Precision Medicine Is Changing the Roles of Healthcare Professionals, Scientists, and Research Staff: Learnings from a Childhood Cancer Precision Medicine Trial
Abstract
1. Introduction
2. Materials and Methods
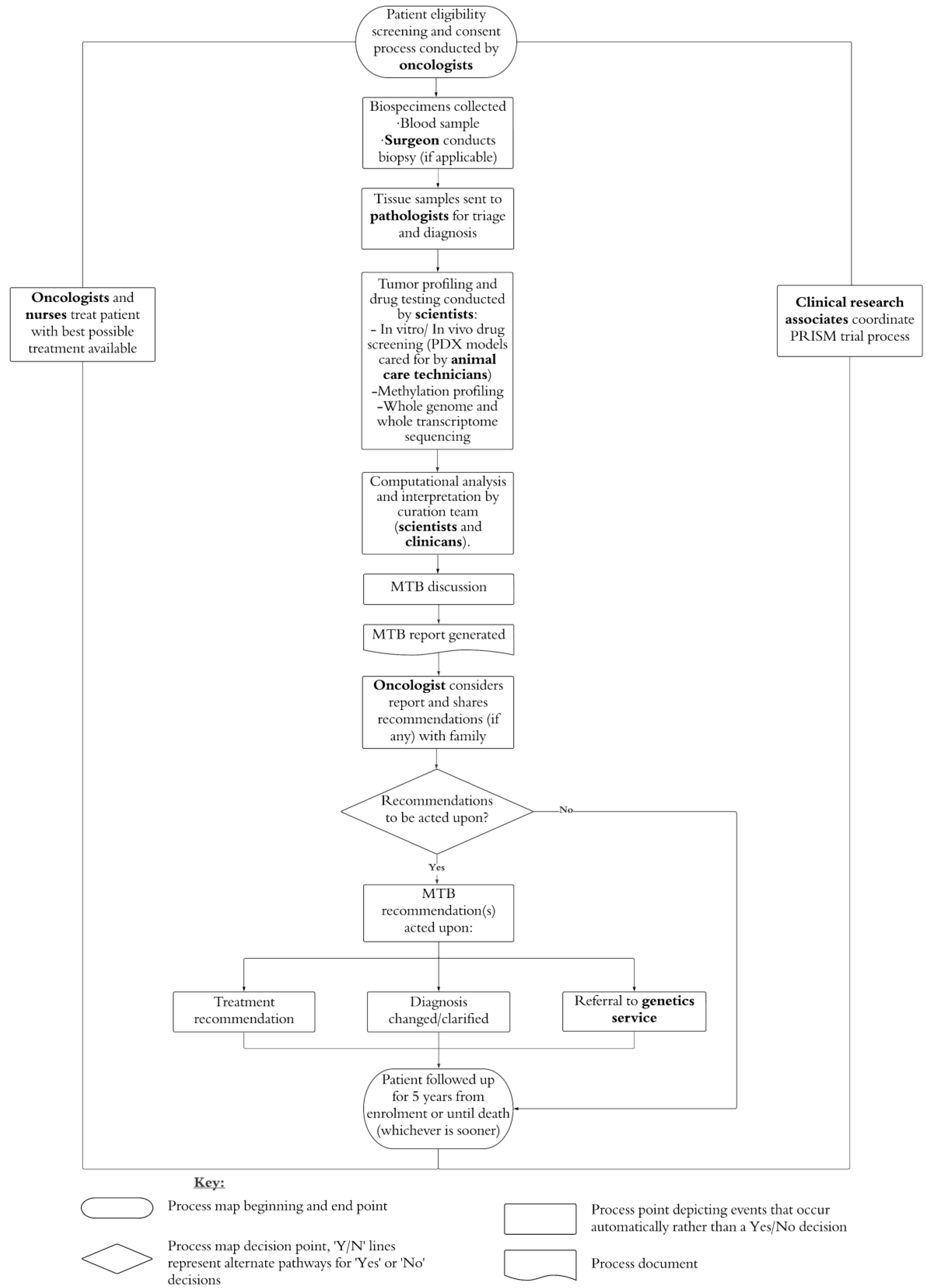
2.1. PRISM and PRISM-Impact
2.2. Participants
2.3. Procedure
2.4. Interview
2.5. Data Analysis
3. Results
3.1. Participants
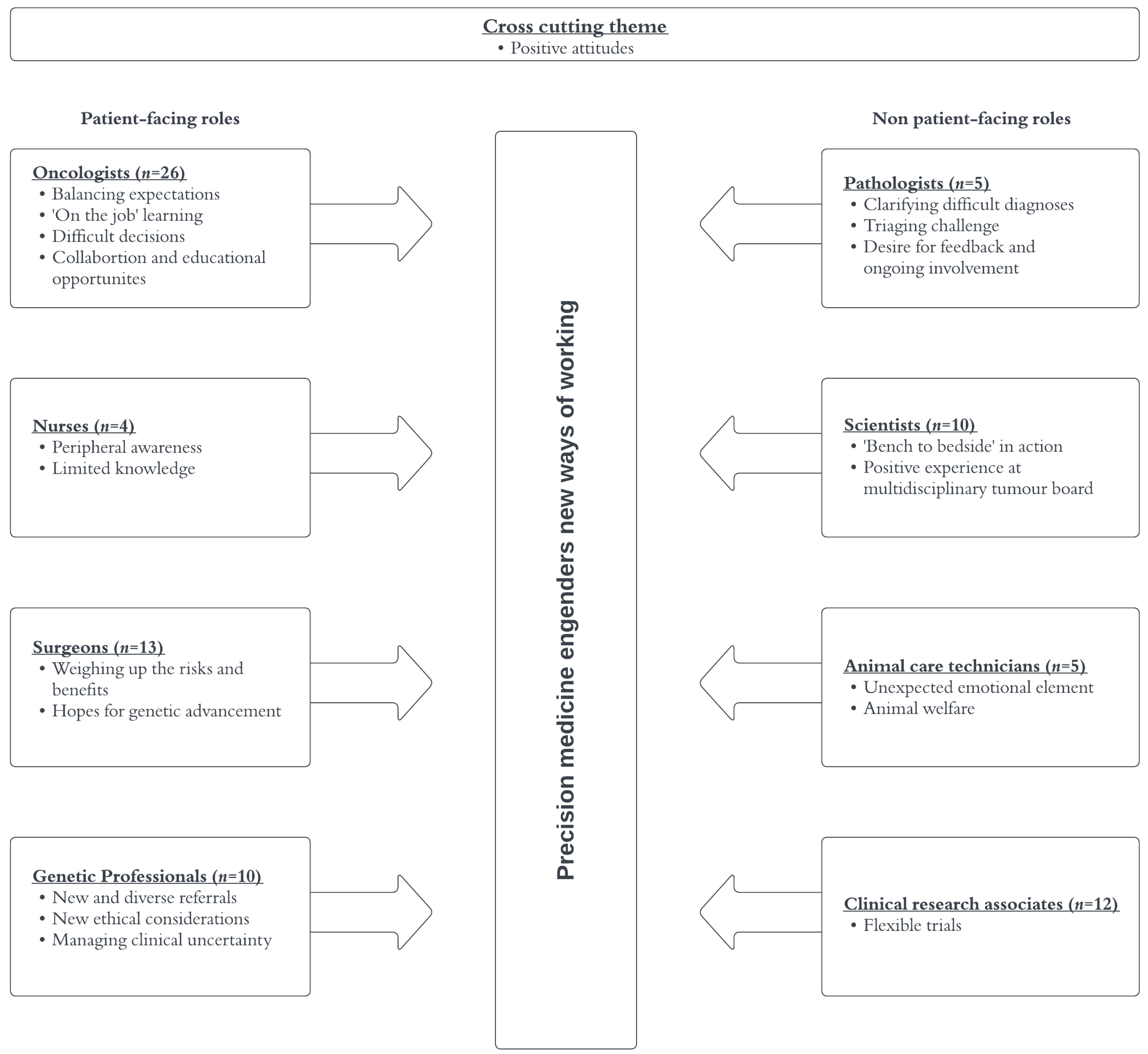
3.2. Themes
3.2.1. Cross-Cutting Theme
3.2.2. Oncologists
3.2.3. Nurses
3.2.4. Surgeons
3.2.5. Genetic Professionals
3.2.6. Pathologists
3.2.7. Scientists
3.2.8. Animal Care Technicians
3.2.9. Clinical Research Associates (CRAs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langenberg, K.P.S.; Looze, E.J.; Molenaar, J.J. The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development. Cancers 2021, 13, 4324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Vundamati, D.S.; Farooqi, M.S.; Guest, E. Precision Medicine in Pediatric Cancer: Current Applications and Future Prospects. High-Throughput 2018, 7, 39. [Google Scholar] [CrossRef]
- Vo, K.T.; Parsons, D.W.; Seibel, N.L. Precision medicine in pediatric oncology. Surg. Oncol. Clin. North Am. 2020, 29, 63. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.R.; George, S.; Angelini, P.; Bruna, A.; Chesler, L. The promise of patient-derived preclinical models to accelerate the implementation of personalised medicine for children with neuroblastoma. J. Pers. Med. 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Kumar, A.; Dolman, M.E.M.; Mayoh, C.; Khuong-Quang, D.; Cadiz, R.; Wong-Erasmus, M.; Mould, E.V.A.; Grebert-Wade, D.; Barahona, P.; et al. The important role of routine cytopathology in pediatric precision oncology. Cancer Cytopathol. 2021, 129, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, C.E.; Doolan, E.L.; Fardell, J.E.; Signorelli, C.; Quinn, V.F.; Tucker, K.M.; Patenaude, A.F.; Marshall, G.M.; Lock, R.B.; Georgiou, G.; et al. The Avatar Acceptability Study: Survivor, parent and community willingness to use patient-derived xen-ografts to personalize cancer care. EBioMedicine 2018, 37, 205–213. [Google Scholar] [CrossRef]
- Lau, L.M.; Mayoh, C.; Xie, J.; Barahona, P.; MacKenzie, K.L.; Wong, M.; Kamili, A.; Tsoli, M.; Failes, T.W.; Kumar, A.; et al. In vitro and in vivo drug screens of tumor cells identify novel therapies for high-risk child cancer. EMBO Mol. Med. 2022, 14, e14608. [Google Scholar] [CrossRef]
- Cahaney, C.; Dhir, A.; Ghosh, T. Role of Precision Medicine in Pediatric Oncology. Pediatr. Ann. 2022, 51, e8–e14. [Google Scholar] [CrossRef] [PubMed]
