Influence of Intraoperative Blood Loss on Tumor Recurrence after Surgical Resection in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Data Collection
2.3. Anesthetic and Surgical Technique
2.4. Statistical Analysis
3. Results
3.1. Clinico-Pathologic Findings of Patients
3.2. Operative Outcomes of HCC Patients
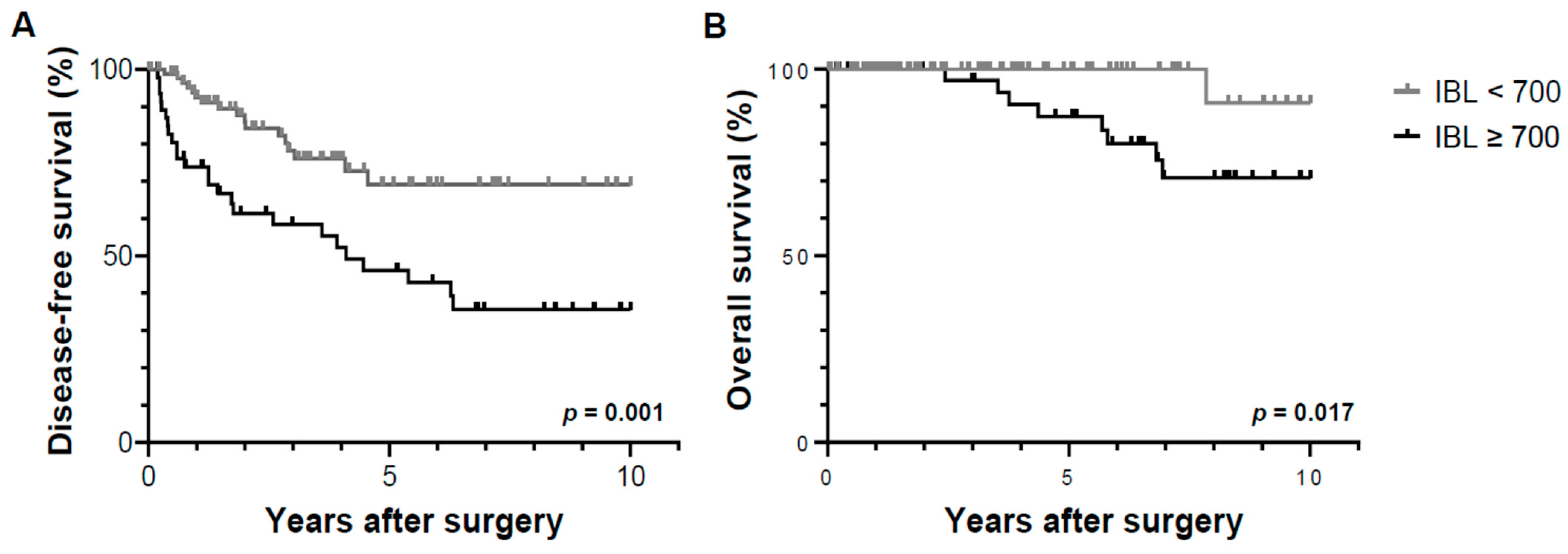
3.3. Cumulative Probability of Tumor Recurrence and Overall Survival by Operative Factors
3.4. Risk Factor Analysis for Recurrence-Free Survival and Overall Survival after Resection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| IBL | intraoperative blood loss |
| CVP | central venous pressure |
| HCC | hepatocellular carcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| BMI | body mass index |
| TB | total bilirubin |
| INR | international normalized ratio |
| AFP | α-fetoprotein |
| PIVKA-II | prothrombin induced by vitamin K absence-II |
| ICU | intensive care unit |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Colombo, M. Hepatocellular carcinoma: Current state of the art in diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 751. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Lee, K.W.; Lee, J.M.; You, T.; Choi, Y.R.; Kim, H.; Lee, H.W.; Lee, J.M.; Yi, N.J.; Suh, K.S. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J. Hepatol. 2014, 60, 1219–1224. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Liu, L.; Miao, R.; Yang, H.; Lu, X.; Zhao, Y.; Mao, Y.; Zhong, S.; Huang, J.; Sang, X.; Zhao, H. Prognostic factors after liver resection for hepatocellular carcinoma: A single-center experience from China. Am. J. Surg. 2012, 203, 741–750. [Google Scholar] [CrossRef]
- Han, D.H.; Choi, G.H.; Park, J.Y.; Ahn, S.H.; Kim, K.S.; Choi, J.S. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J. Surg. Oncol. 2014, 12, 192. [Google Scholar] [CrossRef]
- Kishi, Y.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Hepatocellular carcinoma: Current management and future development-improved outcomes with surgical resection. Int. J. Hepatol. 2011, 2011, 728103. [Google Scholar] [CrossRef]
- Berzigotti, A.; Reig, M.; Abraldes, J.G.; Bosch, J.; Bruix, J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: A systematic review and meta-analysis. Hepatology 2015, 61, 526–536. [Google Scholar] [CrossRef]
- Kingham, T.P.; Correa-Gallego, C.; D’Angelica, M.I.; Gönen, M.; DeMatteo, R.P.; Fong, Y.; Allen, P.J.; Blumgart, L.H.; Jarnagin, W.R. Hepatic parenchymal preservation surgery: Decreasing morbidity and mortality rates in 4,152 resections for malignancy. J. Am. Coll. Surg. 2015, 220, 471–479. [Google Scholar] [CrossRef]
- Topaloglu, S.; Yesilcicek Calik, K.; Calik, A.; Aydın, C.; Kocyigit, S.; Yaman, H.; Kutanis, D.; Karabulut, E.; Dohman, D.; Orem, A.; et al. Efficacy and safety of hepatectomy performed with intermittent portal triad clamping with low central venous pressure. BioMed Res. Int. 2013, 2013, 297971. [Google Scholar] [CrossRef]
