Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review
Abstract
1. Introduction
2. Methods
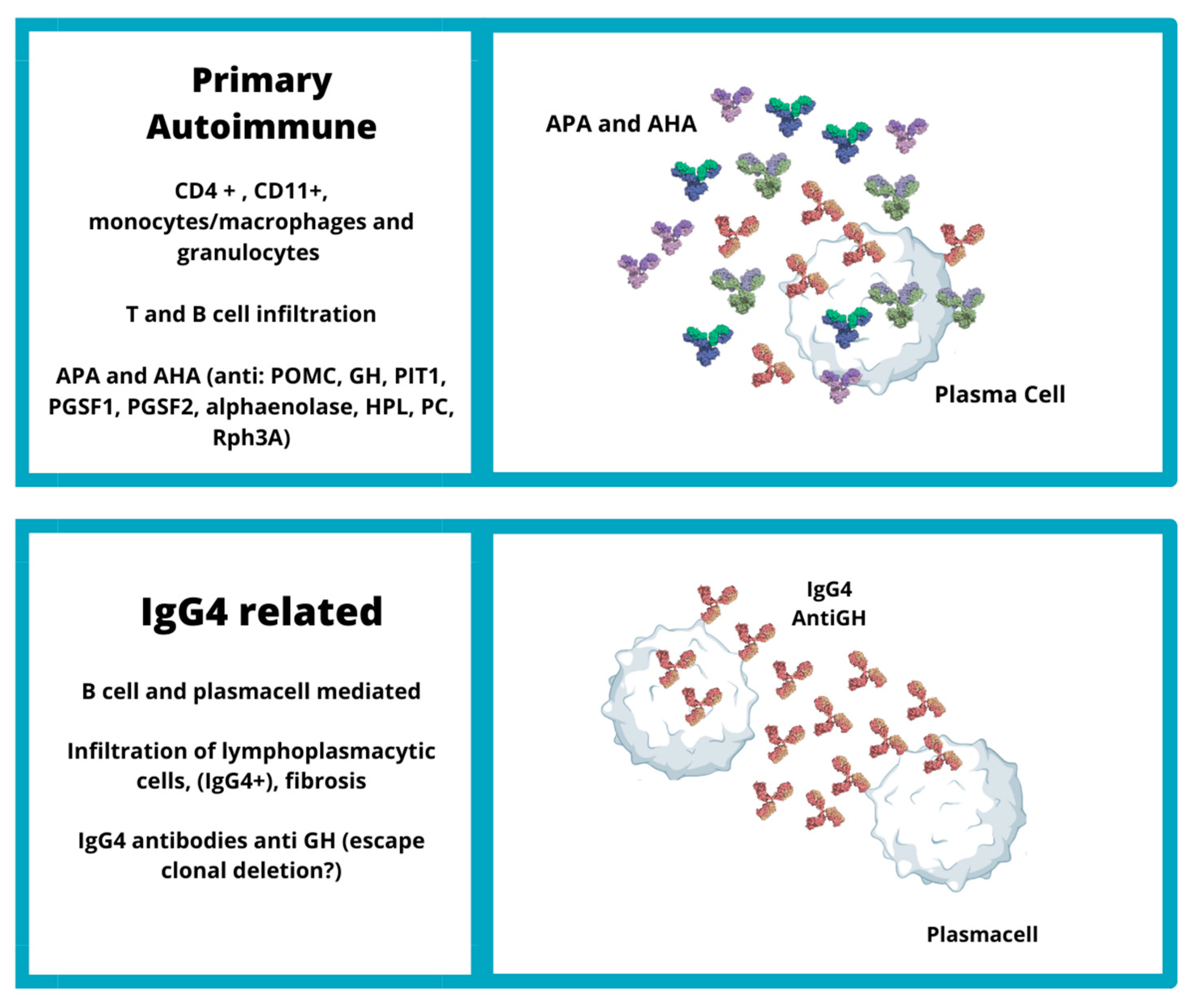
3. Primary Autoimmune Hypophysitis
3.1. Pathogenesis of Primary Autoimmune Hypophysitis
3.2. Classification of Hypophysitis
3.3. Antibodies in Primary Autoimmune Hypophysitis
3.4. Autoantigens of Primary Autoimmune Hypophysitis
3.5. Cell-Mediated Immune Response in Primary Autoimmune Hypophysitis
3.6. The Genetics of Primary Autoimmune Hypophysitis
4. Immunotherapy Induced Hypophysitis
4.1. Pathogenesis of Immunotherapy Induced Hypophysitis
4.2. Genetic Factors of ICI-Induced Hypophysitis
4.3. Paraneoplastic Syndrome Hypothesis
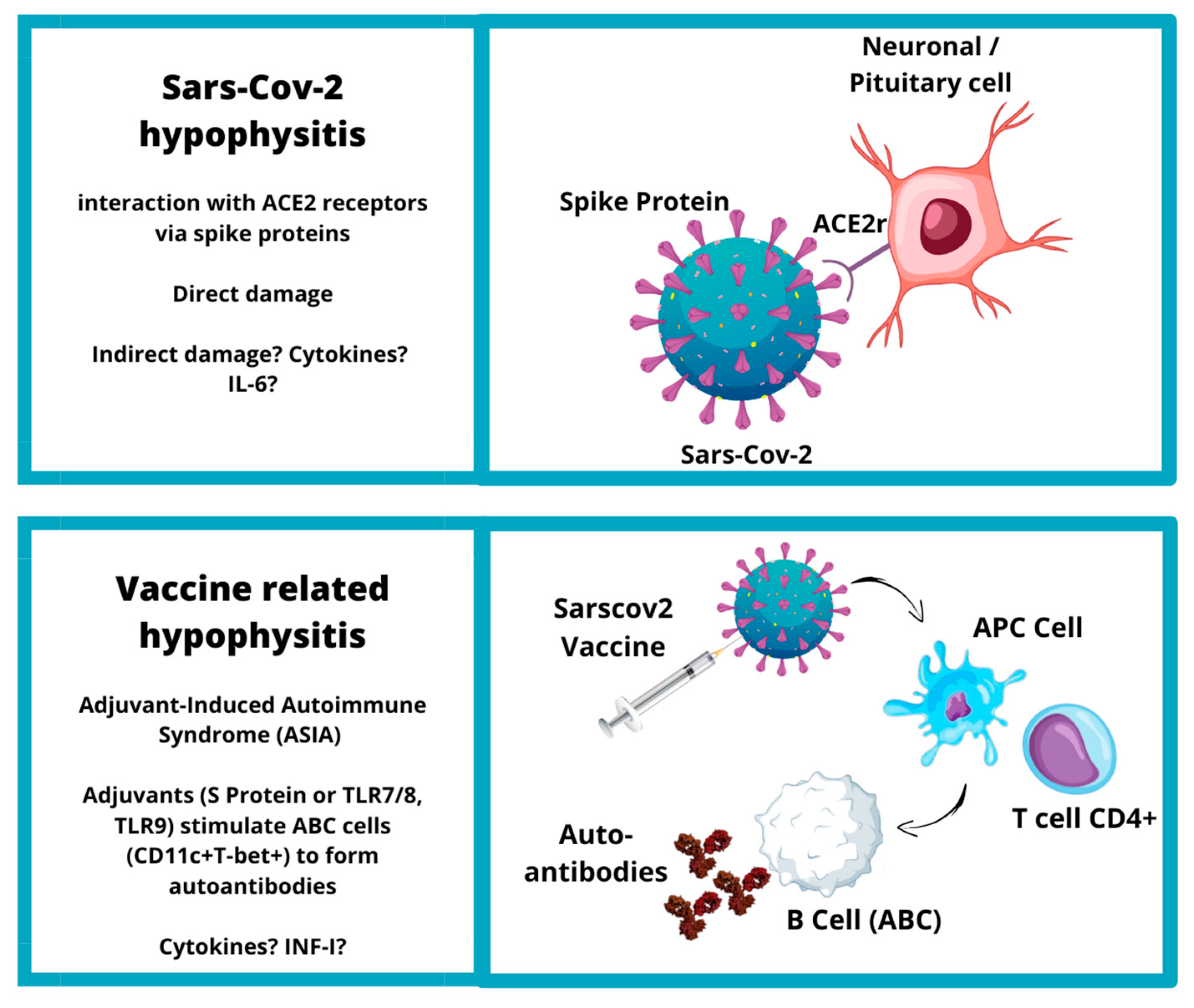
5. SARS-CoV-2 and Vaccine-Related Hypophysitis
5.1. SARS-CoV-2-Related Hypophysitis
5.2. SARS-CoV-2 Vaccine and Hypophysitis
6. Personalized Medicine in the Treatment of Hypophysitis: Is There a Place?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiloiro, S.; Capoluongo, E.D.; Tartaglione, T.; Giampietro, A.; Bianchi, A.; Giustina, A.; Pontecorvi, A.; De Marinis, L. The Changing Clinical Spectrum of Hypophysitis. Trends Endocrinol. Metab. 2019, 30, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.P.; Valadares, L.P.; Moura, A.C.; Oliveira, M.R.F.; Naves, L.A. Frequency and Clinical Characteristics of Hypophysitis and Hypopituitarism in Patients Undergoing Immunotherapy—A Systematic Review. Front. Endocrinol. 2023, 14, 1091185. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-Checkpoint Inhibitors: Long-Term Implications of Toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Langlois, F.; Varlamov, E.V.; Fleseriu, M. Hypophysitis, the Growing Spectrum of a Rare Pituitary Disease. J. Clin. Endocrinol. Metab. 2022, 107, 10–28. [Google Scholar] [CrossRef]
- Rawanduzy, C.A.; Winkler-Schwartz, A.; Couldwell, W.T. Hypophysitis: Defining Histopathologic Variants and a Review of Emerging Clinical Causative Entities. Int. J. Mol. Sci. 2023, 24, 5917. [Google Scholar] [CrossRef]
- Bando, H.; Kanie, K.; Takahashi, Y. Paraneoplastic Autoimmune Hypophysitis: An Emerging Concept. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101601. [Google Scholar] [CrossRef]
- Yamamoto, M.; Iguchi, G.; Bando, H.; Kanie, K.; Hidaka-Takeno, R.; Fukuoka, H.; Takahashi, Y. Autoimmune Pituitary Disease: New Concepts with Clinical Implications. Endocr. Rev. 2020, 41, 261–272. [Google Scholar] [CrossRef]
- Kanie, K.; Iguchi, G.; Bando, H.; Urai, S.; Shichi, H.; Fujita, Y.; Matsumoto, R.; Suda, K.; Yamamoto, M.; Fukuoka, H.; et al. Mechanistic Insights into Immune Checkpoint Inhibitor-Related Hypophysitis: A Form of Paraneoplastic Syndrome. Cancer Immunol. Immunother. 2021, 70, 3669–3677. [Google Scholar] [CrossRef]
- Ach, T.; Kammoun, F.; El Fekih, H.; Slama, N.B.H.; Kahloun, S.; Fredj, F.B.; Laouani, C.; Ach, K. Central Diabetes Insipidus Revealing a Hypophysitis Induced by SARS-CoV-2 Vaccine. Therapies 2022, 78, 453–455. [Google Scholar] [CrossRef]
