Creeping Fat in Crohn’s Disease—Surgical, Histological, and Radiological Approaches
Abstract
1. Introduction
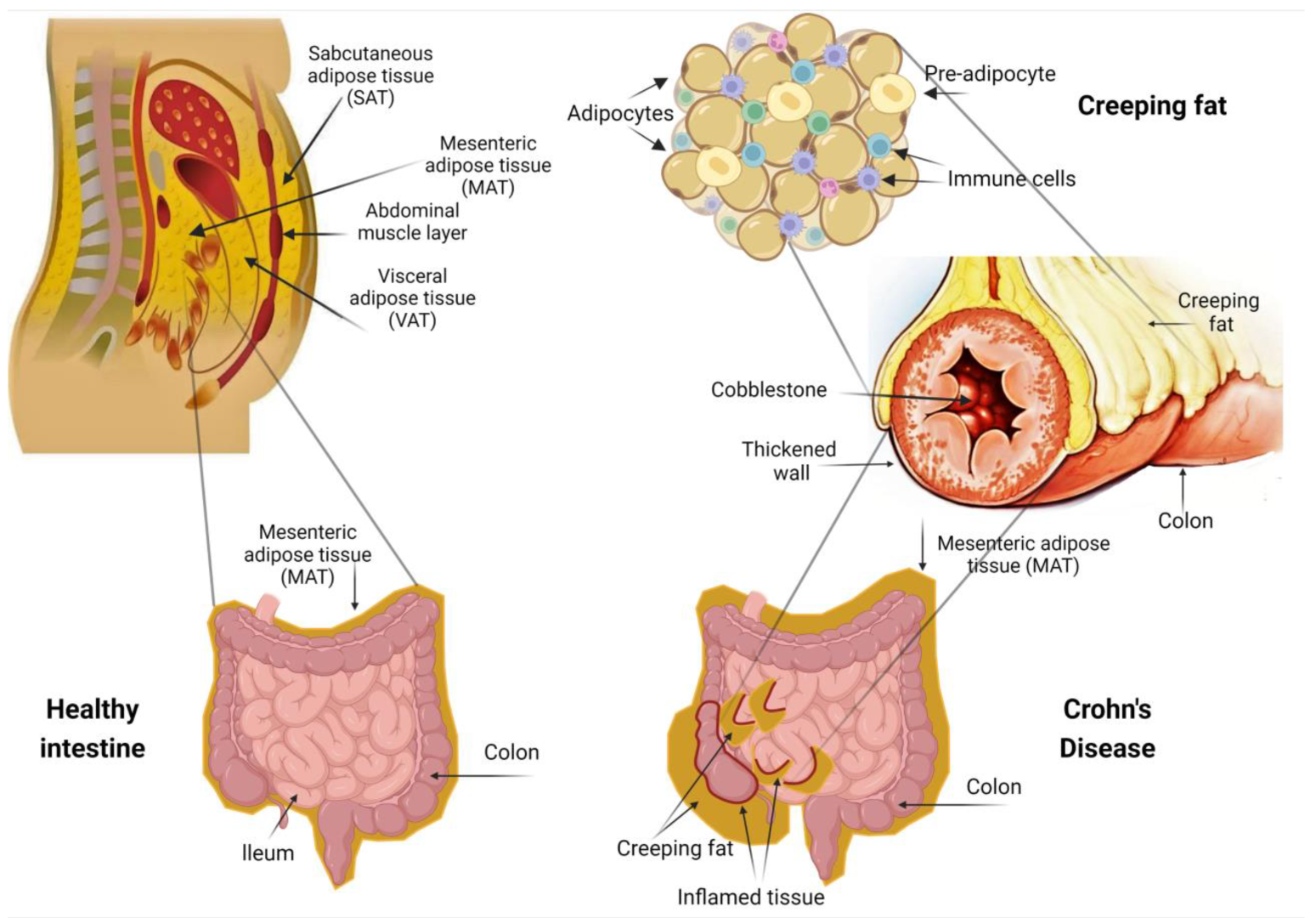
2. Anatomical Relationship between Mesenteric Adipose Tissue and the Intestinal Wall
3. Role of Mesenchymal Stem Cells in the Adipose Tissue
4. The Role of Creeping Fat in Intestinal Inflammation
5. Surgical and Histological Evidence for the Importance of Creeping Fat
6. Creeping Fat: The Radiological Approach
7. The Role of Artificial Intelligence Technology
8. Conclusions—Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef]
- Rustgi, S.D.; Kayal, M. Sex-based differences in inflammatory bowel diseases: A review. Therap. Adv. Gastroenterol. 2020, 13, 1756284820915043. [Google Scholar] [CrossRef]
- Lungaro, L.; Costanzini, A. Impact of Female Gender in Inflammatory Bowel Diseases: A Narrative Review. J. Pers. Med. 2023, 13, 165. [Google Scholar] [CrossRef]
- Severs, M.; Spekhorst, L.M.; Mangen, M.J.; Dijkstra, G.; Löwenberg, M.; Hoentjen, F.; van der Meulen-de Jong, A.E.; Pierik, M.; Ponsioen, C.Y.; Bouma, G.; et al. Sex-Related Differences in Patients with Inflammatory Bowel Disease: Results of 2 Prospective Cohort Studies. Inflamm. Bowel Dis. 2018, 24, 1298–1306. [Google Scholar] [CrossRef]
- Romberg-Camps, M.J.; Dagnelie, P.C.; Kester, A.D.; Hesselink-van de Kruijs, M.A.; Cilissen, M.; Engels, L.G.; Van Deursen, C.; Hameeteman, W.H.; Wolters, F.L.; Russel, M.G.; et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am. J. Gastroenterol. 2009, 104, 371–383. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Harmsen, W.S.; Tremaine, W.J.; Zinsmeister, A.R.; Sandborn, W.J.; Loftus, E.V., Jr. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004). Am. J. Gastroenterol. 2012, 107, 1693–1701. [Google Scholar] [CrossRef]
- Shah, S.C.; Khalili, H.; Gower-Rousseau, C.; Olen, O.; Benchimol, E.I.; Lynge, E.; Nielsen, K.R.; Brassard, P.; Vutcovici, M.; Bitton, A.; et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies from Western Countries. Gastroenterology 2018, 155, 1079–1089.e3. [Google Scholar] [CrossRef]
- Cushing, K.; Higgins, P.D.R. Management of Crohn Disease: A Review. JAMA 2021, 325, 69–80. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Caputo, F.; Zoli, E.; Giancola, F.; Chiarioni, G.; De Giorgio, R. Nutritional Treatment in Crohn’s Disease. Nutrients 2021, 13, 1628. [Google Scholar] [CrossRef]
- Laing, B.B.; Lim, A.G.; Ferguson, L.R. A Personalised Dietary Approach—A Way Forward to Manage Nutrient Deficiency, Effects of the Western Diet, and Food Intolerances in Inflammatory Bowel Disease. Nutrients 2019, 11, 1532. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohns Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Regional ileitis: A pathologic and clinical entity. JAMA 1932, 99, 1323–1329. [Google Scholar] [CrossRef]
- Romacho, T.; Elsen, M.; Röhrborn, D.; Eckel, J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef]
