1. Introduction
Acute myeloid leukemia (AML) is a clonal hematopoietic disorder characterized by the malignant transformation and abnormal proliferation of bone marrow-derived, self-renewing stem cells, or CD34+ CD38− myeloid progenitors [
1,
2,
3]. AML is the most common acute leukemia in adults with an incidence of 2–4/100,000 per year and extremely short survival [
4]. Thanks to advances in modern healthcare, the 5-year overall survival of patients with AML has improved, but it accounts for only 30.5% up to 2023 and differs between various age groups, reaching 50% in younger patients and less than 10% in patients older than 60 years [
5,
6]. The etiology of AML is heterogeneous. In some patients, prior exposure to therapeutic, occupational, or environmental DNA-damaging factors is implicated, but most cases of AML remain without an obvious etiology [
4]. Recently, many recurrent somatic mutations in AML have been identified, including early, disease-initiating driver mutations and germline predisposing mutations/variants, as well as co-occurring passenger lesions [
7].
AML is diagnosed with a blast threshold ≥ 20% in the bone marrow/blood or >10% in the presence of genetic abnormalities that define specific AML subtypes [
8]. The risk stratification of patients diagnosed with AML takes into account numerous disease factors such as the presence/absence of adverse cytogenetic features, germline predisposition, age, poor performance status, prior exposure history to cytotoxic agents or radiotherapy, and prior history of myelodysplasia or myeloproliferative neoplasm, amongst others that are the strongest clinical predictors of early death [
9]. Currently, measurable residual disease (MRD) is the most incorporated into longitudinal risk assessments [
5,
10].
The goal of AML treatment should be the eradication of the disease, whenever possible, accomplished by inducing complete remission (CR) with initial therapy [
8]. However, the response to treatment and the overall prognosis in a particular patient are variable, dependent on the several patient- and tumor-specific factors mentioned above. Treatment failure in AML is represented by two concepts: refractory disease and relapsed disease [
8,
11].
One of the main obstacles in selecting the optimal treatment option for AML therapy is the lack of validated criteria to consider a patient fit or unfit for intensive chemotherapy. The choice of therapy is dictated by many of the same principles that govern risk stratification [
12]. Assigning a patient to a particular treatment group based on the presence/absence of target mutations (so-called, risk stratification by genetics) is an important consideration in determining the proper choice of regimen [
8,
13]. Additionally, managing tactics regarding AML patients may include prognosis stratification by cytogenetic/molecular markers and clinical characteristics [
14]. Patients considered fit for intensive therapy are managed with more aggressive induction regimens that include anthracyclines and cytarabine. Patients considered unsuitable for intensive therapy are managed with lower intensity regimens incorporating hypomethylating agents with venetoclax or low-dose cytarabine [
15,
16].
Despite significant advances in improving the outcomes of hematological disorders over the past decades, mostly due to the development of targeted therapeutics, AML patients respond differently to treatment and prognosis [
17]. Recent studies have explored the relevant contributions of clinical, genetic, demographic, and treatment variables to predict event-free and overall survival in patients with AML [
8,
18]. However, models incorporating all of these factors that aim to predict whether a patient with a definite set of covariates will have a longer remission or life expectancy than a patient with another set of covariates will not always provide the correct results. This emphasizes the necessity of not only evaluating established prognostic factors but also of focusing on new variables and models for the prediction of therapy response and survival in AML patients.
It is known that induction failure due to resistance to chemotherapeutic agents represents a serious obstacle for improving survival outcomes in AML [
19,
20]. Previously, we have demonstrated that the drug responsiveness of tumor cells in AML patients correlates with the response to therapy as well as with the presence of immunophenotypic and cytogenetic prognostic markers [
21]. The prognostic significance of sensitivity or the resistance of leukemic cells to cytotoxic drugs depended on the therapy given to a patient [
22].