- Ach, T.; El Euch, M. The Need to Shed Light on Potential Insidious SARS-CoV-2 Post-Vaccination Pituitary Lesions. Therapies 2022, 78, 456–457. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A. The Pituitary Gland in SARS-CoV-2 Infections, Vaccinations, and Post-COVID Syndrome. Clinics 2023, 78, 100157. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Gunawardena, S.; Goenka, A.; Ey, E.; Kumar, G. Post COVID-19 Lymphocytic Hypophysitis: A Rare Presentation. Child. Neurol. Open 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on Endocrine Side Effects of Immunotherapy. Endocr. Relat. Cancer 2019, 26, G1–G18. [Google Scholar] [CrossRef]
- Husebye, E.S.; Castinetti, F.; Criseno, S.; Curigliano, G.; Decallonne, B.; Fleseriu, M.; Higham, C.E.; Lupi, I.; Paschou, S.A.; Toth, M.; et al. Endocrine-Related Adverse Conditions in Patients Receiving Immune Checkpoint Inhibition: An ESE Clinical Practice Guideline. Eur. J. Endocrinol. 2022, 187, G1–G21. [Google Scholar] [CrossRef]
- Chiloiro, S.; Russo, F.; Tartaglione, T.; Capoluongo, E.D. Molecular and Genetic Immune Biomarkers of Primary and Immune-Therapy Induced Hypophysitis: From Laboratories to the Clinical Practice. J. Pers. Med. 2021, 11, 1026. [Google Scholar] [CrossRef]
- Cironi, K.A.; Decater, T.; Iwanaga, J.; Dumont, A.S.; Tubbs, R.S. Arterial Supply to the Pituitary Gland: A Comprehensive Review. World Neurosurg. 2020, 142, 206–211. [Google Scholar] [CrossRef]
- Tzou, S.-C.; Landek-Salgado, M.A.; Kimura, H.; Caturegli, P. Preparation of Mouse Pituitary Immunogen for the Induction of Experimental Autoimmune Hypophysitis. J. Vis. Exp. 2010, e2181. [Google Scholar] [CrossRef]
- Lupi, I.; Zhang, J.; Gutenberg, A.; Landek-Salgado, M.; Tzou, S.-C.; Mori, S.; Caturegli, P. From Pituitary Expansion to Empty Sella: Disease Progression in a Mouse Model of Autoimmune Hypophysitis. Endocrinology 2011, 152, 4190–4198. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Tartaglione, T.; Capobianco, A.; Anile, C.; De Marinis, L. DIAGNOSIS OF ENDOCRINE DISEASE: Primary Empty Sella: A Comprehensive Review. Eur. J. Endocrinol. 2017, 177, R275–R285. [Google Scholar] [CrossRef] [PubMed]
- Naran, J.; Can, A.S. Lymphocytic Hypophysitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- O’Dwyer, D.T.; Smith, A.I.; Matthew, M.L.; Andronicos, N.M.; Ranson, M.; Robinson, P.J.; Crock, P.A. Identification of the 49-KDa Autoantigen Associated with Lymphocytic Hypophysitis as α-Enolase. J. Clin. Endocrinol. Metab. 2002, 87, 752–757. [Google Scholar] [CrossRef]
- Joshi, M.N.; Whitelaw, B.C.; Carroll, P.V. MECHANISMS IN ENDOCRINOLOGY: Hypophysitis: Diagnosis and Treatment. Eur. J. Endocrinol. 2018, 179, R151–R163. [Google Scholar] [CrossRef] [PubMed]
- Türe, U.; De Bellis, A.; Harput, M.V.; Bellastella, G.; Topcuoglu, M.; Yaltirik, C.K.; Cirillo, P.; Yola, R.N.; Sav, A.; Kelestimur, F. Hypothalamitis: A Novel Autoimmune Endocrine Disease. A Literature Review and Case Report. J. Clin. Endocrinol. Metab. 2021, 106, e415–e429. [Google Scholar] [CrossRef] [PubMed]
- Chalan, P.; Thomas, N.; Caturegli, P. Th17 Cells Contribute to the Pathology of Autoimmune Hypophysitis. J. Immunol. 2021, 206, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Falorni, A.; Minarelli, V.; Bartoloni, E.; Alunno, A.; Gerli, R. Diagnosis and Classification of Autoimmune Hypophysitis. Autoimmun. Rev. 2014, 13, 412–416. [Google Scholar] [CrossRef]
- Force, B.K.; Vogel, T.P.; Nguyen, D.M.; Heck, K.A.; Sebastian, S.; Takashima, M.; Yoshor, D.; Samson, S.L. A Remarkable Response of Granulomatous Hypophysitis to Infliximab in a Patient with a Background of Crohn’s Disease—A Case Report. Front. Endocrinol. 2020, 11, 350. [Google Scholar] [CrossRef]