- Vetsch, J.; Wakefield, C.E.; Techakesari, P.; Warby, M.; Ziegler, D.; O’Brien, T.; Drinkwater, C.; Neeman, N.; Tucker, K. Healthcare professionals’ attitudes toward cancer precision medicine: A systematic review. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wujcik, D. Scientific Advances Shaping the Future Roles of Oncology Nurses. In Seminars in Oncology Nursing; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- McGrath, S.; Ghersi, D. Building towards precision medicine: Empowering medical professionals for the next revolution. BMC Med. Genom. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- McGill, B.C.; Wakefield, C.E.; Hetherington, K.; Munro, L.J.; Warby, M.; Lau, L.; Tyrrell, V.; Ziegler, D.S.; O’brien, T.A.; Marshall, G.M.; et al. “Balancing Expectations with Actual Realities”: Conversations with Clinicians and Scientists in the First Year of a High-Risk Childhood Cancer Precision Medicine Trial. J. Pers. Med. 2020, 10, 9. [Google Scholar] [CrossRef]
- Hsu, R.L.; Gutierrez, A.M.; Schellhammer, S.K.; Robinson, J.O.; Scollon, S.; Street, R.L.; Salisbury, A.N.; Pereira, S.; Plon, S.E.; Malek, J.; et al. Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care. J. Pers. Med. 2021, 11, 570. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Banerjee, S.C.; Carlsson, S.V.; Vera, J.; Lynch, K.A.; Sar-Graycar, L.; Martin, C.M.; Parker, P.A.; Hay, J.L. Clinician perspectives on communication and implementation challenges in precision oncology. Pers. Med. 2021, 18, 559–572. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Shuen, A.Y. In Brief: BRCA1 and BRCA2. J. Pathol. 2013, 230, 347–349. [Google Scholar] [CrossRef]
- Hallowell, N.; Wright, S.; Stirling, D.; Gourley, C.; Young, O.; Porteous, M. Moving into the mainstream: Healthcare profes-sionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam. Cancer 2019, 18, 293–301. [Google Scholar] [CrossRef]
- Wright, H.; Zhao, L.; Birks, M.; Mills, J. Nurses’ competence in genetics: An integrative review. Nurs. Health Sci. 2018, 20, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Stoll, K.; Kubendran, S.; Cohen, S.A. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. In American Journal of Medical Genetics Part C: Seminars in Medical Genetics; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Druker, H.; Zelley, K.; McGee, R.B.; Scollon, S.R.; Kohlmann, W.K.; Schneider, K.A.; Schneider, K.W. Genetic Counselor Recommendations for Cancer Predisposition Evaluation and Surveillance in the Pediatric Oncology Patient. Clin. Cancer Res. 2017, 23, e91–e97. [Google Scholar] [CrossRef]