The present study evaluated the contribution of tumor cell drug responsiveness to predict the therapy response and overall survival of patients with AML. Our study demonstrates that the developed prognostic scale for the risk stratification of AML patients based on cell sensitivity to chemotherapeutic drugs, MDR1 mRNA expression, tumor origin (primary or secondary), unfavorable cytogenetic abnormalities, and aberrant immunophenotype is related to the therapy response and represents an independent predictive factor for the overall survival of newly diagnosed AML patients without complicated clinical and hematological anamnesis.
2. Materials and Methods
2.1. Patients
A total of 53 patients with acute myeloid leukemia (AML) admitted to the Novosibirsk Hematology Center from 1 January 2014 to 31 December 2018 were enrolled in this study. The average age of patients was 51.2 ± 14.5 years (
Table S1). The gender distribution of the patients was as follows: male—25 patients (47.2%), female—28 patients (52.8%) (
Table S2). Anamnesis collection, objective examination, laboratory tests (peripheral blood and urine analysis, biochemical tests (total protein, alanine aminotransferase, aspartate aminotransferase, prothrombin time, thymol test, C-reactive protein, fibrinogen, lactate dehydrogenase, alkaline phosphatase, creatinine, urea, uric acid, Na
+, K
+, Ca
2+)), and computed tomography of the abdomen, chest, pelvis, and retroperitoneal space, as well as bone marrow aspiration/biopsy with myelogram counting, multiparameter flow cytometry using a wide panel of primary antibodies to hematopoietic cell differentiation clusters, and cytogenetic analysis with detection of chromosome abnormalities using metaphase plates and the FISH method were performed. All recruited patients were diagnosed according to the 5th Edition of the World Health Organization classification of hematolymphoid tumors [
23] and received treatment in accordance with the standard clinical recommendations [
8].
Inclusion criteria: patients diagnosed with AML on the base of clinical, morphological, and genetic markers according to the standard diagnostic protocols for AML were included in this study.
Exclusion criteria: patients presenting with hematological disorders (e.g., anemia) because of other non-malignant causes, patients with HIV, hepatitis B and C, and tuberculosis were excluded from this study.
During the study, patients with AML were assigned into two cohorts: patients received intensive induction chemotherapy with anthracycline-based regimens and patients received non-intensive chemotherapy with low-dose cytarabine because of their comorbidity and complicated clinical and hematological anamnesis.
This study was approved by the Institutional Review Board of Novosibirsk State Medical University (protocol No. 80 from 17 December 2015), and informed consent was obtained from the patients. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki in the current version [
24].
2.2. Collection of Bone Marrow Samples
Bone marrow samples were obtained from AML patients at diagnosis before the start of treatment, as described previously [
21,
22], and were transported to the laboratory within 3-4 h of material collection.
2.3. Biobanking
Bone marrow samples were obtained at the time of diagnosis before the treatment and were stored at −80 °C for further in vitro studies.
2.4. Cell Isolation and Culture
Tumor cells were isolated from the bone marrow of AML patients using centrifugation in lymphocyte separation medium (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s protocol and were cultured in the Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% foetal bovine serum and 1% solution of antibiotics and antimycotic (10,000 μg/mL streptomycin, 10,000 IU/mL penicillin, and 25 μg/mL amphotericin; ICN, Eschwege, Germany) at 37 °C in a 5% CO2.
2.5. Water-Soluble Tetrazolium (WST)-Test
Tumor cells were seeded in 96-well plates at a density of 10
5 cells per well and incubated with daunorubicin (0, 0.05, 0.1, 0.2, 0.4, 0.6, 1, and 2 μM) or cytarabine (0, 0.001, 0.01, 0.2, 0.8, 4, 40, and 82 μM) (both from TEVA, Rehovot, Israel) for 72 h at 37 °C. Next, the cells were incubated with WST-1 solution (10 μL in a 0.5 mg/mL, Roche, Basel, Switzerland) for 3 h at 37 °C. The absorbance was measured at 450 and 620 nm with a Multiscan RC spectrophotometer (Labsystems, Vantaa, Finland). The concentration of chemotherapeutic drugs that caused the death of 50% of tumor cells (IC
50) was calculated as described in [
21]. Then, based on IC
50 values, the drug responsiveness of leukemia cells was scaled for subsequent analysis (
Table 1).
