Inhaled Corticosteroids and the Risk of Nontuberculous Mycobacterial Pulmonary Disease in Chronic Obstructive Pulmonary Disease: Findings from a Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
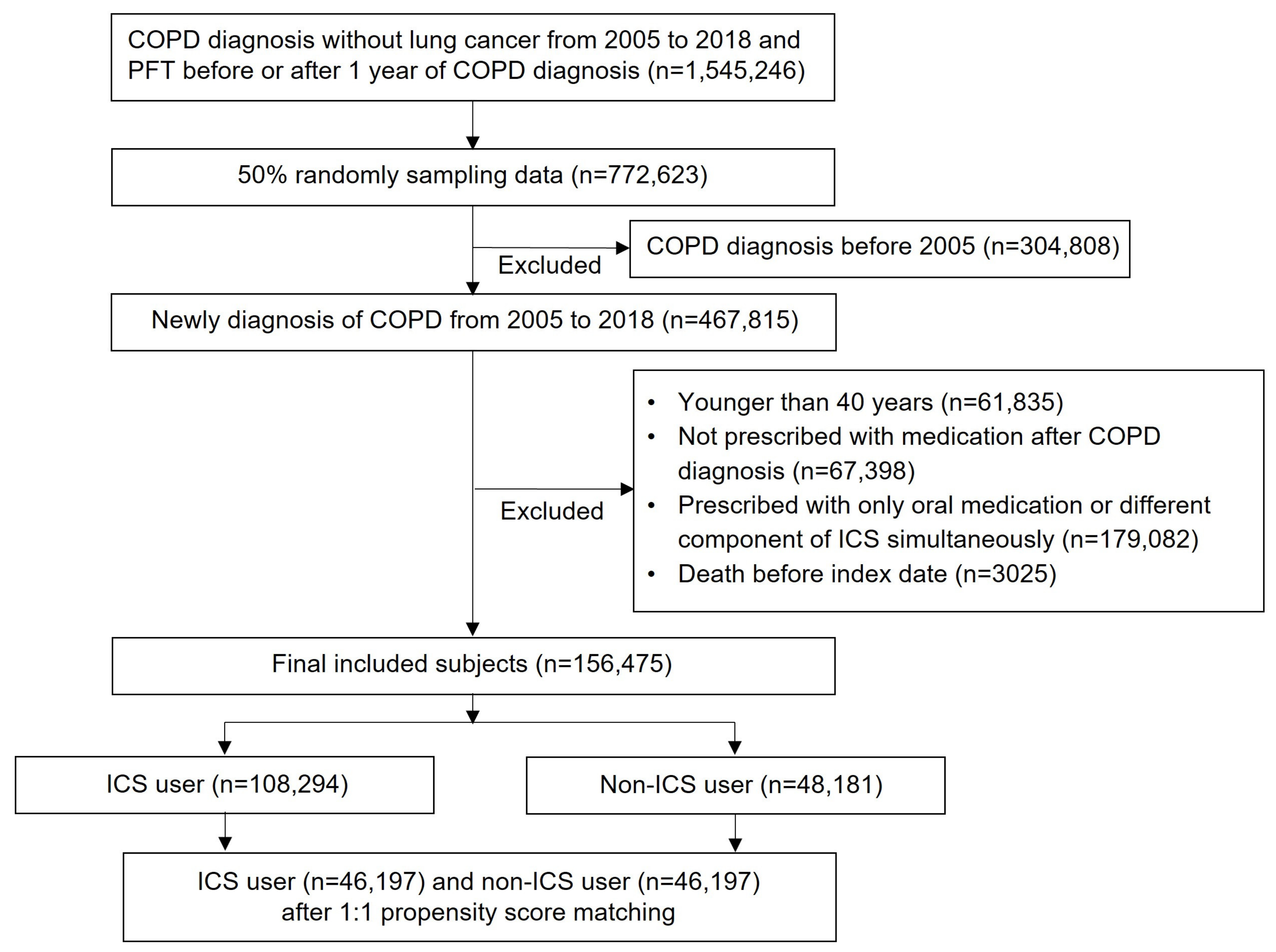
2.2. Selection of Study Participants
2.3. Study Design
2.4. Definition of Outcomes
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Incidence of NTM in ICS Users
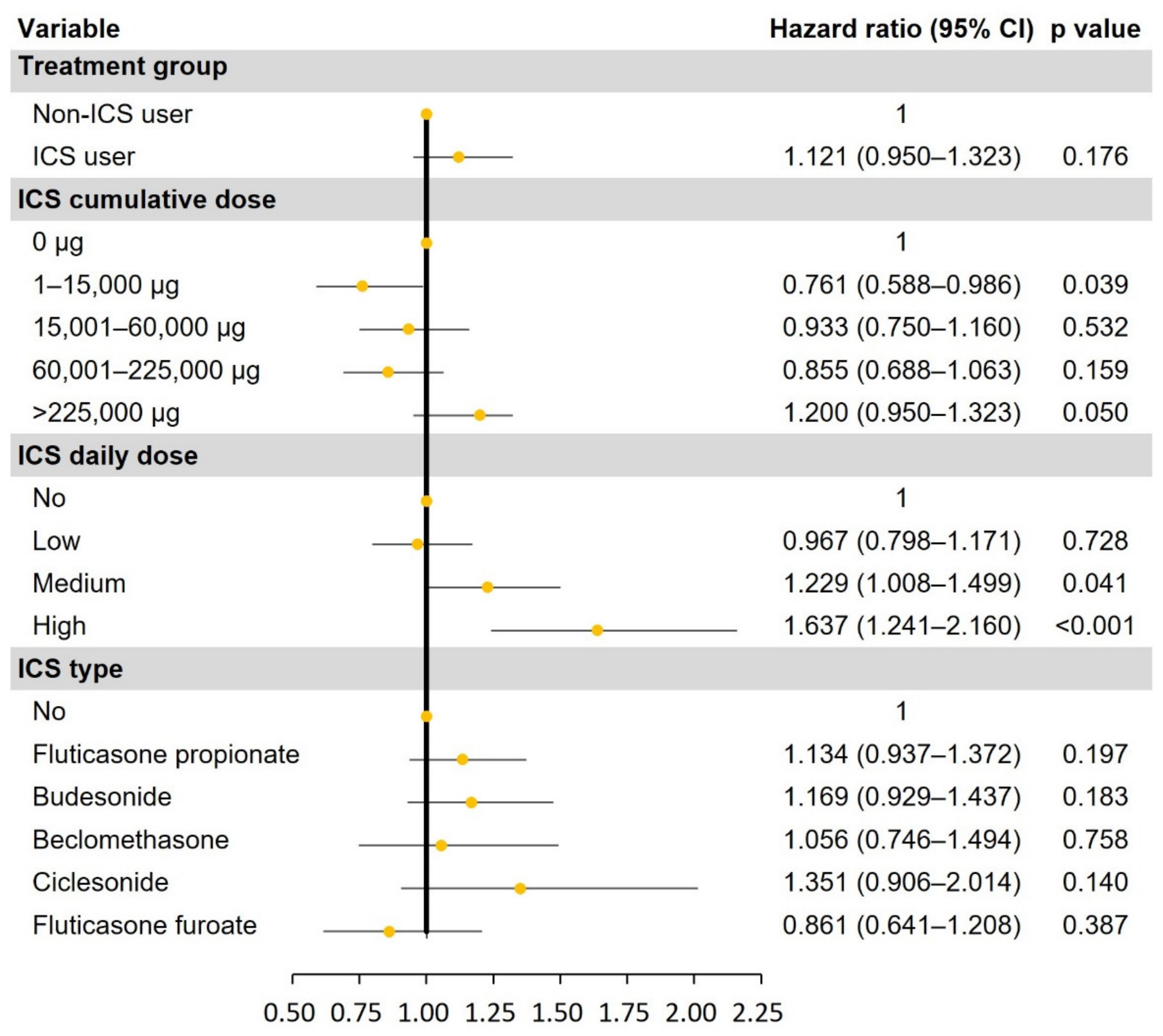
3.3. Risk of NTM in ICS Users
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- GBD Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Koarai, A.; Sugiura, H.; Yamada, M.; Ichikawa, T.; Fujino, N.; Kawayama, T.; Ichinose, M. Treatment with LABA versus LAMA for stable COPD: A systematic review and meta-analysis. BMC Pulm. Med. 2020, 20, 111. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, W.C.; Huang, C.H.; Hsiang, Y.P.; Sheu, C.C.; Chen, Y.C.; Lin, M.C.; Chu, K.A.; Lee, C.H.; Wei, Y.F. LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937194. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Rabe, K.F.; Ferguson, G.T.; Wedzicha, J.A.; Singh, D.; Wang, C.; Rossman, K.; St Rose, E.; Trivedi, R.; Ballal, S.; et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am. J. Respir. Crit. Care Med. 2021, 203, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: Cohort study in real-world clinical practice. Chest 2020, 157, 846–855. [Google Scholar] [CrossRef]
- Cardoso, J.; Ferreira, A.J.; Guimarães, M.; Oliveira, A.S.; Simão, P.; Sucena, M. Treatable traits in COPD—A proposed approach. Int. J. Chron. Obs. Pulmon Dis. 2021, 16, 3167–3182. [Google Scholar] [CrossRef]
- Miravitlles, M.; Auladell-Rispau, A.; Monteagudo, M.; Vázquez-Niebla, J.C.; Mohammed, J.; Nuñez, A.; Urrútia, G. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev. 2021, 30, 2100075. [Google Scholar] [CrossRef]
- Park, S.C.; Kang, M.J.; Han, C.H.; Lee, S.M.; Kim, C.J.; Lee, J.M.; Kang, Y.A. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: A nationwide population-based study. BMC Pulm. Med. 2019, 19, 140. [Google Scholar] [CrossRef]