- Parra-Peralbo, E.; Talamillo, A.; Barrio, R. Origin and Development of the Adipose Tissue, a Key Organ in Physiology and Disease. Front. Cell Dev. Biol. 2021, 9, 786129. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 29, 1741–1755. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sato, S.; Kurashima, Y.; Lai, C.-Y.; Otsu, M.; Hayashi, M.; Yamaguchi, T.; Kiyono, H. Reciprocal inflammatory signaling between intestinal epithelial cells and adipocytes in the absence of immune cells. EBioMedicine 2017, 23, 34–45. [Google Scholar] [CrossRef]
- Serena, C.; Keiran, N.; Madeira, A.; Maymó-Masip, E.; Ejarque, M.; Terrón-Puig, M.; Espin, E.; Martí, M.; Borruel, N.; Guarner, F.; et al. Crohn’s Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation. Stem Cell Rep. 2017, 9, 1109–1123. [Google Scholar] [CrossRef]
- Borley, N.R.; Mortensen, N.J.; Jewell, D.P.; Warren, B.F. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: Evidence for a possible causative link. J. Pathol. 2000, 190, 196–202. [Google Scholar] [CrossRef]
- Zuo, L.; Li, Y.; Zhu, W.; Shen, B.; Gong, J.; Guo, Z.; Zhang, W.; Wu, R.; Gu, L.; Li, N. Mesenteric adipocyte dysfunction in Crohn’s disease is associated with hypoxia. Inflamm. Bowel Dis. 2016, 22, 114–126. [Google Scholar] [CrossRef]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The mesenteric fat and intestinal muscle interface: Creeping fat influencing stricture formation in Crohn’s disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef]
- Cravo, M.L.; Velho, S.; Torres, J.; Santos, M.P.C.; Palmela, C.; Cruz, R.; Strecht, J.; Maio, R.; Baracos, V. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study. Clin. Nutr. ESPEN 2017, 21, 79–85. [Google Scholar] [CrossRef]
- Büning, C.; von Kraft, C.; Hermsdorf, M.; Gentz, E.; Wirth, E.K.; Valentini, L.; Haas, V. Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm. Bowel Dis. 2015, 21, 2590–2597. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Visceral adipose tissue is associated with stricturing Crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm. Bowel Dis. 2019, 25, 592–600. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e17. [Google Scholar] [CrossRef]
- Suau, R.; Pardina, E.; Domènech, E.; Lorén, V.; Manyé, J. The complex relationship between microbiota, immune response and creeping fat in Crohn’s disease. J. Crohn’s Colitis 2022, 16, 472–489. [Google Scholar] [CrossRef]
- Eder, P.; Adler, M.; Dobrowolska, A.; Kamhieh-Milz, J.; Witowski, J. The role of adipose tissue in the pathogenesis and therapeutic outcomes of inflammatory bowel disease. Cells 2019, 8, 628. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Li, C.; Zhang, L.; Zhuoma, D.; Yang, P.; Yan, G.; Chen, C.; Ba, Y.; Du, P.; et al. Single-cell Expression Atlas Reveals Cell Heterogeneity in the Creeping Fat of Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 850–865. [Google Scholar] [CrossRef]
- Coffey, J.C.; Byrnes, K.G.; Walsh, D.J.; Cunningham, R.M. Update on the mesentery: Structure, function, and role in disease. Lancet Gastroenterol. Hepatol. 2022, 7, 96–106. [Google Scholar] [CrossRef]
- Coffey, J.C.; Walsh, D.; Byrnes, K.G.; Hohenberger, W.; Heald, R. Mesentery—A ‘New’ organ. Emerg. Top. Life Sci. 2020, 4, 191–206. [Google Scholar]
