collado, R.; Gimeno, E.; et al. Optical Genome Mapping: A Promising New Tool to Assess Genomic Complexity in Chronic Lymphocytic Leukemia (CLL). Cancers 2022, 14, 3376. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Eichhorst, B.; Hamaker, M.E.; Kaplanov, K.; Morrison, V.A.; Österborg, A.; Poddubnaya, I.; Woyach, J.A.; Shanafelt, T.; Smolej, L.; et al. Management of Chronic Lymphocytic Leukemia (CLL) in the Elderly: A Position Paper from an International Society of Geriatric Oncology (SIOG) Task Force. Ann. Oncol. 2017, 28, 218–227. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Hillmen, P.; Hallek, M.; Buske, C. Chronic Lymphocytic Leukaemia: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26, 78–84. [Google Scholar] [CrossRef]
- Gottardi, M.; Gattei, V.; Degan, M.; Bomben, R.; Zucchetto, A.; Tecchio, C.; Laurino, L.; Zanatta, L.; Dei Tos, A.P.; Mordacchini, M.; et al. Concomitant Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia: Evidence of Simultaneous Expansion of Two Independent Clones. Leuk. Lymphoma. 2006, 47, 885–889. [Google Scholar] [CrossRef]
- Ene, G.; Vlădăreanu, A.M.; niversitatea de MedicinășiFarmacie“Carol Davila”, București, România; Bumbea, H.; Dumitru, I.; Clinica de Hematologie, SpitalulUniversitar de Urgență, București, România. T Lymphocyte Subsets Studied in a Patient with Two Associated Hematologic Neoplasias–Chronic Lymphocytic Leukemia B and Chronic Myeloid Leukemia. Rom. J. Med. Pract. 2019, 14, 325–330. [Google Scholar] [CrossRef]
- Gaidano, G.; Rossi, D. The Mutational Landscape of Chronic Lymphocytic Leukemia and Its Impact on Prognosis and Treatment. Hematology 2017, 2017, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Khalid, K.; Padda, J.; Syam, M.; Moosa, A.; Kakani, V.; Sanka, S.; Zubair, U.; Padda, S.; Cooper, A.C.; Jean-Charles, G. 13q14 Deletion and Its Effect on Prognosis of Chronic Lymphocytic Leukemia. Cureus 2021, 13, e16839. [Google Scholar] [CrossRef]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef] [Green Version]
- GeneCards, T.H.G.D. RB1 Gene—RB Transcriptional Corepressor 1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=RB1&keywords=RB1 (accessed on 1 June 2023).
- Durak Aras, B.; Isik, S.; Uskudar Teke, H.; Aslan, A.; Yavasoglu, F.; Gulbas, Z.; Demirkan, F.; Ozen, H.; Cilingir, O.; Inci, N.S.; et al. Which Prognostic Marker Is Responsible for the Clinical Heterogeneity in CLL with 13q Deletion? Mol. Cytogenet. 2021, 14, 2. [Google Scholar] [CrossRef]
- GeneCards, T.H.G.D. ATM Gene—ATM Serine/Threonine Kinase. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ATM&keywords=ATM (accessed on 1 June 2023).
- Puiggros, A.; Blanco, G.; Espinet, B. Genetic Abnormalities in Chronic Lymphocytic Leukemia: Where We Are and Where We Go. BioMed. Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos-Weil, D.; Nguyen-Khac, F.; Chevret, S.; Roux, C.; Touzeau, C.; Cosson, A.; Cymbalista, F.; Feugier, P.; Leprêtre, S.; Bene, M.-C.; et al. Mutational and Cytogenetic Analyses Of 177 CLL Patients With Trisomy 12: A Retrospective Study Of The CLL French Intergroup. Blood 2013, 122, 4144. [Google Scholar] [CrossRef]
- GeneCards, T.H.G.D. TP53 Gene—Tumor Protein P53 Protein Coding (Updated: Mar 21, 2023; GC17M007661; GIFtS: 61). Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TP53 (accessed on 10 June 2023).