- Harada, N.; Shirabe, K.; Maeda, T.; Kayashima, H.; Ishida, T.; Maehara, Y. Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J. Surg. 2015, 39, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.D.; Salceda, J.A.; Laurent, A.; Tayar, C.; Duvoux, C.; Decaens, T.; Luciani, A.; Van Nhieu, J.T.; Azoulay, D.; Cherqui, D. Liver resection for hepatocellular carcinoma in 313 Western patients: Tumor biology and underlying liver rather than tumor size drive prognosis. J. Hepatol. 2015, 62, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, L.; Yu, H.; Yu, X. Laparoscopic hepatectomy for hepatocellular carcinoma: Short- and long-term outcomes with blood loss. Transl. Cancer Res. 2021, 10, 4303–4315. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Ribero, D.; Reddy, S.K.; Donadon, M.; Zorzi, D.; Gautam, S.; Abdalla, E.K.; Curley, S.A.; Capussotti, L.; Clary, B.M.; et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J. Am. Coll. Surg. 2007, 204, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef]
- Mizuno, A.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Iwata, N.; Yamada, S.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Koike, M.; et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig. Surg. 2016, 33, 121–128. [Google Scholar] [CrossRef]
- Ito, Y.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Intraoperative blood loss is associated with shortened postoperative survival of patients with stage II/III gastric cancer: Analysis of a multi-institutional dataset. World J. Surg. 2019, 43, 870–877. [Google Scholar] [CrossRef]
- Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann. Surg. Oncol. 2013, 20, 1575–1583. [Google Scholar] [CrossRef]
- Arita, T.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Hiramoto, H.; Hamada, J.; Shoda, K.; Kawaguchi, T.; Hirajima, S.; et al. Increase in peritoneal recurrence induced by intraoperative hemorrhage in gastrectomy. Ann. Surg. Oncol. 2015, 22, 758–764. [Google Scholar] [CrossRef]
- Kamei, T.; Kitayama, J.; Yamashita, H.; Nagawa, H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J. Surg. 2009, 33, 1240–1246. [Google Scholar] [CrossRef]
- Kuroda, S.; Tashiro, H.; Kobayashi, T.; Oshita, A.; Amano, H.; Ohdan, H. No impact of perioperative blood transfusion on recurrence of hepatocellular carcinoma after hepatectomy. World J. Surg. 2012, 36, 651–658. [Google Scholar] [CrossRef]
- Torrance, H.D.; Brohi, K.; Pearse, R.M.; Wozniak, E.; Prowle, J.R.; Hinds, C.J.; O’Dwyer, M.J. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Ann. Surg. 2015, 261, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Sasaki, A.; Iwaki, K.; Uchida, H.; Kai, S.; Shibata, K.; Ohta, M.; Kitano, S. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: A retrospective study. Eur. J. Surg. Oncol. 2008, 34, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tang, Y.; Xue, Y.; Chen, L. Impact of intraoperative allogenic and autologous transfusion on immune function and prognosis in patients with hepatocellular carcinoma. Medicine (Baltimore) 2020, 99, e22568. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lu, J.H.; Lau, W.Y.; Zhang, T.Y.; Zhang, H.; Shen, Y.N.; Alshebeeb, K.; Wu, M.C.; Schwartz, M.; Shen, F. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J. Hepatol. 2016, 64, 583–593. [Google Scholar] [CrossRef]
- Hughes, M.J.; Ventham, N.T.; Harrison, E.M.; Wigmore, S.J. Central venous pressure and liver resection: A systematic review and meta-analysis. HPB 2015, 17, 863–871. [Google Scholar] [CrossRef]
- Suh, S.W. Influence of obesity and fluid balance on operative outcomes in hepatic resection. J. Pers. Med. 2022, 12, 1897. [Google Scholar] [CrossRef]