- Coffey, J.C.; Culligan, K.; Walsh, L.G.; Sehgal, R.; Dunne, C.; McGrath, D.; Walsh, D.; Moore, M.; Staunton, M.; Scanlon, T. An appraisal of the computed axial tomographic appearance of the human mesentery based on mesenteric contiguity from the duodenojejunal flexure to the mesorectal level. Eur. Radiol. 2016, 26, 714–721. [Google Scholar] [CrossRef]
- Anthony, A.; Dhillon, A.P.; Pounder, R.E.; Wakefield, A.J. Ulceration of the ileum in Crohn’s disease: Correlation with vascular anatomy. J. Clin. Pathol. 1997, 50, 1013–1017. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Zuo, L.; Shen, B. The role of the mesentery in Crohn’s disease: The contributions of nerves, vessels, lymphatics, and fat to the pathogenesis and disease course. Inflamm. Bowel Dis. 2016, 22, 1483–1495. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Zou, Y.; Cao, L.; Cai, X.; Gong, J.; Xu, Y.; Zhu, W. Mesenteric adipose tissue alterations in Crohn’s disease are associated with the lymphatic system. Inflamm. Bowel Dis. 2019, 25, 283–293. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Pestel, J.; Blangero, F.; Eljaafari, A. Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases. Cells 2023, 12, 348. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef]
- Dave, M.; Dev, A.; Somoza, R.A.; Zhao, N.; Viswanath, S.; Mina, P.R.; Chirra, P.; Obmann, V.C.; Mahabeleshwar, G.H.; Menghini, P.; et al. Mesenchymal stem cells ameliorate inflammation in an experimental model of Crohn’s disease via the mesentery. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tian, C.M.; Zhang, Y.; Yang, M.F.; Xu, H.M.; Zhu, M.Z. Stem Cell Therapy in Inflammatory Bowel Disease: A Review of Achievements and Challenges. J. Inflamm. Res. 2023, 16, 2089–2119. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Dicker, A.; Le Blanc, K.; Åström, G.; van Harmelen, V.; Götherström, C.; Blomqvist, L.; Arner, P.; Rydén, M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005, 308, 283–290. [Google Scholar] [CrossRef]
- Shamsuddin, S.A.; Chan, A.M.L.; Ng, M.H.; Yazid, M.D.; Law, J.X.; Hj Idrus, R.B.; Fauzi, M.B.; Mohd Yunus, M.H.; Lokanathan, Y. Stem cells as a potential therapy in managing various disorders of metabolic syndrome: A systematic review. Am. J. Transl. Res. 2021, 13, 12217–12227. [Google Scholar]
- Tokunaga, M.; Inoue, M.; Jiang, Y.; Barnes, R.H.; Buchner, D.A.; Chun, T.-H. Fat depot-specific gene signature and ECM remodeling of Sca1high adipose-derived stem cells. Matrix Biol. 2014, 36, 28–38. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mikhailova, M.V.; Rasoolzadegan, S.; Falaki, M.; Akhavanfar, R.; Gonzáles, J.L.A.; Rigi, A.; Kiasari, B.A. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur. J. Med. Res. 2023, 28, 47. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Micheletto, G.; Gentilini, D.; Gilardini, L.; Danelli, P.; Invitti, C. Visceral adipocytes: Old actors in obesity and new protagonists in Crohn’s disease? Gut 2012, 61, 86–94. [Google Scholar] [CrossRef]
- Olivier, I.; Théodorou, V.; Valet, P.; Castan-Laurell, I.; Guillou, H.; Bertrand-Michel, J.; Cartier, C.; Bezirard, V.; Ducroc, R.; Segain, J.P.; et al. Is Crohn’s creeping fat an adipose tissue? Inflamm. Bowel Dis. 2011, 17, 747–757. [Google Scholar] [CrossRef]