- Derouault, P.; Chauzeix, J.; Rizzo, D.; Miressi, F.; Magdelaine, C.; Bourthoumieu, S.; Durand, K.; Dzugan, H.; Feuillard, J.; Sturtz, F.; et al. CovCopCan: An Efficient Tool to Detect Copy Number Variation from Amplicon Sequencing Data in Inherited Diseases and Cancer. PLoSComput. Biol. 2020, 16, e1007503. [Google Scholar] [CrossRef] [Green Version]
- Döhner, H.; Fischer, K.; Bentz, M.; Hansen, K.; Benner, A.; Cabot, G.; Diehl, D.; Schlenk, R.; Coy, J.; Stilgenbauer, S. P53 Gene Deletion Predicts for Poor Survival and Non-Response to Therapy with Purine Analogs in Chronic B-Cell Leukemias. Blood 1995, 85, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Malcikova, J.; Tausch, E.; Rossi, D.; Sutton, L.A.; Soussi, T.; Zenz, T.; Kater, A.P.; Niemann, C.U.; Gonzalez, D.; Davi, F.; et al. ERIC Recommendations for TP53 Mutation Analysis in Chronic Lymphocytic Leukemia-Update on Methodological Approaches and Results Interpretation. Leukemia 2018, 32, 1070–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeromin, S.; Weissmann, S.; Haferlach, C.; Dicker, F.; Bayer, K.; Grossmann, V.; Alpermann, T.; Roller, A.; Kohlmann, A.; Haferlach, T.; et al. SF3B1 Mutations Correlated to Cytogenetics and Mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 Untreated CLL Patients. Leukemia 2014, 28, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Dicker, F.; Herholz, H.; Schnittger, S.; Nakao, A.; Patten, N.; Wu, L.; Kern, W.; Haferlach, T.; Haferlach, C. The Detection of TP53 Mutations in Chronic Lymphocytic Leukemia Independently Predicts Rapid Disease Progression and Is Highly Correlated with a Complex Aberrant Karyotype. Leukemia 2009, 23, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, G.; Rasi, S.; Rossi, D.; Trifonov, V.; Khiabanian, H.; Ma, J.; Grunn, A.; Fangazio, M.; Capello, D.; Monti, S.; et al. Analysis of the Chronic Lymphocytic Leukemia Coding Genome: Role of NOTCH1 Mutational Activation. J. Exp. Med. 2011, 208, 1389–1401. [Google Scholar] [CrossRef]
- Fabbri, G.; Dalla-Favera, R. The Molecular Pathogenesis of Chronic Lymphocytic Leukaemia. Nat. Rev. Cancer. 2016, 16, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Putowski, M.; Giannopoulos, K. Perspectives on Precision Medicine in Chronic Lymphocytic Leukemia: Targeting Recurrent Mutations-NOTCH1, SF3B1, MYD88, BIRC3. J. Clin. Med. 2021, 10, 3735. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Rasi, S.; Spina, V.; Bruscaggin, A.; Monti, S.; Ciardullo, C.; Deambrogi, C.; Khiabanian, H.; Serra, R.; Bertoni, F.; et al. Integrated Mutational and Cytogenetic Analysis Identifies New Prognostic Subgroups in Chronic Lymphocytic Leukemia. Blood 2013, 121, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Biswas, A.; Süel, K.E.; Jackson, L.K.; Martinez, R.; Gu, H.; Chook, Y.M. Structural Basis for Leucine-Rich Nuclear Export Signal Recognition by CRM1. Nature 2009, 458, 1136–1141. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The Family of Five: TIR-Domain-Containing Adaptors in Toll-like Receptor Signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- O’Neil, J.; Grim, J.; Strack, P.; Rao, S.; Tibbitts, D.; Winter, C.; Hardwick, J.; Welcker, M.; Meijerink, J.P.; Pieters, R.; et al. FBW7 Mutations in Leukemic Cells Mediate NOTCH Pathway Activation and Resistance to γ-Secretase Inhibitors. J. Exp. Med. 2007, 204, 1813–1824. [Google Scholar] [CrossRef]
- Quesada, V.; Conde, L.; Villamor, N.; Ordóñez, G.R.; Jares, P.; Bassaganyas, L.; Ramsay, A.J.; Beà, S.; Pinyol, M.; Martínez-Trillos, A.; et al. Exome Sequencing Identifies Recurrent Mutations of the Splicing Factor SF3B1 Gene in Chronic Lymphocytic Leukemia. Nat. Genet. 2012, 44, 47–52. [Google Scholar] [CrossRef]
- GeneCards, T.H.G.D. SF3B1 Gene—Splicing Factor 3b Subunit 1 Protein Coding (Updated: Mar 21, 2023; GC02M197447; GIFtS: 52). Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SF3B1&keywords=sf3b1 (accessed on 15 June 2023).
- Leeksma, A.C.; Derks, I.A.M.; Kasem, M.H.; Kilic, E.; de Klein, A.; Jager, M.J.; van de Loosdrecht, A.A.; Jansen, J.H.; Navrkalova, V.; Faber, L.M.; et al. The Effect of SF3B1 Mutation on the DNA Damage Response and Nonsense-Mediated MRNA Decay in Cancer. Front. Oncol. 2021, 10, 609409. [Google Scholar] [CrossRef]
- GeneCards, T.H.G.D. NOTCH1 Gene—Notch Receptor 1 Protein Coding (Updated: Mar 21, 2023; GC09M138053; GIFtS: 59). Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=NOTCH1&keywords=notch1 (accessed on 15 June 2023).
