Pectoralis Major in Salvage Total Laryngectomy after Irradiation: Morbidity, Mortality, Functional, and Oncological Results in a Referral Center in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique and Postoperative Management
2.2. Statistical Analysis
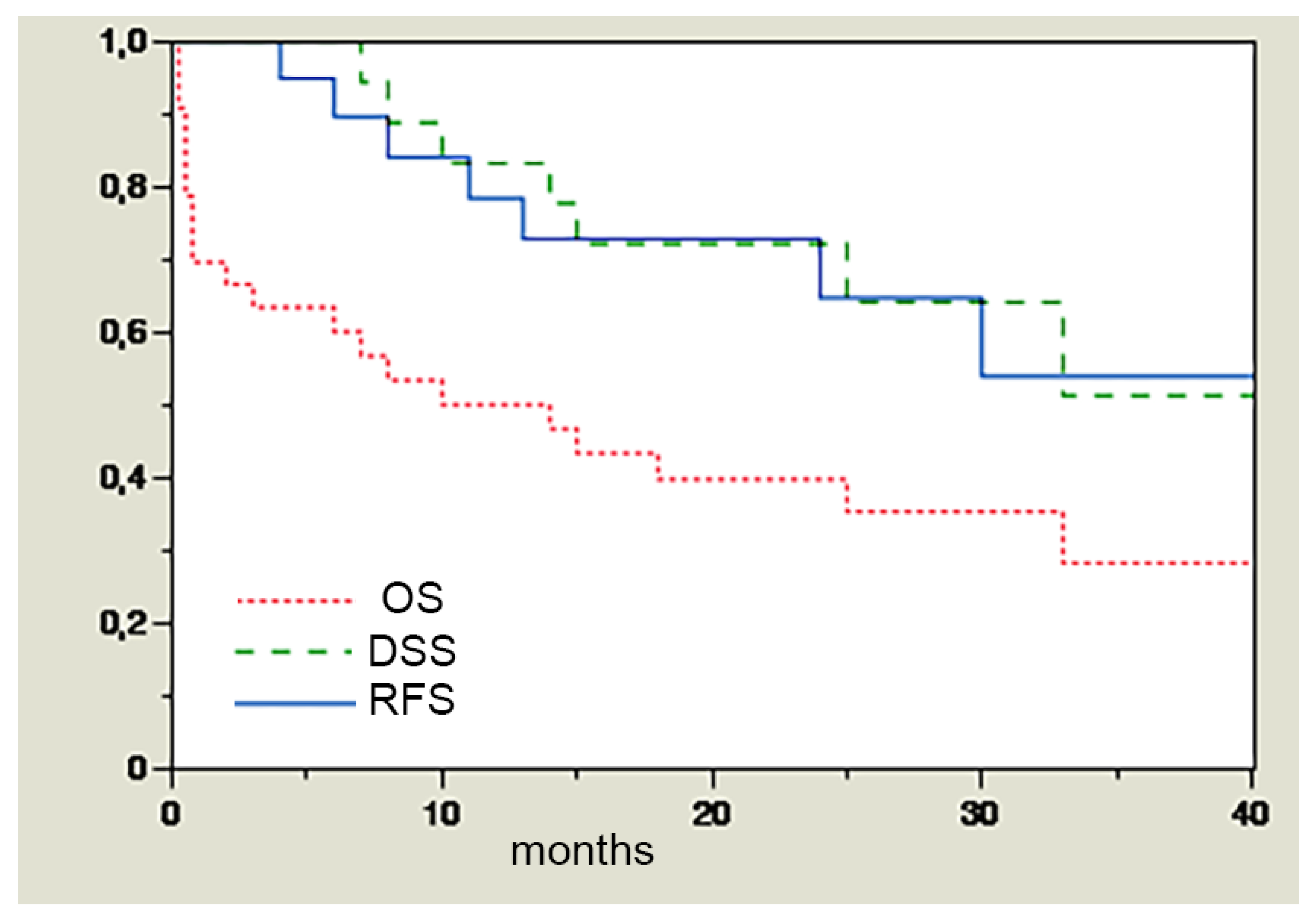
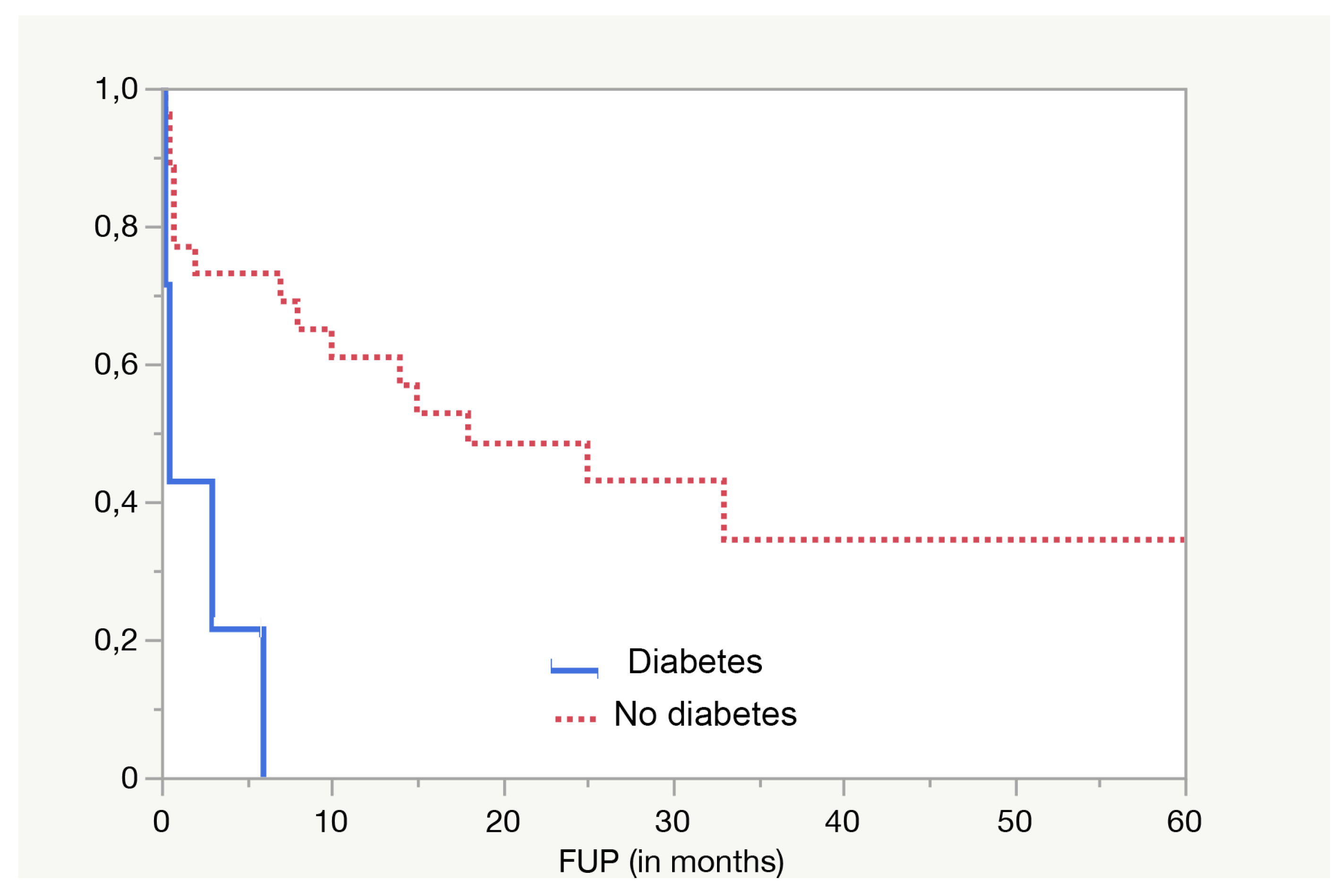
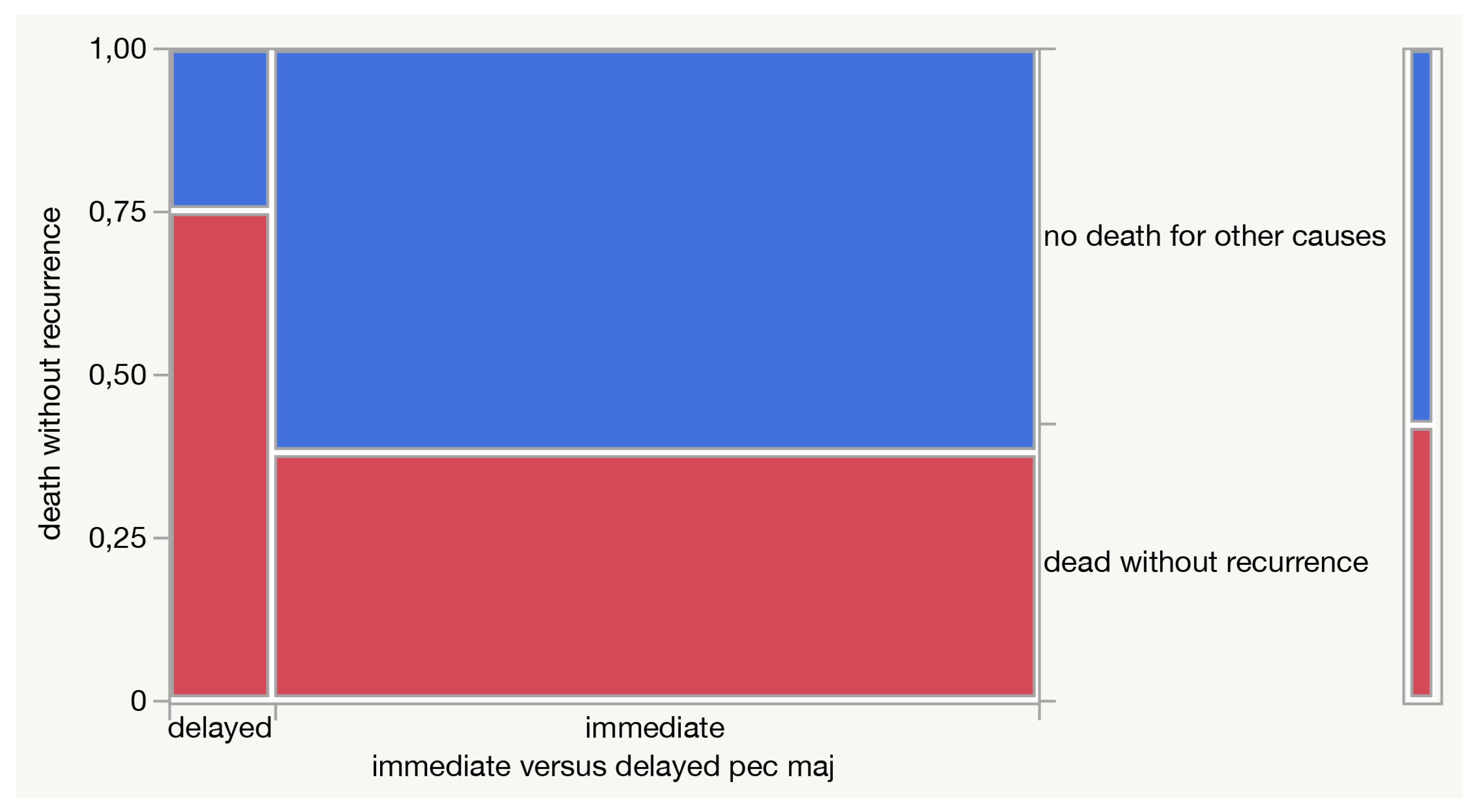
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bussu, F.; Miccichè, F.; Rigante, M.; Dinapoli, N.; Parrilla, C.; Bonomo, P.; Cadoni, G.; Mantini, G.; Galli, J.; Rufini, V.; et al. Oncologic outcomes in advanced laryngeal squamous cell carcinomas treated with different modalities in a single institution: A retrospective analysis of 65 cases. Head Neck 2012, 34, 573–579. [Google Scholar] [CrossRef]
- Cabrera, C.I.; Joseph Jones, A.; Philleo Parker, N.; Emily Lynn Blevins, A.; Weidenbecher, M.S. Pectoralis Major Onlay vs. Interpositional Reconstruction Fistulation After Salvage Total Laryngectomy: Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2021, 164, 972–983. [Google Scholar] [CrossRef]
- Bussu, F.; Paludetti, G.; Almadori, G.; De Virgilio, A.; Galli, J.; Miccichè, F.; Tombolini, M.; Rizzo, D.; Gallo, A.; Giglia, V.; et al. Comparison of total laryngectomy with surgical (cricohyoidopexy) and nonsurgical