- Karagiannides, I.; Kokkotou, E.; Tansky, M.; Tchkonia, T.; Giorgadze, N.; O’Brien, M.; Leeman, S.E.; Kirkland, J.L.; Pothoulakis, C. Induction of colitis causes inflammatory responses in fat depots: Evidence for substance P pathways in human mesenteric preadipocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 5207–5212. [Google Scholar] [CrossRef]
- Sideri, A.; Bakirtzi, K.; Shih, D.Q.; Koon, H.W.; Fleshner, P.; Arsenescu, R.; Arsenescu, V.; Turner, J.R.; Karagiannides, I.; Pothoulakis, C. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 420–432. [Google Scholar] [CrossRef]
- Koon, H.W.; Kim, Y.S.; Xu, H.; Kumar, A.; Zhao, D.; Karagiannides, I.; Dobner, P.R.; Pothoulakis, C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2009, 106, 8766–8771. [Google Scholar] [CrossRef]
- Tsounis, E.P.; Aggeletopoulou, I.; Mouzaki, A.; Triantos, C. Creeping Fat in the Pathogenesis of Crohn’s Disease: An Orchestrator or a Silent Bystander? Inflamm. Bowel Dis. 2023, izad095. [Google Scholar] [CrossRef]
- Pennington, L.; Hamilton, S.R.; Bayless, T.M.; Cameron, J.L. Surgical management of Crohn’s disease. Influence of disease at margin of resection. Ann. Surg. 1980, 192, 311–318. [Google Scholar] [CrossRef]
- Fazio, V.W.; Marchetti, F.; Church, M.; Goldblum, J.R.; Lavery, C.; Hull, T.L.; Milsom, J.W.; Strong, S.A.; Oakley, J.R.; Secic, M. Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. A randomized controlled trial. Ann. Surg. 1996, 224, 563–571. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Gong, J.; Zhu, W. Mesenteric organ lymphatics and inflammatory bowel disease. Ann. Anat. 2018, 218, 199–204. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Shen, B.; Li, N.; Li, J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Color. Dis. 2015, 17, 225–234. [Google Scholar] [CrossRef]
- Holt, D.; Moore, G.; Strauss, B.; Hamilton, A.; De Cruz, P.; Kamm, M. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment. Pharmacol. Ther. 2017, 45, 1255–1264. [Google Scholar] [CrossRef]
- Erhayiem, B.; Dhingsa, R.; Hawkey, C.J.; Subramanian, V. Ratio of Visceral to Subcutaneous Fat Area Is a Biomarker of Complicated Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 684–687.e1. [Google Scholar] [CrossRef]
- Sheehan, A.; Warren, B.; Gear, M.; Shepherd, N. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef]
- Maconi, G.; Greco, S.; Duca, P.; Ardizzone, S.; Massari, A.; Cassinotti, A.; Radice, E.; Bianchi Porro, G. Prevalence and clinical significance of sonographic evidence of mesenteric fat alterations in Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1555–1561. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Cao, L.; Cai, X.; Ge, Y.; Zhu, W. Decreased expression of Prox1 is associated with postoperative recurrence in Crohn’s disease. J. Crohn’s Colitis 2018, 12, 1210–1218. [Google Scholar] [CrossRef]
- Li, Y.; Ge, Y.; Gong, J.; Zhu, W.; Cao, L.; Guo, Z.; Gu, L.; Li, J. Mesenteric lymphatic vessel density is associated with disease behavior and postoperative recurrence in Crohn’s disease. J. Gastrointest. Surg. 2018, 22, 2125–2132. [Google Scholar] [CrossRef]