- Li, Z.; Yu, F.; Ye, W.; Mao, L.; Huang, J.; Shao, Y.; Yan, J.; Yu, W.; Jin, J.; Wang, J. Clinical Features and Prognostic Significance of NOTCH1 Mutations in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 746577. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, F.; Bittolo, T.; Tissino, E.; Zucchetto, A.; Bomben, R.; Polcik, L.; DannewitzProsseda, S.; Hartmann, T.N.; Gattei, V. Multiple Mechanisms of NOTCH1 Activation in Chronic Lymphocytic Leukemia: NOTCH1 Mutations and Beyond. Cancers 2022, 14, 2997. [Google Scholar] [CrossRef]
- Del Papa, B.; Baldoni, S.; Dorillo, E.; De Falco, F.; Rompietti, C.; Cecchini, D.; Cantelmi, M.G.; Sorcini, D.; Nogarotto, M.; Adamo, F.M.; et al. Decreased NOTCH1 Activation Correlates with Response to Ibrutinib in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2019, 25, 7540–7553. [Google Scholar] [CrossRef] [Green Version]
- Roti, G.; Sorrentino, C.; Cuneo, A. Therapeutic targeting of notch signaling pathway in hematological malignancies. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019037. [Google Scholar] [CrossRef]
- Stephens, D.M. NCCN Guidelines Update: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. J. Natl. Compr. Cancer Netw. 2023, 21, 563–566. [Google Scholar] [CrossRef]
- Schouten, J.P. Relative Quantification of 40 Nucleic Acid Sequences by Multiplex Ligation-Dependent Probe Amplification. Nucleic Acids Res. 2002, 30, e57. [Google Scholar] [CrossRef] [Green Version]
- Donahue, A.C.; Abdool, A.K.; Gaur, R.; Wohlgemuth, J.G.; Yeh, C.-H. Multiplex Ligation-Dependent Probe Amplification for Detection of Chromosomal Abnormalities in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Leuk. Res. 2011, 35, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Oldridge, D.; Poptsova, M.; Hussain, W.M.; Chakravarty, D.; Demichelis, F. A Computational Framework Discovers New Copy Number Variants with Functional Importance. PLoS ONE 2011, 6, e17539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Xia, C.; Zhou, Z.; Wei, D.; Xu, K.; Jia, J.; Xu, W.; Zhang, H. A Multiplex Ligation-dependent Probe Amplification-based Next-generation Sequencing Approach for the Detection of Copy Number Variations in the Human Genome. Mol. Med. Rep. 2018, 18, 5823–5833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, J.R.; Ziman, R.; Yuen, R.K.C.; Feuk, L.; Scherer, S.W. The Database of Genomic Variants: A Curated Collection of Structural Variation in the Human Genome. Nucleic Acids Res. 2014, 42, D986–D992. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Lee, S.; Yun, H.; Cho, S.I.; Kim, B.; Lee, J.-S.; Chae, J.H.; Sun, C.; Park, S.S.; Seong, M.-W. Consistent Count Region–Copy Number Variation (CCR-CNV): An Expandable and Robust Tool for Clinical Diagnosis of Copy Number Variation at the Exon Level Using next-Generation Sequencing Data. Genet. Med. 2022, 24, 663–672. [Google Scholar] [CrossRef]
- Bănescu, C.; Tripon, F.; Trifa, A.P.; Crauciuc, A.G.; Bogliș, A.; Lazar, E.; Dima, D.; Macarie, I.; Duicu, C.; Iancu, M. Presence of Copy Number Aberration and Clinical Prognostic Factors in Patients with Acute Myeloid Leukemia: An Analysis of Effect Modification. Pol. Arch. Intern. Med. 2019, 129, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-H.; Lin, T.-K.; Jou, S.-T.; Lin, C.-Y.; Lin, K.-H.; Lu, M.-Y.; Chen, S.-H.; Cheng, C.-N.; Wu, K.-H.; Wang, S.-C.; et al. MLPA and DNA Index Improve the Molecular Diagnosis of Childhood B-Cell Acute Lymphoblastic Leukemia. Sci. Rep. 2020, 10, 11501. [Google Scholar] [CrossRef]