- Kohut, K.; Limb, S.; Crawford, G. The Changing Role of the Genetic Counsellor in the Genomics Era. Curr. Genet. Med. Rep. 2019, 7, 75–84. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, P.; Zhang, X.; Mania-Farnell, B.; Xi, G.; Wan, F. Advanced Pediatric Diffuse Pontine Glioma Murine Models Pave the Way towards Precision Medicine. Cancers 2021, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Alférez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinská, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef]
- Grunfeld, E.; Zitzelsberger, L.; Coristine, M.; Aspelund, F. Barriers and facilitators to enrollment in cancer clinical trials: Qualitative study of the perspectives of clinical research associates. Cancer 2002, 95, 1577–1583. [Google Scholar] [CrossRef]
- Pfister, S.M.; Reyes-Múgica, M.; Chan, J.K.; Hasle, H.; Lazar, A.J.; Rossi, S.; Ferrari, A.; Jarzembowski, J.A.; Pritchard-Jones, K.; Hill, D.A.; et al. A Summary of the Inaugural WHO Classification of Pediatric Tumors: Transitioning from the Optical into the Molecular Era. Cancer Discov. 2021, 12, 331–355. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z. The Role of Pathology in the Era of Personalized (Precision) Medicine: A Brief Review. Acta Med. Acad. 2021, 50, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Virgin, H.W., IV; Asa, S.L. “Next-Generation” Pathology and Laboratory Medicine; College of American Pathologist: Northfield, IL, USA, 2011; pp. 1531–1532. [Google Scholar]
- Kaul, K.L. Preparing pathology for precision medicine: Challenges and opportunities. Virchows Arch. 2017, 471, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rapport, F.; Smith, J.; O’brien, T.A.; Tyrrell, V.J.; Mould, E.V.; Long, J.C.; Gul, H.; Braithwaite, J. Development of an implementation and evaluation strategy for the Australian ‘Zero Childhood Cancer’ (Zero) Program: A study protocol. BMJ Open 2020, 10, e034522. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.-A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Vorderstrasse, A.A.; Hammer, M.J.; Dungan, J.R. Nursing Implications of Personalized and Precision Medicine. Semin. Oncol. Nurs. 2014, 30, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Fanta, P.T.; Boles, S.G.; Daniels, G.A.; Bazhenova, L.A.; Subramanian, R.; Coutinho, A.C.; Ojeda-Fournier, H.; et al. Molecular Tumor Board: The University of California San Diego Moores Cancer Center Experience. Oncologist 2014, 19, 631–636. [Google Scholar] [CrossRef]