- Kiernan, M.G.; Coffey, J.C.; McDermott, K.; Cotter, P.D.; Cabrera-Rubio, R.; Kiely, P.A.; Dunne, C.P. The human mesenteric lymph node microbiome differentiates between Crohn’s disease and ulcerative colitis. J. Crohn’s Colitis 2019, 13, 58–66. [Google Scholar] [CrossRef]
- Coffey, C.J.; Kiernan, M.G.; Sahebally, S.M.; Jarrar, A.; Burke, J.P.; Kiely, P.A.; Shen, B.; Waldron, D.; Peirce, C.; Moloney, M. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J. Crohn’s Colitis 2018, 12, 1139–1150. [Google Scholar] [CrossRef]
- Kusunoki, M.; Ikeuchi, H.; Yanagi, H.; Shoji, Y.; Yamamura, T. A comparison of stapled and hand-sewn anastomoses in Crohn’s disease. Dig. Surg. 1998, 15, 679–682. [Google Scholar] [CrossRef]
- Varma, M.G. Sew or staple: Does it make a difference? Inflamm. Bowel Dis. 2010, 17, 1046–1047. [Google Scholar] [CrossRef]
- Maguire, L.H.; Alavi, K.; Sudan, R.; Wise, P.E.; Kaiser, A.M.; Bordeianou, L. Surgical considerations in the treatment of small bowel Crohn’s disease. J. Gastrointest. Surg. 2017, 21, 398–411. [Google Scholar] [CrossRef]
- Li, Y.; Mohan, H.; Lan, N.; Wu, X.; Zhou, W.; Gong, J.; Shen, B.; Stocchi, L.; Coffey, J.C.; Zhu, W. Mesenteric excision surgery or conservative limited resection in Crohn’s disease: Study protocol for an international, multicenter, randomized controlled trial. Trials 2020, 21, 210. [Google Scholar] [CrossRef]
- Alshantti, A.; Hind, D.; Hancock, L.; Brown, S. The role of Kono-S anastomosis and mesenteric resection in reducing recurrence after surgery for Crohn’s disease: A systematic review. Color. Dis. 2021, 23, 7–17. [Google Scholar] [CrossRef]
- Gauci, J.; Sammut, L.; Sciberras, M.; Piscopo, N.; Micallef, K.; Cortis, K.; Ellul, P. Small bowel imaging in Crohn’s disease patients. Ann. Gastroenterol. 2018, 31, 395–405. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.; Vermeire, S.; Colombel, J. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: A semiquantitative analysis by using a novel histological grading scheme. J. Crohn’s Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef]
- Zhang, X.; Ko, H.M.; Torres, J.; Panchal, H.J.; Cai, Z.; Wagner, M.; Sands, B.E.; Colombel, J.-F.; Cho, J.; Taouli, B. Luminally polarized mural and vascular remodeling in ileal strictures of Crohn’s disease. Hum. Pathol. 2018, 79, 42–49. [Google Scholar] [CrossRef]
- Li, C.; Vu, K.; Hazelgrove, K.; Kuemmerle, J.F. Increased IGF-IEc expression and mechano-growth factor production in intestinal muscle of fibrostenotic Crohn’s disease and smooth muscle hypertrophy. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G888–G899. [Google Scholar] [CrossRef]
- Mao, R.; Doyon, G.; Kurada, S.; Gordon, I.; Zhao, S.; Dejanovic, D.; West, G.A.; Rennison, J.; van Wagoner, D.; Fiocchi, C. 629-Creeping-Fat Derived Free Fatty Acids Induce Hyperplasia of Intestinal Muscularis Propria Muscle Cells–A Novel Link Between Fat and Intestinal Stricture Formation in Crohn’s Disease. Gastroenterology 2018, 154, S-131. [Google Scholar] [CrossRef]