- Rowswell-Turner, R.B.; Barr, P.M. Treatment of Chronic Lymphocytic Leukemia in Older Adults. J. Geriatr. Oncol. 2017, 8, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Monnereau, A.; Troussard, X.; Belot, A.; Guizard, A.-V.; Woronoff, A.-S.; Bara, S.; Lapôtre-Ledoux, B.; Iwaz, J.; Tretarre, B.; Maynadié, M.; et al. Unbiased Estimates of Long-Term Net Survival of Hematological Malignancy Patients Detailed by Major Subtypes in France. Int. J. Cancer 2013, 132, 2378–2387. [Google Scholar] [CrossRef]
- Royo, R.; Magnano, L.; Delgado, J.; Ruiz-Gil, S.; Gelpí, J.L.; Heyn, H.; Taylor, M.A.; Stankovic, T.; Puente, X.S.; Nadeu, F.; et al. ATM Germline Variants in a Young Adult with Chronic Lymphocytic Leukemia: 8 Years of Genomic Evolution. Blood Cancer J. 2022, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Condoluci, A.; Rossi, D. Age-Related Clonal Hematopoiesis and Monoclonal B-Cell Lymphocytosis/Chronic Lymphocytic Leukemia: A New Association? Haematologica 2018, 103, 751–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Ebert, B.L. Clonal Hematopoiesis in Human Aging and Disease. Science 2019, 366, e4673. [Google Scholar] [CrossRef]
- Delgado, J.; Nadeu, F.; Colomer, D.; Campo, E. Chronic Lymphocytic Leukemia: From Molecular Pathogenesis to Novel Therapeutic Strategies. Haematologica 2020, 105, 2205–2217. [Google Scholar] [CrossRef]
- Eid, O.M.; Abdel Kader, R.M.A.; Fathalla, L.A.; Abdelrahman, A.H.; Rabea, A.; Mahrous, R.; Eid, M.M. Evaluation of MLPA as a Comprehensive Molecular Cytogenetic Tool to Detect Cytogenetic Markers of Chronic Lymphocytic Leukemia in Egyptian Patients. J. Genet. Eng. Biotechnol. 2021, 19, 98. [Google Scholar] [CrossRef]
- Al Zaabi, E.A.; Fernandez, L.A.; Sadek, I.A.; Riddell, D.C.; Greer, W.L. Multiplex Ligation-Dependent Probe Amplification Versus Multiprobe Fluorescence in Situ Hybridization To Detect Genomic Aberrations in Chronic Lymphocytic Leukemia. J. Mol. Diagn. 2010, 12, 197–203. [Google Scholar] [CrossRef]
- Urquhart, B.; Bergeron, M.B.; Brierley, K.; Vasto, M.D.; Edissi, C.; Morphy, U.; Ross, M. 57. A Rare Case of near-Tetraploidy in CLL: An Investigation by MLPA, FISH, and Chromosome Analysis. Cancer Genet. 2022, 19, 260–261. [Google Scholar] [CrossRef]
- Reindl, L.; Bacher, U.; Dicker, F.; Alpermann, T.; Kern, W.; Schnittger, S.; Haferlach, T.; Haferlach, C. Biological and Clinical Characterization of Recurrent 14q Deletions in CLL and Other Mature B-Cell Neoplasms: 14q Deletions in Mature B-Cell Neoplasms. Br. J. Haematol. 2010, 151, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Adão, D.C.A.C. Characterization of Chronic Lymphocytic Leukemia by ACGH/MLPA. Ph.D. Thesis, University of Lisbon, Faculty of Sciences, Lisbon, Portugal, 2018. [Google Scholar]
- GeneCards, T.H.G.D. PTEN Gene—Phosphatase And Tensin Homolog Protein Coding (Updated: Mar 21, 2023, GC10P096651; GIFtS: 59). GeneCard Hum. Gene Database 2023. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PTEN&keywords=pten (accessed on 15 June 2023).
- Bernardi, R.; Ghia, P. Reactivating Nuclear PTEN to Treat CLL. Oncotarget 2017, 8, 35486–35487. [Google Scholar] [CrossRef]
- Carrà, G.; Panuzzo, C.; Torti, D.; Parvis, G.; Crivellaro, S.; Brancaccio, M.; Guerrasio, A.; Pandolfi, P.P.; Saglio, G.; Morotti, A. Therapeutic Inhibition of HAUSP-PTEN Network in Chronic Lymphocytic Leukemia. Clin. Lymphoma MyelomaLeuk. 2015, 15, S25. [Google Scholar] [CrossRef]