- Gubbi, S.; Hannah-Shmouni, F.; Verbalis, J.G.; Koch, C.A. Hypophysitis: An Update on the Novel Forms, Diagnosis and Management of Disorders of Pituitary Inflammation. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101371. [Google Scholar] [CrossRef]
- Hunn, B.H.M.; Martin, W.G.; Simpson, S.; Mclean, C.A. Idiopathic Granulomatous Hypophysitis: A Systematic Review of 82 Cases in the Literature. Pituitary 2014, 17, 357–365. [Google Scholar] [CrossRef]
- Radojkovic, D.; Pesic, M.; Dimic, D.; Radjenovic Petkovic, T.; Radenkovic, S.; Velojic-Golubovic, M.; Novak, V.; Ilic, I.; Radojkovic, M. Localised Langerhans Cell Histiocytosis of the Hypothalamic-Pituitary Region: Case Report and Literature Review. Hormones 2018, 17, 119–125. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Bao, X.; Wang, R. Lymphocytic Hypophysitis Secondary to Ruptured Rathke Cleft Cyst: Case Report and Literature Review. World Neurosurg. 2018, 114, 172–177. [Google Scholar] [CrossRef]
- DeCou, S.; Recinos, P.F.; Prayson, R.A.; Karakasis, C.; Haider, A.; Patel, N. Successful Immunomodulatory Treatment for Recurrent Xanthogranulomatous Hypophysitis in an Adolescent: Illustrative Case. J. Neurosurg. Case Lessons 2022, 4, CASE22191. [Google Scholar] [CrossRef]
- Shikuma, J.; Kan, K.; Ito, R.; Hara, K.; Sakai, H.; Miwa, T.; Kanazawa, A.; Odawara, M. Critical Review of IgG4-Related Hypophysitis. Pituitary 2017, 20, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.J.; Popovic, V.; Trainer, P.J. New Causes of Hypophysitis. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101276. [Google Scholar] [CrossRef] [PubMed]
- Landek-Salgado, M.A.; Leporati, P.; Lupi, I.; Geis, A.; Caturegli, P. Growth Hormone and Proopiomelanocortin Are Targeted by Autoantibodies in a Patient with Biopsy-Proven IgG4-Related Hypophysitis. Pituitary 2012, 15, 412–419. [Google Scholar] [CrossRef]
- De Bellis, A.; Bizzarro, A.; Bellastella, A. Pituitary Antibodies and Lymphocytic Hypophysitis. Best. Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 67–84. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, A.; Bizzarro, A.; Perrino, S.; Coronella, C.; Conte, M.; Pasquali, D.; Sinisi, A.A.; Betterle, C.; Bellastella, A. Characterization of Antipituitary Antibodies Targeting Pituitary Hormone-Secreting Cells in Idiopathic Growth Hormone Deficiency and Autoimmune Endocrine Diseases. Clin. Endocrinol. 2005, 63, 45–49. [Google Scholar] [CrossRef]
- Lupi, I.; Manetti, L.; Raffaelli, V.; Grasso, L.; Sardella, C.; Cosottini, M.; Iannelli, A.; Gasperi, M.; Bogazzi, F.; Caturegli, P.; et al. Pituitary Autoimmunity Is Associated with Hypopituitarism in Patients with Primary Empty Sella. J. Endocrinol. Investig. 2011, 34, e240-4. [Google Scholar] [CrossRef]
- Chiloiro, S.; Tartaglione, T.; Angelini, F.; Bianchi, A.; Arena, V.; Giampietro, A.; Mormando, M.; Sciandra, M.; Laino, M.E.; De Marinis, L. An Overview of Diagnosis of Primary Autoimmune Hypophysitis in a Prospective Single-Center Experience. Neuroendocrinology 2017, 104, 280–290. [Google Scholar] [CrossRef]