- Lee, H.; Myung, W.; Lee, E.M.; Kim, H.; Jhun, B.W. Mortality and prognostic factors of nontuberculous mycobacterial infection in Korea: A population-based comparative study. Clin. Infect. Dis. 2021, 72, e610–e619. [Google Scholar] [CrossRef]
- You, Y.; Ni, Y.; Shi, G. Inhaled corticosteroids and mycobacterial infection in patients with chronic airway diseases: A systematic review and meta-analysis. Respiration 2022, 101, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Brode, S.K.; Campitelli, M.A.; Kwong, J.C.; Lu, H.; Marchand-Austin, A.; Gershon, A.S.; Jamieson, F.B.; Marras, T.K. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur. Respir. J. 2017, 50, 1700037. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Khang, Y.H.; Park, J.H.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Bang, J.H.; Ha, S.; et al. Data resource profile: The national health onformation database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, Y.S.; Linton, J.A.; Nam, C.M.; Choi, Y.; Park, E.C. Effects of inhaled corticosteroids /long-acting agonists in a single inhaler versus inhaled corticosteroids alone on all-cause mortality, pneumonia, and fracture in chronic obstructive pulmonary disease: A nationwide cohort study 2002–2013. Respir. Med. 2017, 130, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Suissa, S.; Kezouh, A.; Ernst, P. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am. J. Respir. Crit. Care Med. 2011, 183, 675–678. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, K.; Hyun, M.K.; Jang, E.J.; Lee, N.R.; Yim, J.J. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013, 68, 1105–1113. [Google Scholar] [CrossRef]
- Jeon, D. Infection source and epidemiology of nontuberculous mycobacterial lung disease. Tuberc. Respir. Dis. 2019, 82, 94–101. [Google Scholar] [CrossRef]
- Kim, K.J.; Oh, S.H.; Jeon, D.; Chang, C.L. Isolation and antimicrobial susceptibility of nontuberculous mycobacteria in a tertiary hospital in Korea, 2016 to 2020. Tuberc. Respir. Dis. 2023, 86, 47–56. [Google Scholar] [CrossRef]