- Leupin, N.; Cenni, B.; Novak, U.; Hügli, B.; Graber, H.U.; Tobler, A.; Fey, M.F. Disparate Expression of the PTEN Gene: A Novel Finding in B-Cell Chronic Lymphocytic Leukaemia (B-CLL). Br. J. Haematol. 2003, 121, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, D.; Yehuda, O.; Ben-Neriah, S.; Kapelushnik, Y.; Ben-Yehuda, D. Dup(10q) Lacking α-Satellite DNA in Bone Marrow Cells of a Patient with Acute Myeloid Leukemia. Cancer Genet. Cytogenet. 1996, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Raimond, C.S.; Chang, N.M.; Ravindranath, Y.; Behm, G.F.; Gresik, V.M.; Steuber, C.P.; Weinstein, J.H.; Caroll, J.A. Chromosomal Abnormalities in 478 Children With Acute Myeloid Leukemia: Clinical Characteristics and Treatment Outcome in a Cooperative Pediatric Oncology Group Study—POG 8821. Blood 1999, 94, 3707–3716. [Google Scholar] [CrossRef]
- Abdool, A.; Donahue, A.C.; Wohlgemuth, J.G.; Yeh, C.-H. Detection, Analysis and Clinical Validation of Chromosomal Aberrations by Multiplex Ligation-Dependent Probe Amplification in Chronic Leukemia. PLoS ONE 2010, 5, e15407. [Google Scholar] [CrossRef]
- Sellmann, L.; Gesk, S.; Walter, C.; Ritgen, M.; Harder, L.; Martín-Subero, J.I.; Schroers, R.; Siemer, D.; Nückel, H.; Dyer, M.J.S.; et al. Trisomy 19 Is Associated with Trisomy 12 and Mutated IGHV Genes in B-Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2007, 138, 217–220. [Google Scholar] [CrossRef]
- Srinivasan, V.K.; Naseem, S.; Varma, N.; Lad, D.P.; Malhotra, P. Genomic Alterations in Chronic Lymphocytic Leukemia and Their Correlation with Clinico-Hematological Parameters and Disease Progression. Blood Res. 2020, 55, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zenz, T.; Eichhorst, B.; Busch, R.; Denzel, T.; Häbe, S.; Winkler, D.; Bühler, A.; Edelmann, J.; Bergmann, M.; Hopfinger, G.; et al. TP53 Mutation and Survival in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2010, 28, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Xu, Z.; Scuoppo, C.; Rillahan, C.D.; Gao, J.; Spitzer, B.; Bosbach, B.; Kastenhuber, E.R.; Baslan, T.; et al. Deletions Linked to TP53 Loss Drive Cancer through P53-Independent Mechanisms. Nature 2016, 531, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Soussi, T.; Baliakas, P. Landscape of TP53 Alterations in Chronic Lymphocytic Leukemia via Data Mining Mutation Databases. Front. Oncol. 2022, 12, 808886. [Google Scholar] [CrossRef]
- Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.; Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko, A.; DeLuca, D.S.; Zhang, L.; et al. SF3B1 and Other Novel Cancer Genes in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2011, 365, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, C.J. SF3B1 Mutations in Chronic Lymphocytic Leukemia. Blood 2013, 121, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Balatti, V.; Bottoni, A.; Palamarchuk, A.; Alder, H.; Rassenti, L.Z.; Kipps, T.J.; Pekarsky, Y.; Croce, C.M. NOTCH1 Mutations in CLL Associated with Trisomy 12. Blood 2012, 119, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Vaisitti, T.; Deaglio, S. The NOTCH Pathway and Its Mutations in Mature B Cell Malignancies. Front. Oncol. 2018, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Cravero, K.; Pantone, M.V.; Shin, D.H.; Bergman, R.; Cochran, R.; Chu, D.; Zabransky, D.J.; Karthikeyan, S.; Waters, I.G.; Hunter, N.; et al. NOTCH1 PEST Domain Variants Are Responsive to Standard of Care Treatments despite Distinct Transformative Properties in a Breast Cancer Model. Oncotarget 2022, 13, 373–386. [Google Scholar] [CrossRef]