- van der Velden, D.; van Herpen, C.; van Laarhoven, H.; Smit, E.; Groen, H.; Willems, S.; Nederlof, P.; Langenberg, M.; Cuppen, E.; Sleijfer, S.; et al. Molecular Tumor Boards: Current practice and future needs. Ann. Oncol. 2017, 28, 3070–3075. [Google Scholar] [CrossRef]
- Formea, C.M.; Nicholson, W.T.; Vitek, C.R. An inter-professional approach to personalized medicine education: One institution’s experience. Pers. Med. 2015, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.E.; Moeckel, F.; Villa, M.S.; Housman, L.T.; McCarty, C.A.; McLeod, H.L. Strategies for integrating personalized medicine into healthcare practice. Pers. Med. 2017, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Newsome, J.T.; Clemmons, E.A.; Fitzhugh, D.C.; Gluckman, T.L.; Creamer-Hente, M.A.; Tambrallo, L.J.; Wilder-Kofie, T. Compassion fatigue, euthanasia stress, and their management in laboratory animal research. J. Am.-Can Assoc. Lab. Anim. Sci. 2019, 58, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Tremoleda, J.L.; Kerton, A. Creating space to build emotional resilience in the animal research community. Lab Anim. 2020, 49, 1–3. [Google Scholar] [CrossRef] [PubMed]
| Profession | Invited (n) | Opted in (n) | Participated (n) | Response Rate (%) | Participation Rate (%) |
|---|---|---|---|---|---|
| Oncologist | 57 | 30 | 26 | 52.6 | 86.6 |
| Surgeon | 21 | 17 | 13 | 80.9 | 76.4 |
| Clinical Research Associate | 24 | 13 | 12 | 54.1 | 92.3 |
| Genetic Professionals | 15 | 10 | 10 | 66.6 | 100 |
| Scientist | 24 | 11 | 10 | 45.0 | 90.9 |
| Pathologist | 22 | 7 | 5 | 31.8 | 71.4 |
| Nurse | 15 | 6 | 4 | 40.0 | 66.6 |
| Animal Care Technician | 9 | 7 | 5 | 77.7 | 71.4 |
| Total | 187 | 101 | 85 | - | - |
| Characteristic | Participants (n = 85) |
|---|---|
| Profession, n | |
| Oncologist | 26 |
| Surgeon | 13 |
| Clinical Research Associate | 12 |
| Genetics Professional | 10 |
| Scientist | 10 |
| Pathologist | 5 |
| Nurse | 4 |
| Animal Care Technician | 5 |
| Site, n (%) | |
| Sydney Children’s Hospital | 25 (29.4) |
| Children’s Cancer Institute | 15 (17.6) |
| Royal Children’s Hospital, Melbourne | 9 (10.6) |
| Perth Children’s Hospital | 9 (10.6) |
| Queensland Children’s Hospital | 8 (9.4) |
| The Children’s Hospital, Westmead | 8 (9.4) |
| John Hunter Children’s Hospital, Newcastle | 5 (5.9) |
| Women’s and Children’s Hospital, Adelaide | 4 (4.7) |
| Monash Children’s Hospital, Melbourne | 2 (2.4) |
| Age (years), Mean (SD), Range | 43.1 (11), 24–75 |
| Gender, n (%) | |
| Female | 50 (58.8) |