- Sand, L.; Rizell, M.; Houltz, E.; Karlsen, K.; Wiklund, J.; Odenstedt Herges, H.; Stenqvist, O.; Lundin, S. Effect of patient position and PEEP on hepatic, portal and central venous pressures during liver resection. Acta Anaesthesiol. Scand. 2011, 55, 1106–1112. [Google Scholar] [CrossRef]
- Yu, L.; Sun, H.; Jin, H.; Tan, H. The effect of low central venous pressure on hepatic surgical field bleeding and serum lactate in patients undergoing partial hepatectomy: A prospective randomized controlled trial. BMC Surg. 2020, 20, 25. [Google Scholar] [CrossRef]
- Lee, J.; Kim, W.H.; Ryu, H.G.; Lee, H.C.; Chung, E.J.; Yang, S.M.; Jung, C.W. Stroke Volume Variation-Guided Versus Central Venous Pressure-Guided Low Central Venous Pressure With Milrinone During Living Donor Hepatectomy: A Randomized Double-Blinded Clinical Trial. Anesth. Analg. 2017, 125, 423–430. [Google Scholar] [CrossRef]
- Suh, S.W.; Park, H.J.; Choi, Y.S. Preoperative volume assessment using bioelectrical impedance analysis for minimizing blood loss during hepatic resection. HPB 2022, 24, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W. Bioelectrical impedance analysis for preoperative volemia assessment in living donor hepatectomy. J. Pers. Med. 2022, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Gilbert, R.; Baker, L.; Shorr, R.; Workneh, A.; Turcotte, S.; Bertens, K.A.; Abou-Khalil, J.; Balaa, F.K.; Martel, G. The safety and efficacy of hypovolemic phlebotomy on blood loss and transfusion in liver surgery: A systematic review and meta-analysis. HPB 2020, 22, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ryckx, A.; Christiaens, C.; Clarysse, M.; Vansteenkiste, F.; Jan Steelant, P.; Sergeant, G.; Parmentier, I.; Pottel, H.; D’Hondt, M. Central venous pressure drop after hypovolemic phlebotomy is a strong independent predictor of intraoperative blood loss during liver resection. Ann. Surg. Oncol. 2017, 24, 1367–1375. [Google Scholar] [CrossRef]

| Variables | IBL ≥ 700 (n = 47) | IBL < 700 (n = 95) | p Value |
|---|---|---|---|
| Age, years | 60.1 (±10.7) | 62.2 (±10.8) | 0.270 |
| Male | 40 (85.1%) | 70 (73.7%) | 0.125 |
| Presence of HBV | 27 (57.4%) | 48 (50.5%) | 0.437 |
| Presence of HCV | 6 (12.8%) | 7 (7.4%) | 0.294 |
| BMI | 25.6 (±4.5) | 24.5 (±3.5) | 0.113 |
| Hemoglobin | 13.5 (±1.7) | 13.1 (±2.0) | 0.226 |
| Total bilirubin | 0.8 (±0.3) | 0.7 (±0.4) | 0.058 |
| Albumin | 4.0 (±0.4) | 4.2 (±0.4) | 0.074 |
| PT-INR | 1.14 (±0.11) | 1.09 (±0.12) | 0.031 |
| Tumor size | 3.5 (±2.2) | 3.1 (±2.3) | 0.321 |
| AFP | 78 (±237) | 125 (±349) | 0.414 |
| PIVKA-II | 492 (±1612) | 368 (±1140) | 0.622 |
| Microvascular invasion | 9 (20.0%) | 13 (14.0%) | 0.365 |
| Poor histologic grade (III or IV) | 37 (82.2%) | 58 (63.7%) | 0.027 |
| IBL ≥ 700 (n = 47) | IBL < 700 (n = 95) | p Value | |
|---|---|---|---|
| Operative duration | 244 (±94) | 169 (±58) | <0.001 |
| Major resection | 32 (68.1%) | 65 (68.4%) | 0.968 |
| IBL | 1351 (±698) | 354 (±166) | <0.001 |
| Blood transfusion | 30 (63.8%) | 6 (6.3%) | <0.001 |
| ICU admission | 11 (23.4%) | 9 (9.5%) | 0.025 |
| Postoperative complications | |||
| Wound infection | 3 (6.4%) | 8 (8.4%) | 0.669 |
| Pneumonia | 1 (2.1%) | 2 (2.1%) | 0.993 |
| Acute kidney injury | 5 (10.6%) | 7 (7.4%) | 0.510 |
| Liver insufficiency | 3 (6.4%) | 5 (5.3%) | 0.785 |
| Abdominal wall hernia | 1 (2.1%) | 1 (1.1%) | 0.609 |