- Helbig, D.R.; Abu Zeinah, G.F.; Bhavsar, E.B.; Allan, J.N. Outcomes in Chronic Lymphocytic Leukemia (CLL) Patients with NOTCH1 Signaling Pathway Mutations. J. Clin. Oncol. 2019, 37, 7524. [Google Scholar] [CrossRef]
- Yi, S.; Li, Z.; Zou, D.; An, G.; Cui, R.; Zhong, S.; Li, H.; Xiong, W.; Li, C.; Chen, W.; et al. Intratumoral Genetic Heterogeneity and Number of Cytogenetic Aberrations Provide Additional Prognostic Significance in Chronic Lymphocytic Leukemia. Genet. Med. 2017, 19, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Del Giudice, I.; Rossi, D.; Chiaretti, S.; Marinelli, M.; Tavolaro, S.; Gabrielli, S.; Laurenti, L.; Marasca, R.; Rasi, S.; Fangazio, M.; et al. NOTCH1 Mutations in +12 Chronic Lymphocytic Leukemia (CLL) Confer an Unfavorable Prognosis, Induce a Distinctive Transcriptional Profiling and Refine the Intermediate Prognosis of +12 CLL. Haematologica 2012, 97, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Fuka, G.; Farias-Vieira, T.M.; Hummel, L.; Blunck, C.B.; Santoro, J.C.; Terra-Granado, E.; Conceição Barbosa, T.; Emerenciano, M.; Pombo-de-Oliveira, M.S. Evaluation of Multiplex Ligation Dependent Probe Amplification (MLPA) for Identification of Acute Lymphoblastic Leukemia with an Intrachromosomal Amplification of Chromosome 21 (IAMP21) in a Brazilian Population. Mol. Cytogenet. 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhourani, E.; Rincic, M.; Othman, M.A.; Pohle, B.; Schlie, C.; Glaser, A.; Liehr, T. Comprehensive Chronic Lymphocytic Leukemia Diagnostics by Combined Multiplex Ligation Dependent Probe Amplification (MLPA) and Interphase Fluorescence in Situ Hybridization (IFISH). Mol. Cytogenet. 2014, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielaite, M.; Torp, M.H.; Rasmussen, M.S.; Andreu-Sánchez, S.; Vieira, F.G.; Pedersen, C.B.; Kinalis, S.; Madsen, M.B.; Kodama, M.; Demircan, G.S.; et al. A Comparison of Tools for Copy-Number Variation Detection in Germline Whole Exome and Whole Genome Sequencing Data. Cancers 2021, 13, 6283. [Google Scholar] [CrossRef]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A Copy Number Variation Map of the Human Genome. Nat. Rev. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef]
- Ionita-Laza, I.; Rogers, A.J.; Lange, C.; Raby, B.A.; Lee, C. Genetic Association Analysis of Copy-Number Variation (CNV) in Human Disease Pathogenesis. Genomics 2009, 93, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardinelli, L.; Giorgi, R.; Ruiz, A.; Leal, A.; Pinotti, P.; Ferreira, A.; Velloso, E.; Buccheri, V.; Rocha, V.; Bendit, I. Comparison between multiplex ligation-dependent probe amplification (mlpa) and cytogenetics for validation of chromosomal aberrations in chronic lymphocytic leukemia. Hematol. Transfus. Cell Ther. 2021, 43, S423–S424. [Google Scholar] [CrossRef]
- Braggio, E.; Fonseca, R.; Kay, N.E. CGH Protocols: Chronic Lymphocytic Leukemia. In Array Comparative Genomic Hybridization; Banerjee, D., Shah, S.P., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 973, pp. 87–98. ISBN 978-1-62703-280-3. [Google Scholar]