organ-preservation modalities in advanced laryngeal squamous cell carcinomas: A multicenter retrospective analysis. Head Neck 2013, 35, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Berkey, B.A.; Forastiere, A.; Cooper, J.; Maor, M.; Goepfert, H.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Outcome of salvage total laryngectomy following organ preservation therapy: The Radiation Therapy Oncology Group trial 91-11. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 44–49. [Google Scholar] [CrossRef]
- Anschütz, L.; Nisa, L.; Elicin, O.; Bojaxhiu, B.; Caversaccio, M.; Giger, R. Pectoralis major myofascial interposition flap prevents postoperative pharyngocutaneous fistula in salvage total laryngectomy. Eur. Arch. Otorhinolaryngol. 2016, 273, 3943–3949. [Google Scholar] [CrossRef]
- Sassler, A.M.; Esclamado, R.M.; Wolf, G.T. Surgery after organ preservation therapy. Analysis of wound complications. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 162–165. [Google Scholar] [CrossRef]
- Patel, U.A.; Moore, B.A.; Wax, M.; Rosenthal, E.; Sweeny, L.; Militsakh, O.N.; Califano, J.A.; Lin, A.C.; Hasney, C.P.; Butcher, R.B.; et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1156–1162. [Google Scholar] [CrossRef]