| No. years working in pediatric oncology by profession, mean (SD), range 1 | |
| Oncologist | 15.61 (10.9), 6–50 |
| Nurse | 17.25 (12), 6–30 |
| Genetics Professional | 12.93 (14.2), 1–40 |
| Clinical Research Associate | 4.20 (4.1) 0–14 |
| No. years of practice by profession, mean (SD), range 1 | |
| Pathologist | 17.13 (9.5), 9–30 |
| Scientist | 8.52 (10), 1–30 |
| Animal Care Technician | 8.60 (9.5), 1–25 |
| Percentage of their time dedicated to research by profession, mean (SD), range 1 | |
| Oncologist | 35.4 (27.7), 5–100 |
| Nurse | 37.5 (12.5), 20–50 |
| Surgeon | 8.71 (7.6), 2–30 |
| Genetics Professional | 22.75 (23.9), 2–60 |
| Pathologist | 8.4 (7), 2–20 |
| Scientist | 100 (100), 100–100 |
| Animal Care Technician | 47.5 (33), 10–90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daly, R.; Hetherington, K.; Hazell, E.; Wadling, B.R.; Tyrrell, V.; Tucker, K.M.; Marshall, G.M.; Ziegler, D.S.; Lau, L.M.S.; Trahair, T.N.; et al. Precision Medicine Is Changing the Roles of Healthcare Professionals, Scientists, and Research Staff: Learnings from a Childhood Cancer Precision Medicine Trial. J. Pers. Med. 2023, 13, 1033. https://doi.org/10.3390/jpm13071033
Daly R, Hetherington K, Hazell E, Wadling BR, Tyrrell V, Tucker KM, Marshall GM, Ziegler DS, Lau LMS, Trahair TN, et al. Precision Medicine Is Changing the Roles of Healthcare Professionals, Scientists, and Research Staff: Learnings from a Childhood Cancer Precision Medicine Trial. Journal of Personalized Medicine. 2023; 13(7):1033. https://doi.org/10.3390/jpm13071033
Chicago/Turabian StyleDaly, Rebecca, Kate Hetherington, Emily Hazell, Bethany R. Wadling, Vanessa Tyrrell, Katherine M. Tucker, Glenn M. Marshall, David S. Ziegler, Loretta M. S. Lau, Toby N. Trahair, and et al. 2023. "Precision Medicine Is Changing the Roles of Healthcare Professionals, Scientists, and Research Staff: Learnings from a Childhood Cancer Precision Medicine Trial" Journal of Personalized Medicine 13, no. 7: 1033. https://doi.org/10.3390/jpm13071033
APA StyleDaly, R., Hetherington, K., Hazell, E., Wadling, B. R., Tyrrell, V., Tucker, K. M., Marshall, G. M., Ziegler, D. S., Lau, L. M. S., Trahair, T. N., O’Brien, T. A., Collins, K., Gifford, A. J., Haber, M., Pinese, M., Malkin, D., Cowley, M. J., Karpelowsky, J., Drew, D., ... Wakefield, C. E. (2023). Precision Medicine Is Changing the Roles of Healthcare Professionals, Scientists, and Research Staff: Learnings from a Childhood Cancer Precision Medicine Trial. Journal of Personalized Medicine, 13(7), 1033. https://doi.org/10.3390/jpm13071033