- Mele, C.; Pingue, V.; Caputo, M.; Zavattaro, M.; Pagano, L.; Prodam, F.; Nardone, A.; Aimaretti, G.; Marzullo, P. Neuroinflammation and Hypothalamo-Pituitary Dysfunction: Focus of Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 2686. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Angelini, F.; Arena, V.; Stigliano, E.; Tartaglione, T.; Mattogno, P.P.; D’Alessandris, Q.G.; Lauretti, L.; Pontecorvi, A.; et al. Markers of Humoral and Cell-Mediated Immune Response in Primary Autoimmune Hypophysitis: A Pilot Study. Endocrine 2021, 73, 308–315. [Google Scholar] [CrossRef]
- Crock, P.A. Cytosolic Autoantigens in Lymphocytic Hypophysitis. J. Clin. Endocrinol. Metab. 1998, 83, 609–618. [Google Scholar] [CrossRef]
- Chiloiro, S.; Tartaglione, T.; Capoluongo, E.D.; Angelini, F.; Arena, V.; Giampietro, A.; Bianchi, A.; Zoli, A.; Pontecorvi, A.; Colosimo, C.; et al. Hypophysitis Outcome and Factors Predicting Responsiveness to Glucocorticoid Therapy: A Prospective and Double-Arm Study. J. Clin. Endocrinol. Metab. 2018, 103, 3877–3889. [Google Scholar] [CrossRef]
- Smith, C.J.A.; Bensing, S.; Burns, C.; Robinson, P.J.; Kasperlik-Zaluska, A.A.; Scott, R.J.; Kämpe, O.; Crock, P.A. Identification of TPIT and Other Novel Autoantigens in Lymphocytic Hypophysitis; Immunoscreening of a Pituitary CDNA Library and Development of Immunoprecipitation Assays. Eur. J. Endocrinol. 2012, 166, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Iwama, S.; Sugimura, Y.; Kiyota, A.; Kato, T.; Enomoto, A.; Suzuki, H.; Iwata, N.; Takeuchi, S.; Nakashima, K.; Takagi, H.; et al. Rabphilin-3A as a Targeted Autoantigen in Lymphocytic Infundibulo-Neurohypophysitis. J. Clin. Endocrinol. Metab. 2015, 100, E946–E954. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, A.; Iwama, S.; Sugimura, Y.; Takeuchi, S.; Takagi, H.; Iwata, N.; Nakashima, K.; Suzuki, H.; Nishioka, T.; Kato, T.; et al. Identification of the Novel Autoantigen Candidate Rab GDP Dissociation Inhibitor Alpha in Isolated Adrenocorticotropin Deficiency. Endocr. J. 2015, 62, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kume, Y.; Sakuma, H.; Sekine, H.; Sumikoshi, M.; Sugimura, Y.; Hosoya, M. Lymphocytic Infundibuloneurohypophysitis with Positive Anti-Rabphilin-3A Antibodies Nine Years Post-Onset of Central Diabetes Insipidus. Clin. Pediatr. Endocrinol. 2021, 30, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Hansen, D.; Husby, S.; Jacobsen, B.B.; Lillevang, S.T. Association of a Putative Regulatory Polymorphism in the PD-1 Gene with Susceptibility to Type 1 Diabetes. Tissue Antigens 2003, 62, 492–497. [Google Scholar] [CrossRef]
- Lin, H.-H.; Gutenberg, A.; Chen, T.-Y.; Tsai, N.-M.; Lee, C.-J.; Cheng, Y.-C.; Cheng, W.-H.; Tzou, Y.-M.; Caturegli, P.; Tzou, S.-C. In Situ Activation of Pituitary-Infiltrating T Lymphocytes in Autoimmune Hypophysitis. Sci. Rep. 2017, 7, 43492. [Google Scholar] [CrossRef]
- Chiloiro, S.; Capoluongo, E.D.; Angelini, F.; Mariotti, F.; Grande, G.; Stigliano, E.; Vincenzoni, F.; Bianchi, A.; Giampietro, A.; Milardi, D.; et al. Autoantibody Reactivity Profile of Primary Autoimmune Hypophysitis Patients: Preliminary Results. Endocrine 2022, 76, 224–227. [Google Scholar] [CrossRef]