- Ikiz, A.O.; Uça, M.; Güneri, E.A.; Erdağ, T.K.; Sütay, S. Pharyngocutaneous fistula and total laryngectomy: Possible predisposing factors, with emphasis on pharyngeal myotomy. J. Laryngol. Otol. 2000, 114, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Paydarfar, J.A.; Birkmeyer, N.J. Complications in head and neck surgery: A meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Puram, S.V.; Rocco, J.W.; Old, M.O.; Kang, S.Y. Salvage laryngectomy following organ-preservation therapy—An evidence-based review. Oral. Oncol. 2019, 88, 137–144. [Google Scholar] [CrossRef]
- Bussu, F.; Gallus, R.; Navach, V.; Bruschini, R.; Tagliabue, M.; Almadori, G.; Paludetti, G.; Calabrese, L. Contemporary role of pectoralis major regional flaps in head and neck surgery. Acta Otorhinolaryngol. Ital. 2014, 34, 327–341. [Google Scholar] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Cann, C.I.; Fried, M.P.; Rothman, K.J. Epidemiology of squamous cell cancer of the head and neck. Otolaryngol. Clin. N. Am. 1985, 18, 367–388. [Google Scholar] [CrossRef]
- Barclay, T.H.; Rao, N.N. The incidence and mortality rates for laryngeal cancer from total cancer registries. Laryngoscope 1975, 85, 254–258. [Google Scholar] [CrossRef]
- Almadori, G.; Bussu, F.; Cadoni, G.; Galli, J.; Paludetti, G.; Maurizi, M. Molecular markers in laryngeal squamous cell carcinoma: Towards an integrated clinicobiological approach. Eur. J. Cancer 2005, 41, 683–693. [Google Scholar] [CrossRef]