- Pathak, K.; Hart, S.; Lande, L. Nontuberculous mycobacteria lung disease (NTM-LD): Current recommendations on diagnosis, treatment, and patient management. Int. J. Gen. Med. 2022, 15, 7619–7629. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef]
- Andrejak, C.; Nielsen, R.; Thomsen, V.O.; Duhaut, P.; Sorensen, H.T.; Thomsen, R.W. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013, 68, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.X.; Winthrop, K.L.; Lu, Y.; Sharifi, H.; Nasiri, H.U.; Ruoss, S.J. Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann. Am. Thorac. Soc. 2018, 15, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Wei, Y.F.; Chen, K.H.; Chuang, S.; Wang, Y.H.; Wang, C.Y.; Wang, H.C. Inhaled corticosteroids increase risk of nontuberculous mycobacterial lung disease: A nested case-control study and meta-analysis. J. Infect. Dis. 2022, 225, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Zhou, W.; Yang, X.; Li, J.; Cao, J. Risk of pneumonia with different inhaled corticosteroids in COPD patients: A meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Belchamber, K.B.; Thomas, C.M.; Dunne, A.E.; Barnes, P.J.; Donnelly, L.E. Comparison of fluticasone propionate and budesonide on COPD macrophage and neutrophil function. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 2883–2897. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Huang, Q.; Liu, Y.; Yuan, M.; Ma, C.; Yan, H. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Front. Pharmacol. 2021, 12, 691621. [Google Scholar] [CrossRef]
- Yu, I.; Park, S.; Hong, S.H.; Chang, M.S.; Lee, S.J.; Yong, S.J.; Lee, W.Y.; Kim, S.H.; Lee, J.H. Risk of tuberculosis caused by fluticasone propionate versus budesonide in chronic obstructive pulmonary disease: A nationwide population-based study. J. Pers. Med. 2022, 12, 1189. [Google Scholar] [CrossRef]
| ICS Users (n = 46,197) | Non-ICS Users (n = 46,197) | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| Mean (SD) | 66.59 (10.94) | 66.68 (10.89) | 0.186 | ||
| 40–49 | 3348 | 7.25 | 3315 | 7.18 | 0.371 |
| 50–59 | 8483 | 18.36 | 8602 | 18.62 | |
| 60–69 | 14,467 | 31.32 | 14,661 | 31.74 | |
| 70–79 | 14,343 | 31.05 | 14,141 | 30.61 | |
| ≥80 | 5556 | 12.03 | 5478 | 11.86 | |