| Bile leakage | 1 (2.1%) | 0 | 0.154 |
| Hospital stay | 15.1 (±6.5) | 11.6 (±4.7) | <0.001 |
| IBL ≥ 700 (n = 47) | IBL < 700 (n = 95) | p Value | |
|---|---|---|---|
| Recurrence | 25 (53.2%) | 17 (17.9%) | <0.001 |
| Number | |||
| Single | 13 (52.0%) | 8 (47.1%) | |
| Multiple | 12 (48.0%) | 9 (52.9%) | 0.753 |
| Site | |||
| Intrahepatic | 20 (80.0%) | 13 (76.5%) | |
| Extrahepatic | 5 (20.0%) | 4 (23.5%) | 0.784 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age ≥ 60 years | 0.621 (0.333–1.159) | 0.134 | ||
| Male | 0.529 (0.223–1.258) | 0.150 | ||
| Presence of HBV | 0.720 (0.392–1.321) | 0.289 | ||
| Presence of HCV | 2.519 (1.111–5.709) | 0.027 | ||
| BMI ≥ 25 | 1.170 (0.637–2.150) | 0.613 | ||
| Total bilirubin | 1.463 (0.704–3.038) | 0.308 | ||
| Albumin | 0.402 (0.217–0.742) | 0.004 | 0.471 (0.244–0.907) | 0.024 |
| PT-INR | 4.707 (0.420–52.783) | 0.209 | ||
| Tumor size ≥ 5 cm | 0.499 (0.154–1.616) | 0.246 | ||
| AFP ≥ 400 | 1.347 (0.480–3.783) | 0.572 | ||
| PIVKA-II ≥ 80 | 0.828 (0.412–1.666) | 0.597 | ||
| Microvascular invasion | 2.594 (1.288–5.222) | 0.008 | 2.616 (1.298–5.273) | 0.007 |
| Poor histologic grade (III or IV) | 1.426 (0.694–2.931) | 0.334 | ||
| Major resection | 0.676 (0.332–1.379) | 0.282 | ||
| IBL ≥ 700 mL | 2.788 (1.500–5.181) | 0.001 | 2.325 (1.202–4.497) | 0.012 |
| Transfusion | 1.528 (0.818–2.857) | 0.184 | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age ≥ 60 years | 1.188 (0.318–4.434) | 0.798 | ||
| Male | 0.627 (0.077–5.106) | 0.663 | ||
| Presence of HBV | 0.365 (0.091–1.461) | 0.154 | ||
| Presence of HCV | 2.203 (0.453–10.713) | 0.328 | ||
| BMI ≥ 25 | 2.630 (0.654–10.568) | 0.173 | ||
| Total bilirubin | 1.084 (0.256–4.597) | 0.912 | ||
| Albumin | 0.509 (0.139–1.865) | 0.308 | ||
| PT-INR | 10.127 (0.126–814.239) | 0.301 | ||
| Tumor size ≥ 5 cm | 0.043 (0.000–1729.022) | 0.561 | ||
| AFP ≥ 400 | 1.772 (0.218–14.392) | 0.593 | ||
| PIVKA-II ≥ 80 | 0.580 (0.117–2.885) | 0.506 | ||
| Microvascular invasion | 4.214 (0.992–17.897) | 0.051 | 4.695 (1.091–20.199) | 0.038 |
| Poor histologic grade (III or IV) | 3.272 (0.402–26.609) | 0.268 | ||
| Major resection | 0.353 (0.044–2.833) | 0.327 | ||
| IBL ≥ 700 mL | 8.390 (1.044–67.408) | 0.045 | ||
| Transfusion | 1.618 (0.430–6.090) | 0.477 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.-W.; Lee, S.E.; Choi, Y.S. Influence of Intraoperative Blood Loss on Tumor Recurrence after Surgical Resection in Hepatocellular Carcinoma. J. Pers. Med. 2023, 13, 1115. https://doi.org/10.3390/jpm13071115
Suh S-W, Lee SE, Choi YS. Influence of Intraoperative Blood Loss on Tumor Recurrence after Surgical Resection in Hepatocellular Carcinoma. Journal of Personalized Medicine. 2023; 13(7):1115. https://doi.org/10.3390/jpm13071115
Chicago/Turabian StyleSuh, Suk-Won, Seung Eun Lee, and Yoo Shin Choi. 2023. "Influence of Intraoperative Blood Loss on Tumor Recurrence after Surgical Resection in Hepatocellular Carcinoma" Journal of Personalized Medicine 13, no. 7: 1115. https://doi.org/10.3390/jpm13071115
APA StyleSuh, S.-W., Lee, S. E., & Choi, Y. S. (2023). Influence of Intraoperative Blood Loss on Tumor Recurrence after Surgical Resection in Hepatocellular Carcinoma. Journal of Personalized Medicine, 13(7), 1115. https://doi.org/10.3390/jpm13071115