- Pfister, D.G.; Laurie, S.A.; Weinstein, G.S.; Mendenhall, W.M.; Adelstein, D.J.; Ang, K.K.; Clayman, G.L.; Fisher, S.G.; Forastiere, A.A.; Harrison, L.B.; et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J. Clin. Oncol. 2006, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef]
- de Vincentiis, M.; Minni, A.; Gallo, A. Supracricoid laryngectomy with cricohyoidopexy (CHP) in the treatment of laryngeal cancer: A functional and oncologic experience. Laryngoscope 1996, 106, 1108–1114. [Google Scholar] [CrossRef]
- de Vincentiis, M.; Minni, A.; Gallo, A.; Di Nardo, A. Supracricoid partial laryngectomies: Oncologic and functional results. Head Neck 1998, 20, 504–509. [Google Scholar] [CrossRef]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner HJr Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef]
- Pfister, D.G.; Strong, E.; Harrison, L.; Haines, I.E.; Pfister, D.A.; Sessions, R.; Spiro, R.; Shah, J.; Gerold, F.; McLure, T.; et al. Larynx preservation with combined chemotherapy and radiation therapy in advanced but resectable head and neck cancer. J. Clin. Oncol. 1991, 9, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.D.; Vaughan, C.W.; Carter, R.; Willett, B.; Heeren, T.; Calarese, P.; Zeitels, S.; Strong, M.S.; Hong, W.K. Larynx preservation using induction chemotherapy plus radiation therapy as an alternative to laryngectomy in advanced head and neck cancer. A long-term follow-up report. Am. J. Clin. Oncol. 1991, 14, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Laccourreye, H.; Laccourreye, O.; Weinstein, G.; Menard, M.; Brasnu, D. Supracricoid laryngectomy with cricohyoidopexy: A partial laryngeal procedure for selected supraglottic and transglottic carcinomas. Laryngoscope 1990, 100, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar]
- Bussu, F.; Almadori, G.; De Corso, E.; Rizzo, D.; Rigante, M.; Parrilla, C.; Valentini, V.; Paludetti, G. Endoscopic horizontal partial laryngectomy by CO2 laser in the management of supraglottic squamous cell carcinoma. Head Neck. 2009, 31, 1196–1206. [Google Scholar] [CrossRef]
- Bussu, F.; Mura, F.; Miccichè, F.; Bertino, G.; Occhini, A.; Almadori, G.; Galli, J.; Pandolfini, M.; Gallus, R.; Autorino, R.; et al. Oncologic outcome of hypopharyngeal carcinoma treated with different modalities at 2 different university hospitals. Head Neck. 2016, 38, 606–612. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Ang, K.K.; Brizel, D. NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancer. Available online: www.nccn.org (accessed on 1 May 2023).