| Sex | |||||
| Male | 33,641 | 72.82 | 33,927 | 73.44 | 0.034 |
| Female | 12,556 | 27.18 | 12,270 | 26.56 | |
| Comorbidity | |||||
| Bronchiectasis | 4223 | 4.68 | 4307 | 4.80 | 0.340 |
| Diabetes | 16,028 | 17.77 | 15,935 | 17.76 | 0.520 |
| Hypertension | 28,593 | 31.70 | 28,395 | 31.64 | 0.180 |
| Heart failure | 10,531 | 11.68 | 10,497 | 11.69 | 0.754 |
| Stroke | 11,369 | 12.60 | 11,233 | 12.52 | 0.298 |
| Chronic kidney disease | 3009 | 3.34 | 3002 | 3.35 | 0.926 |
| Chronic liver disease | 16,442 | 18.23 | 16,381 | 18.25 | 0.675 |
| CCI | |||||
| Mean (SD) | 3.05 (2.09) | 3.10 (2.13) | <0.001 | ||
| <2 | 12,644 | 27.37 | 12,050 | 26.08 | <0.001 |
| ≥2 | 33,553 | 72.63 | 24,147 | 73.92 | |
| Bronchodilator | |||||
| SABA | 1593 | 3.45 | 2368 | 38.94 | <0.001 |
| LAMA | 7261 | 15.72 | 18,258 | 39.52 | <0.001 |
| LABA | 37,871 | 68.99 | 2421 | 5.24 | <0.001 |
| LABA/LAMA | 5472 | 11.84 | 12,150 | 26.30 | <0.001 |
| Hospitalization before index date | |||||
| Yes | 2950 | 6.39 | 2426 | 5.25 | <0.001 |
| No | 43,247 | 93.61 | 43,771 | 94.75 | |
| OCS prescription | |||||
| Yes | 34,764 | 75.25 | 34,416 | 74.50 | 0.008 |
| No | 11,433 | 24.75 | 11,781 | 25.50 | |
| OCS prescription day | |||||
| Mean (SD) | 15.64 (26.86) | 6.07 (11.87) | <0.001 | ||
| Interval from COPD diagnosis to index date | |||||
| Mean (SD) | 378.8 (842.4) | 400.9 (866.8) | <0.001 | ||
| ICS Use (n = 46,197) | ||
|---|---|---|
| n | % | |
| ICS cumulative dose (μg) | ||
| Mean (SD) | 242,280.8 (616,995.24) | |
| Median (Q1, Q3) | 60,000 (15,000, 225,000) | |
| 0–5000 | 11,751 | 25.77 |
| 15,001–60,000 | 11,749 | 25.77 |
| 60,001–225,000 | 10,814 | 23.72 |
| >225,000 | 11,277 | 24.74 |
| ICS daily dose | ||
| Low | 26,919 | 59.04 |
| Medium | 15,202 | 33.34 |
| High | 3470 | 7.61 |
| Type of ICS | ||
| Fluticasone propionate | 21,698 | 46.97 |
| Budesonide | 10,201 | 22.08 |
| Beclomethasone | 4714 | 10.26 |
| Ciclesonide | 1889 | 4.09 |
| Fluticasone furoate | 7668 | 16.60 |
| Person Year | NTM Patients | Incidence Rate (Per 100,000) | |
|---|---|---|---|
| Non-ICS users | 192,202.11 | 440 | 228.93 |