- Leeksma, A.C.; Baliakas, P.; Moysiadis, T.; Puiggros, A.; Plevova, K.; Van der Kevie-Kersemaekers, A.-M.; Posthuma, H.; Rodriguez-Vicente, A.E.; Tran, A.N.; Barbany, G.; et al. Genomic Arrays Identify High-Risk Chronic Lymphocytic Leukemia with Genomic Complexity: A Multi-Center Study. Haematologica 2020, 106, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Rasi, S.; Fabbri, G.; Spina, V.; Fangazio, M.; Forconi, F.; Marasca, R.; Laurenti, L.; Bruscaggin, A.; Cerri, M.; et al. Mutations of NOTCH1 Are an Independent Predictor of Survival in Chronic Lymphocytic Leukemia. Blood 2012, 119, 521–529. [Google Scholar] [CrossRef] [Green Version]
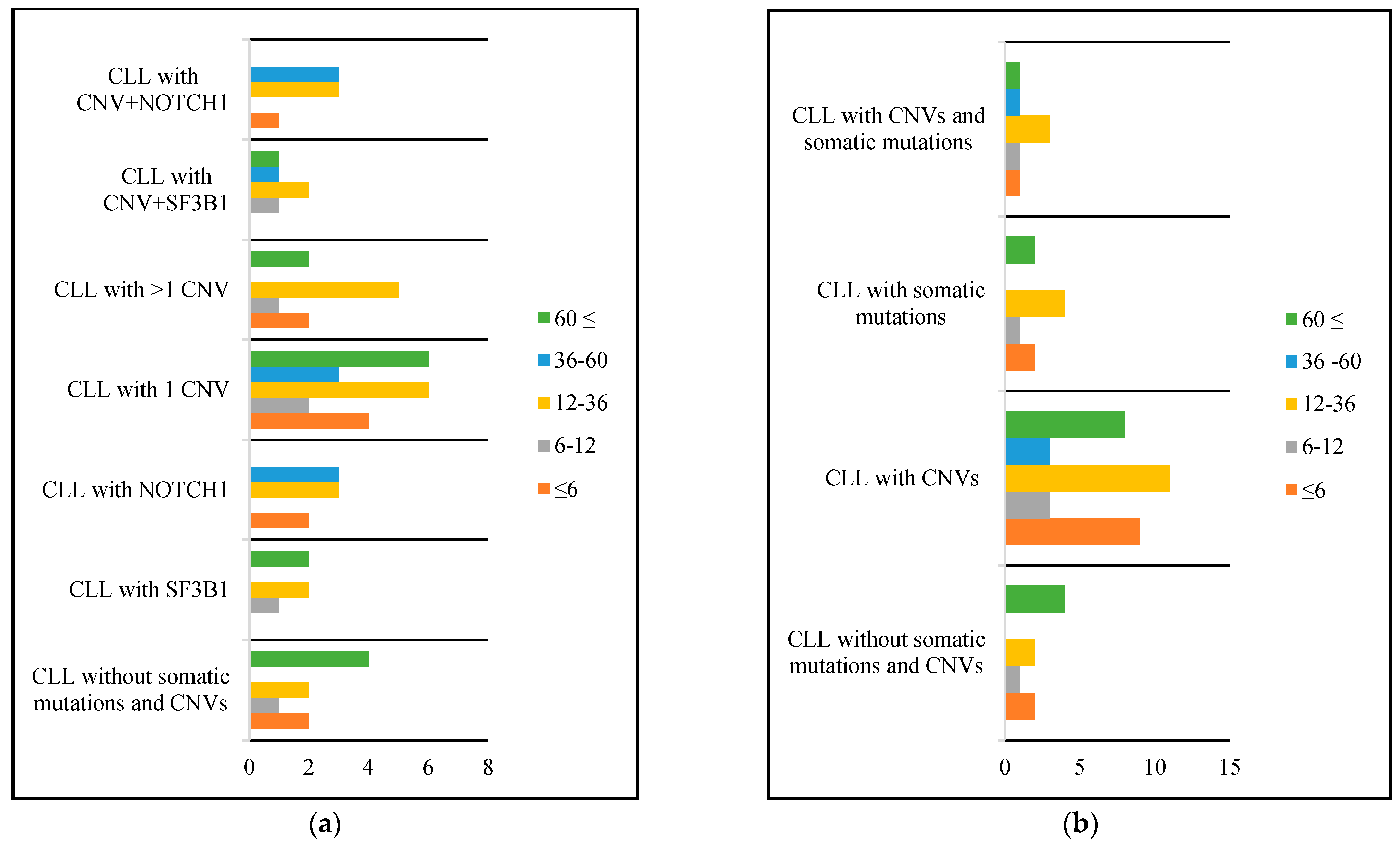
| Variable | All CLL (n = 110) | CLL without Somatic Mutations and CNVs (n = 42) | CLL with CNVs (n = 52) | p Value | CLL with Somatic Mutation (n = 26) | p Value | CLL with CNVs and Somatic Mutation (n = 10) | p Value |
|---|---|---|---|---|---|---|---|---|
| Age, y | ||||||||
| 30–39 | 1 (1) | 1 (2) | 0 (0) | 0.12 | 0 (0) | 0.04 | 0 (0) | 0.20 |
| 40–49 | 7 (6) | 4 (10) | 3 (6) | 0 (0) | 0 (0) | |||
| 50–59 | 28 (26) | 13 (31) | 14 (27) | 2 (8) | 1 (10) | |||
| 60–69 | 34 (31) | 14 (33) | 10 (19) | 13 (50) | 3 (30) | |||
| ≥70 | 40 (36) | 10 (24) | 25 (48) | 11 (42) | 6 (60) | |||
| Sex | ||||||||
| Men | 70 (64) | 26 (62) | 35 (67) | 0.59 | 10 (39) | 0.06 | 7 (70) | 0.63 |
| Women | 40 (36) | 16 (38) | 17 (33) | 16 (62) | 3 (30) | |||
| WBC, cells/µL | ||||||||
| <9000 | 14 (13) | 10 (24) | 4 (8) | 0.03 | 1 (4) | 0.03 | 1 (10) | 0.34 |
| ≥9000 | 96 (87) | 32 (76) | 48 (92) | 25 (96) | 9 (90) | |||
| PLT, cells/µL | ||||||||
| <150,000 | 42 (38) | 14 (33) | 23 (44) | 0.28 | 8 (31) | 0.83 | 3 (30) | 0.84 |
| ≥150,000 | 68 (62) | 28 (67) | 29 (56) | 18 (69) | 7 (70) | |||
| Hemoglobin, g/dL | ||||||||
| <13 | 74 (67) | 31 (74) | 33 (64) | 0.28 | 18 (69) | 0.68 | 8 (80) | 0.68 |
| ≥13 | 36 (33) | 11 (26) | 19 (37) | 8 (31) | 2 (20) | |||
| LDH, IU/L | ||||||||
| <480 | 90 (82) | 36 (86) | 40 (77) | 0.28 | 23 (89) | 0.75 | 9 (90) | 0.72 |
| ≥480 | 20 (18) | 6 (14) | 12 (23) | 3 (12) | 1 (10) | |||
| LYMPH, % | ||||||||
| <25 | 5 (5) | 3 (7) | 1 (2) | 0.20 | 1 (4) | 0.31 | 0 (0) | 0.45 |
| 25–40 * | 4 (4) | 3 (7) | 1 (2) | 0 (0.) | 0 (0) | |||
| ≥40 | 101 (92) | 36 (86) | 50 (96) | 25 (96) | 10 (100) | |||
| Alive | 43 (39) | 23 (55) | 4 (8) | <0.001 | 6 (23) | 0.01 | 2 (20) | 0.05 |
| Deceased | 67 (61) | 19 (45) | 48 (92) | 20 (77) | 8 (80) | |||
| Variable | CLL without CNVs | 1 CNV | p Value | >1CNV | p Value |
|---|---|---|---|---|---|
| 110 (100) | 42 (38) | 35 (32) | 17 (16) | ||
| Age, y | |||||
| 30–39 | 1 (2) | 0 (0) | 0.12 | 0 (0) | 0.46 |
| 40–49 | 4 (10) | 2 (6) | 1 (6) | ||
| 50–59 | 13 (31) | 11 (31) | 3 (18) | ||
| 60–69 | 14 (33) | 5 (14) | 5 (29) | ||
| ≥70 | 10 (24) | 17 (49) | 8 (47) | ||
| Sex | |||||
| Men | 26 (62) | 21 (60) | 0.86 | 14 (82) | 0.13 |
| Women | 16 (38) | 14 (40) | 3 (18) | ||
| WBC, cells/µL | |||||
| <9000 | 10 (24) | 4 (11) | 0.16 | 0 (0) | 0.03 |
| ≥9000 | 32 (76) | 31 (89) | 17 (100) | ||
| PLT, cells/µL | |||||
| <150,000 | 14 (33) | 14 (40) | 0.54 | 9 (53) | 0.16 |
| ≥150,000 | 28 (67) | 21 (60) | 8 (47) | ||
| Hemoglobin, g/dL | |||||
| <13 | 31 (74) | 22 (63) | 0.30 | 11 (65) | 0.48 |
| ≥13 | 11 (26) | 13 (37) | 6 (35) | ||
| LDH, IU/L | |||||
| <480 | 36 (86) | 27 (77) | 0.33 | 13 (77) | 0.39 |
| ≥480 | 6 (14) | 8 (23) | 4 (24) | ||
| LYMPH, % | |||||
| <25 | 3 (7) | 1 (3) | 0.47 | 0 (0) | 0.26 |
| 25–40 * | 3 (7) | 1 (3) | 0 (0) | ||
| ≥40 | 36 (86) | 33 (94) | 17 (100) | ||
| Alive | 23 (55) | 23 (66) | 0.33 | 5 (29) | 0.08 |
| Deceased | 19 (45) | 12 (34) | 12 (71) | ||
| Variable | CLL without Somatic Mutations | SF3B1 | p Value | NOTCH1 | p Value | SF3B1 + CNV | p Value | NOTCH1 + CNV | p Value |