- Richard, J.M.; Sancho-Garnier, H.; Pessey, J.J.; Luboinski, B.; Lefebvre, J.L.; Dehesdin, D.; Stromboni-Luboinski, M.; Hill, C. Randomized trial of induction chemotherapy in larynx carcinoma. Oral. Oncol. 1998, 34, 224–228. [Google Scholar] [CrossRef]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Cooper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.-K.; Gay, G.; Stewart, A.; Robinson, R.A.; et al. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope 2006, 116, 1–13. [Google Scholar] [CrossRef]
- Laudadio, P.; Presutti, L.; Dall’olio, D.; Cunsolo, E.; Consalici, R.; Amorosa, L.; Cancellieri, A.; Bocciolini, C. Supracricoid laryngectomies: Long-term oncological and functional results. Acta Otolaryngol. 2006, 126, 640–649. [Google Scholar] [CrossRef]
- Nakayama, M.; Okamoto, M.; Miyamoto, S.; Takeda, M.; Yokobori, S.; Masaki, T.; Seino, Y. Supracricoid laryngectomy with cricohyoidoepiglotto-pexy or cricohyoido-pexy: Experience on 32 patients. Auris Nasus Larynx. 2008, 35, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.; Crescio, C.; Tramaloni, P.; De Luca, L.M.; Turra, N.; Manca, A.; Crivelli, P.; Tiana, C.R.; Fara, A.; Cossu, A.; et al. Reliability of a Multidisciplinary Multiparametric Approach in the Surgical Planning of Laryngeal Squamous Cell Carcinomas: A Retrospective Observational Study. J. Pers. Med. 2022, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, F.C.; Weinstein, G.S.; Laccourreye, O. Supracricoid partial laryngectomy: An organ-preservation surgery for laryngeal malignancy. Curr. Probl. Cancer 2005, 29, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Hutcheson, K.A.; Lewin, J.S.; Barringer, D.A.; Lisec, A.; Gunn, G.B.; Moore, M.W.S.; Holsinger, F.C. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer 2012, 118, 5793–5799. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Bettini, M.; Molteni, G.; Piccinini, A.; Valoriani, F.; Gabriele, S.; Presutti, L. Analysis of risk factors for pharyngocutaneous fistula after total laryngectomy with particular focus on nutritional status. Acta Otorhinolaryngol. Ital. 2015, 35, 243–248. [Google Scholar]
- Guimarães, A.V.; Aires, F.T.; Dedivitis, R.A.; Kulcsar, M.A.V.; Ramos, D.M.; Cernea, C.R.; Brandão, L.G. Efficacy of pectoralis major muscle flap for pharyngocutaneous fistula prevention in salvage total laryngectomy: A systematic review. Head Neck 2016, 38 (Suppl. S1), E2317–E2321. [Google Scholar] [CrossRef]
- Gendreau-Lefèvre, A.-K.; Audet, N.; Maltais, S.; Thuot, F. Prophylactic pectoralis major muscle flap in prevention of pharyngocutaneous fistula in total laryngectomy after radiotherapy. Head Neck 2015, 37, 1233–1238. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]

| Age at Diagnosis | |
| Mean | 55.5 |
| Range | 30–71 |
| Sex no. (%) | |
| Male | 36 (97.3%) |
| Female | 1 (2.7%) |
| Cigarette smoking no. (%) | |
| Yes | 35 (94.6%) |
| No | 2 (5.4%) |
| Diabetes no. (%) | |
| Yes | 7 (18.9%) |
| No | 30 (81.1%) |
| T classification of primary tumor (before irradiation) no. (%) | |
| 1 | 2 (5.4%) |
| 2 | 22 (59.5%) |
| 3 | 13 (35.1%) |
| N classification of primary tumor (before irradiation) no. (%) | |
| 0 | 35 (94.6%) |
| 1 | 2 (5.4%) |
| Site of primary tumor no. (%) | |