- Mao, R.; Doyon, G.; Gordon, I.O.; Li, J.; Lin, S.; Wang, J.; Le, T.H.N.; Elias, M.; Kurada, S.; Southern, B. Activated intestinal muscle cells promote preadipocyte migration: A novel mechanism for creeping fat formation in Crohn’s disease. Gut 2022, 71, 55–67. [Google Scholar] [CrossRef]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Klopfenstein, B.J.; Kim, M.; Krisky, C.; Szumowski, J.; Rooney, W.; Purnell, J. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol. 2012, 85, e826–e830. [Google Scholar] [CrossRef]
- Rowan, C.R.; McManus, J.; Boland, K.; O’Toole, A. Visceral adiposity and inflammatory bowel disease. Int. J. Color. Dis. 2021, 36, 2305–2319. [Google Scholar] [CrossRef]
- Magro, D.O.; Barreto, M.R.L.; Cazzo, E.; Camargo, M.G.; Kotze, P.G.; Coy, C.S.R. Visceral fat is increased in individuals with Crohn’s disease: A comparative analysis with healthy controls. Arq. Gastroenterol. 2018, 55, 142–147. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Y.; Cai, X.; Ge, Y.; Zhu, W. Visceral fat is associated with mucosal healing of infliximab treatment in Crohn’s disease. Dis. Colon Rectum 2018, 61, 706–712. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, F.; Gong, J.; Yang, J.; Zhang, T.; Gu, L.; Zhu, W.; Guo, Z.; Li, Y.; Li, N. High visceral to subcutaneous fat ratio is associated with increased postoperative inflammatory response after colorectal resection in inflammatory bowel disease. Gastroenterol. Res. Pract. 2018, 2018, 6270514. [Google Scholar] [CrossRef]
- Connelly, T.M.; Juza, R.M.; Sangster, W.; Sehgal, R.; Tappouni, R.F.; Messaris, E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig. Surg. 2014, 31, 219–224. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, X.R.; Remer, E.; Lian, L.; Stocchi, L.; Li, Y.; McCullough, A.; Remzi, F.; Shen, B. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Color. Dis. 2016, 18, 163–172. [Google Scholar] [CrossRef]
- Argeny, S.; Tamandl, D.; Scharitzer, M.; Stift, A.; Bergmann, M.; Riss, S. Visceral fat area measured with computed tomography does not predict postoperative course in Crohn’s disease patients. J. Ren. Nutr. 2018, 13, e0202220. [Google Scholar]
- Thiberge, C.; Charpentier, C.; Gillibert, A.; Modzelewski, R.; Dacher, J.-N.; Savoye, G.; Savoye-Collet, C. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn’s disease. J. Crohn’s Colitis 2018, 12, 1429–1437. [Google Scholar] [CrossRef]
- Dai, C.; Jiang, M.; Huang, Y.-H. The Association between Visceral Adipose Tissue and Stricturing Crohn’s Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm. Bowel Dis. 2019, 25, e61. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Al-Hawary, M.; Anupindi, S.; Baker, M.E.; Bruining, D. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology 2018, 154, 1172–1194. [Google Scholar] [CrossRef]
- Li, X.-H.; Feng, S.-T.; Cao, Q.-H.; Coffey, J.C.; Baker, M.E.; Huang, L.; Fang, Z.-N.; Qiu, Y.; Lu, B.-L.; Chen, Z.-H. Degree of Creeping Fat Assessed by CT Enterography is Associated with Intestinal Fibrotic Stricture in Patients with Crohn’s Disease: A Potentially Novel Mesenteric Creeping Fat Index. J. Crohn’s Colitis 2021, 15, 1161–1173. [Google Scholar] [CrossRef]