| ICS users | 205,324.15 | 495 | 241.08 |
| ICS cumulative dose (μg) | |||
| 0–15,000 | 35,558.75 | 67 | 188.42 |
| 15,001–60,000 | 45,879.23 | 99 | 215.78 |
| 60,001–225,000 | 48,856.66 | 100 | 204.68 |
| >225,000 | 71,228.87 | 225 | 315.88 |
| ICS daily dose | |||
| Low | 105,955.62 | 220 | 207.63 |
| Medium | 78,401.15 | 205 | 261.48 |
| High | 17,166.75 | 66 | 384.46 |
| Types of ICS | |||
| Fluticasone propionate | 116,934.20 | 275 | 235.17 |
| Budesonide | 47,242.98 | 114 | 241.31 |
| Beclomethasone | 15,614.55 | 39 | 249.77 |
| Ciclesonide | 8499.94 | 26 | 305.88 |
| Fluticasone furoate | 17,032.48 | 41 | 240.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, I.; Hong, S.H.; Chang, M.-S.; Lee, S.J.; Yong, S.J.; Lee, W.-Y.; Kim, S.-H.; Lee, J.-H. Inhaled Corticosteroids and the Risk of Nontuberculous Mycobacterial Pulmonary Disease in Chronic Obstructive Pulmonary Disease: Findings from a Nationwide Population-Based Study. J. Pers. Med. 2023, 13, 1088. https://doi.org/10.3390/jpm13071088
Yu I, Hong SH, Chang M-S, Lee SJ, Yong SJ, Lee W-Y, Kim S-H, Lee J-H. Inhaled Corticosteroids and the Risk of Nontuberculous Mycobacterial Pulmonary Disease in Chronic Obstructive Pulmonary Disease: Findings from a Nationwide Population-Based Study. Journal of Personalized Medicine. 2023; 13(7):1088. https://doi.org/10.3390/jpm13071088
Chicago/Turabian StyleYu, Iseul, Se Hwa Hong, Min-Seok Chang, Seok Jeong Lee, Suk Joong Yong, Won-Yeon Lee, Sang-Ha Kim, and Ji-Ho Lee. 2023. "Inhaled Corticosteroids and the Risk of Nontuberculous Mycobacterial Pulmonary Disease in Chronic Obstructive Pulmonary Disease: Findings from a Nationwide Population-Based Study" Journal of Personalized Medicine 13, no. 7: 1088. https://doi.org/10.3390/jpm13071088
APA StyleYu, I., Hong, S. H., Chang, M.-S., Lee, S. J., Yong, S. J., Lee, W.-Y., Kim, S.-H., & Lee, J.-H. (2023). Inhaled Corticosteroids and the Risk of Nontuberculous Mycobacterial Pulmonary Disease in Chronic Obstructive Pulmonary Disease: Findings from a Nationwide Population-Based Study. Journal of Personalized Medicine, 13(7), 1088. https://doi.org/10.3390/jpm13071088