2.6. Real-Time qPCR
Total RNA was extracted from tumor cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quantification was performed with real-time qPCR using BioMaster HS-qPCR SYBR Blue master mix with SYBR Green I fluorescent dye (Biolabmix, Novosibirsk, Russia) and IQ5 Cycler (Bio-Rad, Hercules, CA, USA). The following primers were used: MDR1_F: 5′-AGAGAATCCCCTCCAGATAAGA-3′, MDR1_R: 5′-AAGCCTATTCCATTTTGAACTTTCT-3′, GAPDH_F: 5′-GTGAAGGTCGGAGTCAAC-3′, and GAPDH_R: 5′-TGGAATTTGCCATGGGTG-3′. All measurements were performed in triplicate. Relative level of gene expression was normalized to the level of GAPDH according to the ∆∆Ct method and was determined with CFX96
TM Real-Time system (C1000 Touch
TM, Hercules, CA, USA). MDR1 mRNA expression levels in tumor cells were assessed as described previously [
21].
2.7. Immunocytochemistry
Bone marrow smears of AML patients were incubated with anti-P-glycoprotein (P-gp) antibody [EPR10364-57] (ab170904, Abcam, Cambridge, UK) according to the manufacturer’s protocol. UltraVision Quanto Detection System (Thermo Fisher Scientific, Waltham, MA, USA) and Permanent Fast Red Quanto Substrate System (Thermo Fisher Scientific, USA) were used. Finally, samples were counterstained with Romanovsky–Giemsa and visualized using an Axiostar Plus microscope equipped with an Axiocam MRc5 digital camera (Carl Zeiss, Jena, Germany). P-gp staining was assessed as described previously [
21].
2.8. Statistical Analysis
Correlation analysis was performed using Spearman’s rank order correlation coefficient r, which reflects the strength of the statistical relationships between the studied variables. A value of r 0.01−0.29 indicates a weak positive correlation, 0.30–0.69—moderate positive correlation, and 0.70−1.0—strong positive correlation.
Overall survival was evaluated using the Kaplan–Meier method, and comparisons between groups were performed using the log-rank test.
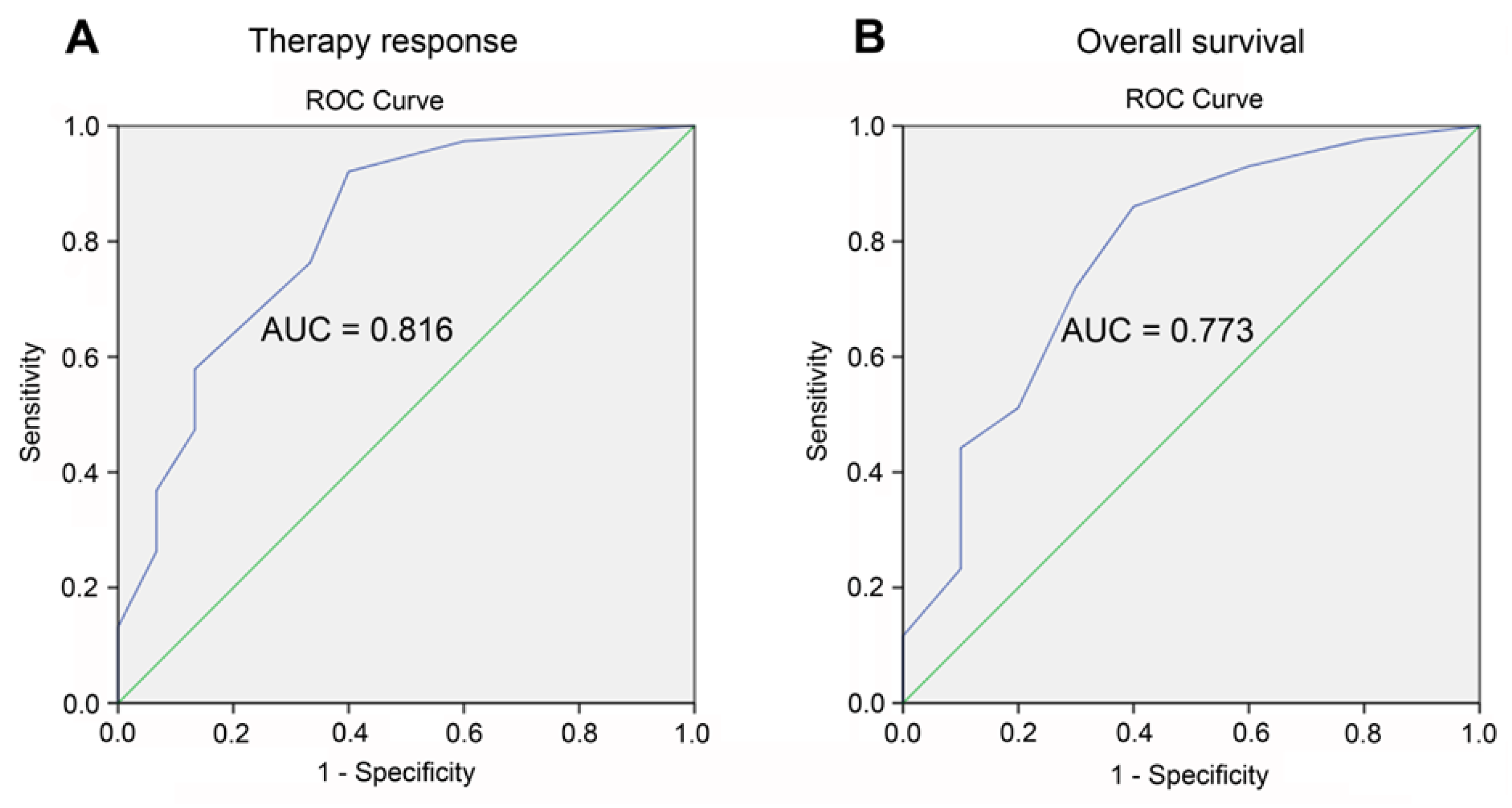
Receiver operating characteristic (ROC) analysis was performed to assess the predictive value of our prognostic scale for risk stratification with respect to therapy response and overall survival of AML patients. ROC curves were constructed and the area under the curve (AUC) reflecting model quality was evaluated as follows: 0.9−1.0—excellent, 0.8−0.9—very good, 0.7−0.8—good, 0.6−0.7—moderate, 0.5−0.6—bad. The optimal cutoff was selected using the Youden index, which maximizes the difference between the true positive rate and the false positive rate. The model was considered statistically significant at p value < 0.05, area under the curve (AUC) > 0.5, and sensitivity and specificity > 60%.