- Sakurai, T.; Katsuno, T.; Saito, K.; Yoshihama, S.; Nakagawa, T.; Koseki, H.; Taida, T.; Ishigami, H.; Okimoto, K.I.; Maruoka, D.; et al. Mesenteric findings of CT enterography are well correlated with the endoscopic severity of Crohn’s disease. Eur. J. Radiol. 2017, 89, 242–248. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, X.T.; Zhou, Y.; Yan, Y.Q.; Ran, Z.H.; Zhu, J. Creeping fat in patients with ileo-colonic Crohn’s disease correlates with disease activity and severity of inflammation: A preliminary study using energy spectral computed tomography. J. Dig. Dis. 2018, 19, 475–484. [Google Scholar] [CrossRef]
- Althoff, P.; Schmiegel, W.; Lang, G.; Nicolas, V.; Brechmann, T. Creeping fat assessed by small bowel MRI is linked to bowel damage and abdominal surgery in Crohn’s disease. Dig. Dis. Sci. 2019, 64, 204–212. [Google Scholar] [CrossRef]
- Meng, J.; Mao, Y.; Zhou, J.; Chen, Z.; Huang, S.; Wang, Y.; Huang, L.; Zhang, R.; Shen, X.; Lv, W.; et al. Mesenteric abnormalities play an important role in grading intestinal fibrosis in patients with Crohn’s disease: A computed tomography and clinical marker-based nomogram. Therap. Adv. Gastroenterol. 2022, 15, 17562848221122504. [Google Scholar] [CrossRef]
- Rimola, J.; Alfaro, I.; Fernández-Clotet, A.; Castro-Poceiro, J.; Vas, D.; Rodríguez, S.; Masamunt, M.C.; Ordás, I.; Ricart, E.; Panés, J.; et al. Persistent damage on magnetic resonance enterography in patients with Crohn’s disease in endoscopic remission. Aliment. Pharmacol. Ther. 2018, 48, 1232–1241. [Google Scholar] [CrossRef]
- Koh, D.; Miao, Y.; Chinn, R.; Amin, Z.; Zeegen, R.; Westaby, D.; Healy, J. MR imaging evaluation of the activity of Crohn’s disease. Am. J. Roentgenol. 2001, 177, 1325–1332. [Google Scholar] [CrossRef]
- Rimola, J.; Fernàndez-Clotet, A.; Capozzi, N.; Rojas-Farreras, S.; Alfaro, I.; Rodríguez, S.; Masamunt, M.C.; Ricart, E.; Ordás, I.; Panés, J. Pre-treatment magnetic resonance enterography findings predict the response to TNF-alpha inhibitors in Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 52, 1563–1573. [Google Scholar] [CrossRef]
- Barajas Ordonez, F.; Melekh, B.; Rodríguez-Feria, P.; Damm, R.; Thormann, M.; March, C.; Omari, J.; Pech, M.; Surov, A. Parameters of body composition and creeping fat are associated with activity of Crohn’s disease. Magn. Reson. Imaging 2023, 98, 1–6. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Bang, C.S.; Lee, J.J. Computer-Aided Diagnosis of Gastrointestinal Ulcer and Hemorrhage Using Wireless Capsule Endoscopy: Systematic Review and Diagnostic Test Accuracy Meta-analysis. J. Med. Internet Res. 2021, 23, e33267. [Google Scholar] [CrossRef]
- Chahal, D.; Byrne, M.F. A primer on artificial intelligence and its application to endoscopy. Gastroint. Endosc. 2020, 92, 813–820.e4. [Google Scholar] [CrossRef]
- Klang, E.; Grinman, A.; Soffer, S. Automated Detection of Crohn’s Disease Intestinal Strictures on Capsule Endoscopy Images Using Deep Neural Networks. J. Crohns Colitis 2021, 15, 749–756. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef]
- Kiyokawa, H.; Abe, M.; Matsui, T.; Kurashige, M.; Ohshima, K.; Tahara, S.; Nojima, S.; Ogino, T.; Sekido, Y.; Mizushima, T.; et al. Deep Learning Analysis of Histologic Images from Intestinal Specimen Reveals Adipocyte Shrinkage and Mast Cell Infiltration to Predict Postoperative Crohn Disease. Am. J. Pathol. 2022, 192, 904–916. [Google Scholar] [CrossRef]