- Raffin-Sanson, M.-L.; Massias, J.F.; Ankotche, A.; Coste, J.; De Keyzer, Y.; Oliver, C.; Dumont, C.; Cabrol, D.; Ferré, F.; Bertagna, X. High Precursor Level in Maternal Blood Results from the Alternate Mode of Proopiomelanocortin Processing in Human Placenta. Clin. Endocrinol. 1999, 50, 85–94. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Menotti, S.; Angelini, F.; Tartaglione, T.; Antonini Cappellini, G.C.; De Galitiis, F.; Rossi, E.; Schinzari, G.; et al. Pituitary Enlargement and Hypopituitarism in Patients Treated with Immune Checkpoint Inhibitors: Two Sides of the Same Coin? J. Pers. Med. 2023, 13, 415. [Google Scholar] [CrossRef]
- Penta, L.; Bizzarri, C.; Panichi, M.; Novelli, A.; Lepri, F.R.; Cappa, M.; Esposito, S. Identification of a Novel PROP1 Mutation in a Patient with Combined Pituitary Hormone Deficiency and Enlarged Pituitary. Int. J. Mol. Sci. 2019, 20, 1875. [Google Scholar] [CrossRef] [PubMed]
- Bertko, E.; Klammt, J.; Dusatkova, P.; Bahceci, M.; Gonc, N.; ten Have, L.; Kandemir, N.; Mansmann, G.; Obermannova, B.; Oostdijk, W.; et al. Combined Pituitary Hormone Deficiency Due to Gross Deletions in the POU1F1 (PIT-1) and PROP1 Genes. J. Hum. Genet. 2017, 62, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Beressi, N.; Beressi, J.P.; Cohen, R.; Modigliani, E. Lymphocytic Hypophysitis. A Review of 145 Cases. Ann. Med. Interne 1999, 150, 327–341. [Google Scholar]
- Heaney, A.P.; Sumerel, B.; Rajalingam, R.; Bergsneider, M.; Yong, W.H.; Liau, L.M. HLA Markers DQ8 and DR53 Are Associated with Lymphocytic Hypophysitis and May Aid in Differential Diagnosis. J. Clin. Endocrinol. Metab. 2015, 100, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Capoluongo, E.D.; Tartaglione, T.; Bianchi, A.; Giampietro, A.; Angelini, F.; Arena, V.; Pontecorvi, A.; De Marinis, L. Human Leucocyte Antigens Coeliac Haplotypes and Primary Autoimmune Hypophysitis in Caucasian Patients. Clin. Endocrinol. 2018, 88, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Megiorni, F.; Mora, B.; Bonamico, M.; Barbato, M.; Nenna, R.; Maiella, G.; Lulli, P.; Mazzilli, M.C. HLA-DQ and Risk Gradient for Celiac Disease. Hum. Immunol. 2009, 70, 55–59. [Google Scholar] [CrossRef]
- Schwartz, J.-C.D.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural Basis for Co-Stimulation by the Human CTLA-4/B7-2 Complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef]
- Bellastella, G.; Carbone, C.; Scappaticcio, L.; Cirillo, P.; Troiani, T.; Morgillo, F.; Vietri, M.T.; Della Corte, C.M.; De Falco, V.; Napolitano, S.; et al. Hypothalamic–Pituitary Autoimmunity in Patients Treated with Anti-PD-1 and Anti-PD-L1 Antibodies. Cancers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 Immunoreceptor Inhibits B Cell Receptor-Mediated Signaling by Recruiting Src Homology 2-Domain-Containing Tyrosine Phosphatase 2 to Phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871. [Google Scholar] [CrossRef]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal Structure of the B7-1/CTLA-4 Complex That Inhibits Human Immune Responses. Nature 2001, 410, 608–611. [Google Scholar] [CrossRef]
- Anderson, B.; Morganstein, D.L. Endocrine Toxicity of Cancer Immunotherapy: Clinical Challenges. Endocr. Connect. 2021, 10, R116–R124. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 Axis Converts Human TH1 Cells into Regulatory T Cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Yao, S.; Zhu, G.; Flies, A.S.; Flies, S.J.; Chen, L. B7-H1 Is a Ubiquitous Antiapoptotic Receptor on Cancer Cells. Blood 2008, 111, 3635–3643. [Google Scholar] [CrossRef]