| Glottic | 27 (73%) |
| Supraglottic | 10 (27%) |
| Pathology of primary tumor no. (%) | |
| Squamous cell carcinoma | 36 (97.3%) |
| Adenocarcinoma | 1 (2.7%) |
| Grading of primary tumor no. (%) | |
| I | 4 (10.8%) |
| II | 31 (83.8%) |
| III | 2 (5.4%) |
| Duration between diagnosis and beginning of radiotherapy (months) | |
| Mean | 3.2 |
| Range | 1–15 |
| Primary organ preservation protocol, chemoradiotherapy no. (%) | |
| Radiotherapy alone | 14 (37.8%) |
| Concomitant chemoradiotherapy | 18 (48.7%) |
| Induction chemotherapy followed by concomitant chemoradiotherapy | 5 (13.5%) |
| Duration between end of irradiation and recurrence (months) | |
| Mean | 11.5 |
| Range | 1–65 |
| T classification of recurrence, yT no. (%) | |
| 2 | 2 (5.4%) |
| 3 | 19 (51.4%) |
| 4 | 16 (43.2%) |
| N classification of recurrence, yN no. (%) | |
| 0 | 34 (91.9%) |
| 1 | 3 (8.1%) |
| Duration between diagnosis of recurrence and operation (months) | |
| Mean | 2.6 |
| Range | 1–7 |
| Partial pharyngectomy no. (%) | |
| Yes | 19 (51.4%) |
| No | 18 (48.6%) |
| Cervical skin excision no. (%) | |
| Yes | 3 (8.1%) |
| No | 34 (91.9%) |
| Pharyngeal closure of total laryngectomy defect no. (%) | |
| Interpositioned myocutaneous flap for mucosal defect | 18 (48.7%) |
| Myocutaneous flap, onlay insetting for pharynx, skin for external surfacing | 3 (8.1%) |
| Primary closure with onlay myofascial flap | 12 (32.4%) |
| Primary closure | 4 (10.8%) |
| Type of pectoralis major flap for total laryngectomy defect no. (%) | |
| Myocutaneous | 21 (56.8%) |
| Myofascial | 12 (32.4%) |
| Not performed | 4 (10.8%) |
| Thyroidectomy no. (%) | |
| Total | 7 (19%) |
| Hemithyroidectomy | 13 (35.1%) |
| Not performed | 17 (45.9%) |
| Neck dissection no. (%) | |
| Bilateral | 25 (67.6%) |
| Monolateral | 3 (8.1%) |
| Not performed | 9 (24.3%) |
| Surgical time (h) | |
| Mean | 4.3 |
| Range | 3–5 |
| Date of neck drain removal postsurgery (d) | |
| Mean | 9 |
| Range | 6–20 |
| T classification of total laryngectomy sample, pT no. (%) | |
| 0 (necrotic tissue) | 1 (2.7%) |
| 3 | 11 (29.7%) |
| 4 | 25 (67.6%) |
| N classification of neck dissection sample, pN (n = 28) no. (%) | |
| 0 | 20 (71.4%) |
| 1 | 8 (28.6%) |
| Margins no. (%) | |
| R1 | 8 (21.6%) |
| R0 | 28 (75.7%) |
| Necrotic tissue | 1 (2.7%) |
| Thyroid gland infiltration (n-20) no. (%) | |
| Infiltrated | 4 (20%) |
| Not infiltrated | 16 (80%) |
| Duration of hospital stay postoperative (days) | |
| Mean | 14 |
| Range | 6–30 |
| Postoperative albumin level (gh\dL) | |
| Mean | 3.6 |
| Range | 2–5 |
| Pharyngocutaneous fistula no. (%) | |
| Yes | 20 (54.1%) |
| No | 13 (35.1%) |
| No data | 4 (10.8%) |
| Postoperative day of appearance of fistula | |
| Mean | 8.5 |
| Range | 4–14 |
| Effects of fistula no. (%) | |
| Minor fistula | 7 (35%) |
| Wound dehiscence | 11 (55%) |
| Bleeding and death | 2 (10%) |
| Date of repair of fistula since appearance (d) | |
| Mean | 31.5 |
| Range | 3–113 |
| Management of fistula no. (%) | |
| Conservative treatment | 10 (55.6%) |
| Trimming and primary closure | 3 (16.7%) |
| Trimming and repair by PMMF | 5 (27.7%) |
| Results of management no. (%) | |
| Closed | 10 (55.5%) |