Univariate and multivariate Cox regression analyses were conducted to estimate the predictive value of our prognostic scale for risk stratification and other studied variables with respect to the overall survival of AML patients.
Statistical analysis was performed using MS Excel, OriginPro 7.5, Statistica v13.1, and IBM SPSS software platform. All results with p value ≤ 0.05 were considered statistically significant.
4. Discussion
Progress in acute myeloid leukemia (AML) treatment is occurring at an unprecedented pace. The past decades have been marked by an increasing progress in the scientific understanding of AML biology, leading to enhanced prognostication tools and risk assessments [
5,
29]. Due to a combination of advances in supportive care and the availability of novel, often molecularly targeted, therapies, the life expectancy of AML patients continues to improve [
30,
31]. However, despite obvious progress in the diagnosis and management of AML, some obstacles in the successful achievement of the therapy response exist, one of which is the initial resistance of leukemic cells to cytotoxic drugs [
3,
32]. Therefore, an initial assessment of the sensitivity of tumor cells to chemotherapeutic agents used in the induction therapy of leukemia and other parameters reflecting the multidrug resistance phenotype, such as drug efflux transporters, may be useful in predicting the patient’s response to antitumor therapy. Previously, in our studies related to acute leukemias [
21,
22], as well as in the works related to a wide range of neoplastic diseases of various histological origins (gastric cancer [
33], renal cancer [
34], colorectal cancer [
35], osteosarcoma [
36]), correlations of the therapy response and the clinical outcome of tumor patients with the drug sensitivity of their tumor cells were demonstrated. As for hematological disorders and particularly AML, many works and reviews show the clinical and prognostic relevance of drug transporter proteins [
37,
38,
39,
40,
41]. However, in most studies, drug resistance marker characteristics were considered separately.