|---|---|---|---|---|---|---|---|---|---|
| 110 (100) | 42 (38) | 13 (12) | 13 (12) | 5 (5) | 5 (5) | ||||
| Age, y | |||||||||
| 30–39 | 1 (2) | 0 (0) | 0.15 | 0 (0) | 0.16 | 0 (0) | 0.86 | 0 (0) | 0.13 |
| 40–49 | 4 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| 50–59 | 13 (31) | 1 (8) | 1 (8) | 1 (20) | 0 (0) | ||||
| 60–69 | 14 (33) | 9 (69) | 5 (39) | 2 (40) | 1 (20) | ||||
| ≥70 | 10 (24) | 3 (23) | 7 (54) | 2 (40) | 4 (80) | ||||
| Sex | |||||||||
| Men | 26 (62) | 9 (69) | 0.63 | 7 (54) | 0.60 | 4 (80) | 0.43 | 3 (60) | 0.93 |
| Women | 16 (38) | 4 (31) | 6 (46) | 1 (20) | 2 (40) | ||||
| WBC, cells/µL | |||||||||
| <9000 | 10 (24) | 0 (0) | 0.05 | 1 (8) | 0.20 | 0 (0) | 0.22 | 1 (20) | 0.85 |
| ≥9000 | 32 (76) | 13 (100) | 12 (92) | 5 (100) | 4 (80) | ||||
| PLT, cells/µL | |||||||||
| <150,000 | 14 (33) | 4 (31) | 0.86 | 1 (8) | 0.07 | 3 (60) | 0.24 | 0 (0) | 0.12 |
| ≥150,000 | 28 (67) | 9 (69) | 12 (92) | 2 (40) | 5 (100) | ||||
| Hemoglobin, g/dL | |||||||||
| <13 | 31 (74) | 10 (77) | 0.82 | 9 (69) | 0.75 | 4 (80) | 0.76 | 4 (80) | 0.76 |
| ≥13 | 11 (26) | 3 (23) | 4 (31) | 1 (20) | 1 (20) | ||||
| LDH, IU/L | |||||||||
| <480 | 36 (86) | 13 (100) | 0.15 | 10 (77) | 0.45 | 5 (100) | 0.37 | 4 (80) | 0.73 |
| ≥480 | 6 (14) | 0 (0) | 3 (23) | 0 (0) | 1 (20) | ||||
| LYMPH, % | |||||||||
| <25 | 3 (7) | 1 (8) | 0.61 | 0 (0) | 0.35 | 0 (0) | 0.66 | 0 (0) | 0.66 |
| 25–40 * | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| ≥40 | 36 (86) | 12 (92) | 13 (100) | 5 (100) | 5 (100) | ||||
| Alive | 23 (55) | 3 (23) | 0.05 | 3 (23) | 0.05 | 2 (40) | 0.53 | 0 (00) | 0.02 |
| Deceased | 19 (45) | 10 (77) | 10 (77) | 3 (60) | 5 (100) | ||||
| Genetic Alteration Identified | No. Patients (%) | |
|---|---|---|
| Somatic mutations | NOTCH1 p.P2514*fs | 12 (11) |
| SF3B1 p.K700E | 12 (11) | |
| MYD 88 p.L265P | 1 (1) | |
| NOTCH1, SF3B1 | 1 (1) | |
| CNVs | del(13q) | 19 (17) |
| del(11q), del(13q) | 9 (8) | |
| dup(12q) | 9 (8) | |
| del(11q) | 4 (4) | |
| del(13q), del(14q) | 2 (2) | |
| del(17p) | 2 (2) | |
| del(14q), del(17p) | 1(1) | |
| del(13q), del(17p) | 1 (1) | |
| del(11q), del(17p), del(19p) | 1(1) | |
| dup(10q), del(13q) | 1(1) | |
| dup(10q), dup(12q) | 1(1) | |
| del(14q) | 1 (1) | |
| trisomy 12, trisomy 13, trisomy 19 | 1(1) | |
| Somatic mutations and CNVs associated | NOTCH1, trisomy 12 | 3 (3) |
| NOTCH1, del(13q) | 2 (2) | |
| SF3B1, del(13q), del(11q) | 2 (2) | |
| SF3B1, del(13q) | 1 (1) | |
| SF3B1, dup(10q), dup(12q) SF3B1, del(11q) | 1 (1) 1 (1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, B.; Tripon, F.; Candea, M.; Banescu, C. Copy Number Variations and Gene Mutations Identified by Multiplex Ligation-Dependent Probe Amplification in Romanian Chronic Lymphocytic Leukemia Patients. J. Pers. Med. 2023, 13, 1239. https://doi.org/10.3390/jpm13081239
Balla B, Tripon F, Candea M, Banescu C. Copy Number Variations and Gene Mutations Identified by Multiplex Ligation-Dependent Probe Amplification in Romanian Chronic Lymphocytic Leukemia Patients. Journal of Personalized Medicine. 2023; 13(8):1239. https://doi.org/10.3390/jpm13081239
Chicago/Turabian StyleBalla, Beata, Florin Tripon, Marcela Candea, and Claudia Banescu. 2023. "Copy Number Variations and Gene Mutations Identified by Multiplex Ligation-Dependent Probe Amplification in Romanian Chronic Lymphocytic Leukemia Patients" Journal of Personalized Medicine 13, no. 8: 1239. https://doi.org/10.3390/jpm13081239