| Persistence and follow-up | 3 (16.7%) |
| Necrosis of flap and repair by LD flap | 1 (5.6%) |
| Bleeding and death | 4 (22.2%) |
| Postoperative bleeding no. (%) | |
| Yes | 11 (29.7%) |
| No | 22 (59.5%) |
| No data | 4 (10.8%) |
| Site no. (%) | |
| Donor site | 3 (27.3%) |
| Neck | 8 (72.7%) |
| Timing of bleeding (postoperative day) | |
| Mean | 10.3 |
| Range | 4–21 |
| Outcome of bleeding no. (%) | |
| Controlled by operation under GA | 4 (36.4%) |
| Death | 7 (63.6%) |
| Recurrent malignancy no. (%) | |
| Yes | 7 (18.9%) |
| No | 26 (70.3%) |
| No data | 4 (10.8%) |
| Type of recurrence no. (%) | |
| Local | 5 (71.4%) |
| Distant | 2 (28.6%) |
| Management of recurrence no. (%) | |
| Palliative chemotherapy | 7 (100%) |
| Outcome of recurrent malignancy after surgical salvage no. (%) | |
| Death | 7 (100%) |
| Postoperative swallowing problems no. (%) | |
| Normal oral feeding | 11 (29.7%) |
| Tube feeding | 12 (32.4%) |
| Dysphagia requiring dilatation | 4 (10.8%) |
| Dysphagia not requiring dilatation | 6 (16.2%) |
| No data | 4 (10.8%) |
| Postoperative speech rehabilitation by tracheoesophageal puncture and prosthesis no. (%) | |
| Inserted and functioning | 15 (40.5%) |
| Not inserted | 18 (48.7%) |
| No data | 4 (10.8%) |
| Timing of TEP (months after surgery) | |
| Mean | 6.9 |
| Range | 6–12 |
| Outcome of follow up no. (%) | |
| Under follow-up | 12 (32.4%) |
| Died | 21 (56.8%) |
| No data | 4 (10.8%) |
| Cause of death no. (%) | |
| Carotid blowout | 6 (28.6%) |
| Venous blowout | 1 (4.8%) |
| Local recurrence | 5 (23.8%) |
| Distant metastases | 2 (9.4%) |
| Myocardial infarction | 2 (9.4%) |
| Septic shock | 1 (4.8%) |
| Intracranial hemorrhage | 1 (4.8%) |
| Sudden arrest | 1 (4.8%) |
| Obstruction of tracheostomy by secretions | 1 (4.8%) |
| COVID-19 | 1 (4.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghany, M.; Amin, A.; Degni, E.; Crescio, C.; Hassan, A.E.M.A.; Ftohy, T.; Bussu, F. Pectoralis Major in Salvage Total Laryngectomy after Irradiation: Morbidity, Mortality, Functional, and Oncological Results in a Referral Center in Egypt. J. Pers. Med. 2023, 13, 1223. https://doi.org/10.3390/jpm13081223
Abdelghany M, Amin A, Degni E, Crescio C, Hassan AEMA, Ftohy T, Bussu F. Pectoralis Major in Salvage Total Laryngectomy after Irradiation: Morbidity, Mortality, Functional, and Oncological Results in a Referral Center in Egypt. Journal of Personalized Medicine. 2023; 13(8):1223. https://doi.org/10.3390/jpm13081223
Chicago/Turabian StyleAbdelghany, Mahmoud, Ayman Amin, Emilia Degni, Claudia Crescio, Asem Elsani M. A. Hassan, Tarek Ftohy, and Francesco Bussu. 2023. "Pectoralis Major in Salvage Total Laryngectomy after Irradiation: Morbidity, Mortality, Functional, and Oncological Results in a Referral Center in Egypt" Journal of Personalized Medicine 13, no. 8: 1223. https://doi.org/10.3390/jpm13081223
APA StyleAbdelghany, M., Amin, A., Degni, E., Crescio, C., Hassan, A. E. M. A., Ftohy, T., & Bussu, F. (2023). Pectoralis Major in Salvage Total Laryngectomy after Irradiation: Morbidity, Mortality, Functional, and Oncological Results in a Referral Center in Egypt. Journal of Personalized Medicine, 13(8), 1223. https://doi.org/10.3390/jpm13081223