Currently, the main approach for predicting the therapy response and overall survival of AML patients is represented in the risk stratification by genetics based on the cytogenetic abnormalities in leukemic cells [
8]. However, it should be emphasized that, for AML patients fit for allogeneic hematopoietic cell transplantation (HCT), decision-making information takes into account the complexity of different risk factors: disease-specific risk factors (e.g., chromosomal aberrations and gene expression profiles), transplantation-specific risk factors (e.g., the choice of donor grafts or stem-cell source), and in some cases patient-specific risk factors (e.g., age and compromised organ functions (comorbidities)), which guide the choice of appropriate HCT regimen [
42]. In this way, a distribution of patients into risk categories (favorable, intermediate, adverse) according to the risk stratification by genetics is complemented by the clinical scoring systems, such as the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) and the Disease Risk Index (DRI), which focus on particular contributing risk factors, such as comorbid conditions or the course of the underlying disease, as well as European Group for Blood and Marrow Transplantation (EBMT), Acute Leukemia (AL)-EMBT, and Pretransplantation Assessment of Mortality (PAM) scores, which take a more holistic approach, seeking to explain the variability of outcomes in broader terms [
43,
44].
Recently, similar combined scoring systems for survival and prognosis prediction have also appeared for patients with AML who are not eligible for HCT. Silveira et al. demonstrated that the distribution of AML patients in accordance with the created novel scoring system integrating both cytogenetic/molecular information and clinical prognostic features such as age (>45 years), white blood cell count (<1.5 or >30.0 × 10
3/μL), and low albumin levels (<3.8 g/dL) were associated with worse overall survival in test cohorts [
45]. In the study of Tsai et al., the incorporation of long non-coding RNA (lncRNA) expression profiles in the 2017 ELN risk classification by genetics was shown to improve the prognostic prediction of AML patients: higher lncRNA scores were significantly associated with older age, adverse gene mutations, and shorter overall and disease-free survival [
46]. Such a combined scoring approach can be useful for informed decision making, better patient counseling, the design of interventional trials, treatment allocation, and personalization according to predicted risk for AML patients.
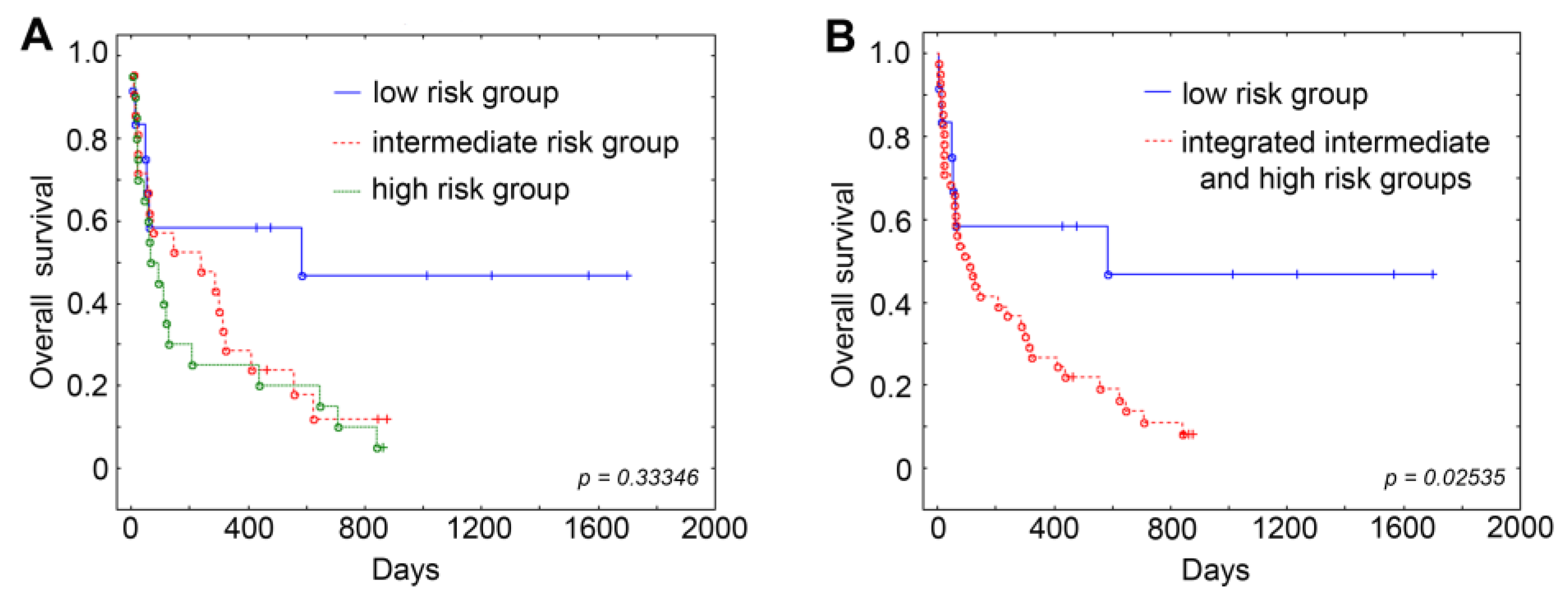
In our study, we attempted to enhance the survival and therapy response prediction for AML patients through a combined approach that incorporates information on drug responsiveness and the