- Queirolo, P.; Dozin, B.; Morabito, A.; Banelli, B.; Carosio, R.; Fontana, V.; Ferrucci, P.F.; Martinoli, C.; Cocorocchio, E.; Ascierto, P.A.; et al. CTLA-4 Gene Variant -1661A>G May Predict the Onset of Endocrine Adverse Events in Metastatic Melanoma Patients Treated with Ipilimumab. Eur. J. Cancer 2018, 97, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, T.; Fukuoka, H.; Takahashi, Y. Immune Checkpoint Inhibitor-Related Hypophysitis. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101668. [Google Scholar] [CrossRef]
- Wölffer, M.; Battke, F.; Schulze, M.; Feldhahn, M.; Flatz, L.; Martus, P.; Forschner, A. Biomarkers Associated with Immune-Related Adverse Events under Checkpoint Inhibitors in Metastatic Melanoma. Cancers 2022, 14, 302. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Sugiyama, D.; Yasuda, Y.; Okuji, T.; Ito, M.; Ito, S.; Sugiyama, M.; Onoue, T.; Takagi, H.; et al. Anti-Pituitary Antibodies and Susceptible Human Leukocyte Antigen Alleles as Predictive Biomarkers for Pituitary Dysfunction Induced by Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002493. [Google Scholar] [CrossRef]
- Kula, A.; Dawidowicz, M.; Kiczmer, P.; Prawdzic Seńkowska, A.; Świętochowska, E. The Role of Genetic Polymorphism within PD-L1 Gene in Cancer. Review. Exp. Mol. Pathol. 2020, 116, 104494. [Google Scholar] [CrossRef]
- Pulichino, A.-M.; Vallette-Kasic, S.; Couture, C.; Gauthier, Y.; Brue, T.; David, M.; Malpuech, G.; Deal, C.; Van Vliet, G.; De Vroede, M.; et al. Human and Mouse TPIT Gene Mutations Cause Early Onset Pituitary ACTH Deficiency. Genes Dev. 2003, 17, 711–716. [Google Scholar] [CrossRef]
- Quandt, Z.; Kim, S.; Villanueva-Meyer, J.; Coupe, C.; Young, A.; Kang, J.H.; Yazdany, J.; Schmajuk, G.; Rush, S.; Ziv, E.; et al. Spectrum of Clinical Presentations, Imaging Findings, and HLA Types in Immune Checkpoint Inhibitor–Induced Hypophysitis. J. Endocr. Soc. 2023, 7, bvad012. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. The Novel Concept of “Onco-Immuno-Endocrinology” Led to the Discovery of New Clinical Entity “Paraneoplastic Autoimmune Hypophysitis”. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101663. [Google Scholar] [CrossRef]
- Frara, S.; Allora, A.; Castellino, L.; di Filippo, L.; Loli, P.; Giustina, A. COVID-19 and the Pituitary. Pituitary 2021, 24, 465–481. [Google Scholar] [CrossRef]
- Capatina, C.; Poiana, C.; Fleseriu, M. Pituitary and SARS-CoV-2: An Unremitting Conundrum. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 101752, in press. [Google Scholar] [CrossRef]
- Wei, L.; Sun, S.; Zhang, J.; Zhu, H.; Xu, Y.; Ma, Q.; McNutt, M.A.; Korteweg, C.; Gu, J. Endocrine Cells of the Adenohypophysis in Severe Acute Respiratory Syndrome (SARS)This Paper Is One of a Selection of Papers Published in This Special Issue Entitled “Second International Symposium on Recent Advances in Basic, Clinical, and Social Medicine” and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2010, 88, 723–730. [Google Scholar] [CrossRef]
- Leow, M.K.-S.; Kwek, D.S.-K.; Ng, A.W.-K.; Ong, K.-C.; Kaw, G.J.-L.; Lee, L.S.-U. Hypocortisolism in Survivors of Severe Acute Respiratory Syndrome (SARS). Clin. Endocrinol. 2005, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Gopalakrishnan, M.; Yadav, P.; Misra, S. Endocrine Involvement in COVID-19: Mechanisms, Clinical Features, and Implications for Care. Indian. J. Endocrinol. Metab. 2020, 24, 381. [Google Scholar] [CrossRef]
- Fitzek, A.; Gerling, M.; Püschel, K.; Saeger, W. Post-Mortem Histopathology of Pituitary and Adrenals of COVID-19 Patients. Leg. Med. 2022, 57, 102045. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Nakayama, Y.; Saitoh, Y.; Ariga, H.; Enokida, T.; Ishihara, T.; Sano, T.; Hirata, Y.; Katano, H.; Suzuki, T.; et al. Pathologic and Neuropathologic Study of a Case of COVID-19. JMA J. 2022, 5, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Misgar, R.A.; Rasool, A.; Wani, A.I.; Bashir, M.I. Central Diabetes Insipidus (Infundibuloneuro Hypophysitis): A Late Complication of COVID-19 Infection. J. Endocrinol. Investig. 2021, 44, 2855–2856. [Google Scholar] [CrossRef]
- Berni, A.; Malandrino, D.; Parenti, G.; Maggi, M.; Poggesi, L.; Peri, A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) Infection: May All Fit Together? J. Endocrinol. Investig. 2020, 43, 1137–1139. [Google Scholar] [CrossRef]
- Park, M.; Cook, A.R.; Lim, J.T.; Sun, Y.; Dickens, B.L. A Systematic Review of COVID-19 Epidemiology Based on Current Evidence. J. Clin. Med. 2020, 9, 967. [Google Scholar] [CrossRef]
- Sachinidis, A.; Garyfallos, A. COVID-19 Vaccination Can Occasionally Trigger Autoimmune Phenomena, Probably via Inducing Age-associated B Cells. Int. J. Rheum. Dis. 2022, 25, 83–85. [Google Scholar] [CrossRef]
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune Post-COVID Vaccine Syndromes: Does the Spectrum of Autoimmune/Inflammatory Syndrome Expand? Clin. Rheumatol. 2022, 41, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Amereller, F.; Küppers, A.-M.; Schilbach, K.; Schopohl, J.; Störmann, S. Clinical Characteristics of Primary Hypophysitis—A Single-Centre Series of 60 Cases. Exp. Clin. Endocrinol. Diabetes 2021, 129, 234–240. [Google Scholar] [CrossRef]
- Krishnappa, B.; Shah, R.; Sarathi, V.; Lila, A.R.; Sehemby, M.K.; Patil, V.A.; Sankhe, S.; Shah, N.; Bandgar, T. Early Pulse Glucocorticoid Therapy and Improved Hormonal Outcomes in Primary Hypophysitis. Neuroendocrinology 2022, 112, 186–195. [Google Scholar] [CrossRef]
- Imga, N.N.; Yildirim, A.E.; Baser, O.O.; Berker, D. Clinical and Hormonal Characteristics of Patients with Different Types of Hypophysitis: A Single-Center Experience. Arch. Endocrinol. Metab. 2019, 63, 47–52. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis Is Associated with Reduced Survival in Patients with Melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef]
- Faje, A. Hypophysitis: Evaluation and Management. Clin. Diabetes Endocrinol. 2016, 2, 15. [Google Scholar] [CrossRef]
- Ko, J.J.; Wu, C.; Mehta, N.; Wald-Dickler, N.; Yang, W.; Qiao, R. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients with COVID-19. J. Intensive Care Med. 2021, 36, 673–680. [Google Scholar] [CrossRef]