MDR1 mRNA/P-gp expression of leukemic cells at diagnosis into the standard protocol for risk and prognosis stratification. Primary chemoresistance to the induction therapy of several AML patients assigned to the favorable risk category (
Tables S7 and S9) may indicate an insufficient predictive value of the risk stratification by genetics, whereas the distribution of the patients into risk groups, considering the indicators of MDR phenotype such as sensitivity to chemotherapeutic drugs and
MDR1 mRNA/P-gp expression in their tumor cells, demonstrated a stronger correlation with response to therapy and the survival of leukemia patients (
Table 5,
Table 6,
Table 7,
Figure 2). It is noteworthy that the consideration of the sensitivity of leukemic cells to only two cytotoxic drugs used in both intensive and non-intensive induction therapy schemes (daunorubicin and cytarabine) improved the predictive power of standard scoring systems for the ranging of AML patients.
In the last few years, a lot of information, based mainly on the computational approach concerning the predictive role of individual genetic and molecular markers for leukemia patients resistant to chemotherapy, has appeared. For example, in the study of Xu et al., tight associations between the high expression of TSC22D3 domain family genes playing an essential role in tumor progression with poor overall and event-free survival and drug resistance to BCL2 inhibitors in adult AML patients, especially in the chemotherapy group, have been reported [
47]. The study of Liu et al. demonstrated that high miR-107 or miR-17 expression levels were associated with poorer overall survival and event-free survival in the chemotherapy cohort of patients with de novo AML [
48]. A special place is occupied by immunity-associated biomarkers, such as immune-related long noncoding RNAs [
49] and immune microenvironment genes [
50], which are closely related to resistance to immunotherapy and poor prognosis for AML patients. Such molecular and genetic features are designed to complement the risk stratification by genetics and represent high-tech approaches with high cost and the need for specially trained staff. The evaluation of the drug responsiveness of the leukemic cells in vitro does not require expensive equipment, takes a short time, and in general can be useful for a preliminary assessment of the response of AML patients to a number of components of standard induction chemotherapy (both intensive and non-intensive), which may be of particular value and applicability in low- and middle-income countries that are limited in full cytogenetic and molecular information at diagnosis and can be one of the steps towards personalized therapy for leukemia patients.