- Taieb, A.; Mounira, E.E. Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology. Vaccines 2022, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Pilli, T.; Dalmiglio, C.; Dalmazio, G.; Sagnella, A.; Forleo, R.; Brilli, L.; Maino, F.; Ciuoli, C.; Castagna, M.G. No Need of Glucocorticoid Dose Adjustment in Patients with Adrenal Insufficiency before COVID-19 Vaccine. Eur. J. Endocrinol. 2022, 187, K7–K11. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, M.N.; Topazian, M.D.; Khosroshahi, A.; Witzig, T.E.; Wallace, Z.S.; Hart, P.A.; Deshpande, V.; Smyrk, T.C.; Chari, S.; Stone, J.H. Rituximab for IgG4-Related Disease: A Prospective, Open-Label Trial. Ann. Rheum. Dis. 2015, 74, 1171–1177. [Google Scholar] [CrossRef]

| Etiology | Sex | Histology | References | |
|---|---|---|---|---|
| Autoimmune | APA and AHA | >F | Lymphocitic infiltration | [4] |
| Lymphocytic | Autoimmune, pregnancy, and peripartum T-cell-mediated | F (30 years) pregnancy and peripartum M (40 years) autoimmunity | Lymphocitic infiltration, plasmacells, histocites, and fibrosis | [4,5] |
| Granulomatous | Primary: idiopathic (APA? cronic LH?) Secondary: sarcoidosis, granulomatosis with polyangitiis, langherans cells histocystosis, tubercolosis, Wegener’s granulomatosis, Erdheim–Chester disease, Crohn’s disease, Takayasu arteritis, Cogan’s syndrome, and other vasculites | >F | Multinucleated giant cells, histiocytes, lymphocytes, granulomas | [4,5] |
| Xanthomatous | Local: hemorrage, cyst ropture, craniopharingioma Xanthogranulomatous: chronic? | >F (40 years) | CD68-positive foamy, macrophages, colesterol clefts, hemosiderin | [4,5] |
| Necrotizing | Unknown (autoimmune?) | >F very rare | Extensive necrosis, lymphocytes, plasmacytes, few eosinophlis | [4,5] |
| IgG4-related | IgG4 disease, POMC, and GH autoantigen? | >M (50–70 years) | >10 high-power field, IgG4 plasma cells, fibrosis | [4,5] |
| ICI induced | Anti-CTLA4 and anti-PD1/PDL1 | >M | Diffuse infiltration with lymphocytes and macrophages | [2] |
| Paraneoplastic | Anti-Pit1, anti-POMC, or anti-ACTHEctopic expression of ACTH, Pit1, or other | ? | Lymphocitic infiltration | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menotti, S.; Giampietro, A.; Raia, S.; Veleno, M.; Angelini, F.; Tartaglione, T.; Gaudino, S.; Doglietto, F.; De Marinis, L.; Pontecorvi, A.; et al. Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review. J. Pers. Med. 2023, 13, 1210. https://doi.org/10.3390/jpm13081210
Menotti S, Giampietro A, Raia S, Veleno M, Angelini F, Tartaglione T, Gaudino S, Doglietto F, De Marinis L, Pontecorvi A, et al. Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review. Journal of Personalized Medicine. 2023; 13(8):1210. https://doi.org/10.3390/jpm13081210
Chicago/Turabian StyleMenotti, Sara, Antonella Giampietro, Salvatore Raia, Miriam Veleno, Flavia Angelini, Tommaso Tartaglione, Simona Gaudino, Francesco Doglietto, Laura De Marinis, Alfredo Pontecorvi, and et al. 2023. "Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review" Journal of Personalized Medicine 13, no. 8: 1210. https://doi.org/10.3390/jpm13081210
APA StyleMenotti, S., Giampietro, A., Raia, S., Veleno, M., Angelini, F., Tartaglione, T., Gaudino, S., Doglietto, F., De Marinis, L., Pontecorvi, A., Bianchi, A., & Chiloiro, S. (2023). Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review. Journal of Personalized Medicine, 13(8), 1210. https://doi.org/10.3390/jpm13081210