- Weidinger, C.; Siegmund, B. Deciphering Cellular Networks in Creeping Fat. Inflamm. Bowel Dis. 2023, izad046. [Google Scholar] [CrossRef]
| Authors (Ref.) | Year | Number of Patients | Type of Study | Outcomes |
|---|---|---|---|---|
| Coffey et al. [67] | 2018 | 158 | Prospective cohort study |
|
| Li et al. [65] | 2018 | 63 | Prospective study |
|
| Li et al. [71] | 2020 | 116 | Multicenter, randomized controlled trial | Ongoing trial examining:
|
| Alshantti et al. [72] | 2021 | 896 | Meta-analysis |
|
| Authors (Ref) | Year | Number of Patients | Type of Study | Index | Method Description | Results |
|---|---|---|---|---|---|---|
| Erhayiem et al. [61] | 2011 | 50 | Retrospective cohort study | Visceral to subcutaneous fat area ratio | Cross-sectional scan at the level of the umbilicus was used to determine the areas of subcutaneous and visceral fat. MFI was defined as the ratio of areas of visceral to subcutaneous fat. |
|
| Li et al. [92] | 2021 | 91 | Retrospective cohort study |
| ||
| 30 | Prospective cohort study | |||||
| Sakurai et al. [93] | 2017 | 41 | NR | Fibrofatty proliferation score | Fibrofatty proliferation score was evaluated according to the increase in mesenteric fat degree around the affected intestine and the degree of displacement of the adjacent intestine. A score of 0-1-2 points corresponded to none, mild, and moderate-to-severe, respectively. |
|
| Coffey et al. [68] | 2018 | 158 | Prospective cohort study | Mesenteric disease activity index | Mesenteric disease activity index was developed using fat wrapping and mesenteric thickening. Fat wrapping was graded in accordance with the proportion of intestinal circumference affected. Mesenteric thickening was graded in accordance with vascular and avascular mesenteric regions appearance. |
|
| Feng et al. [94] | 2018 | 80 | Retrospective cohort study | Energy spectral computed tomography | The slope of the λHU was measured and calculated on energy spectral CT images. |
|
| Althoff et al. [95] | 2019 | 90 | Retrospective observational cohort study | Small bowel magnetic resonance imaging | Site of inflammation and involvement of mesenteric and peri-intestinal fat were taken into consideration. |
|
| Li et al. [92] | 2021 | 91 | Retrospective cohort study | MCFI | Creeping fat severity was graded based on the extension of mesenteric fat around the intestinal circumference. MCFI accuracy was evaluated by comparing it with the creeping fat degree in surgical specimens. |
|
| 30 | Prospective cohort study | |||||
| Meng et al. [96] | 2022 | 174 | Retrospective multicenter study | Model 1 | MCFI |
|
| Model 2 | Mesenteric oedema and MCFI | |||||
| Model 3 | Mesenteric oedema, MCFI, and disease duration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Creeping Fat in Crohn’s Disease—Surgical, Histological, and Radiological Approaches. J. Pers. Med. 2023, 13, 1029. https://doi.org/10.3390/jpm13071029
Aggeletopoulou I, Tsounis EP, Mouzaki A, Triantos C. Creeping Fat in Crohn’s Disease—Surgical, Histological, and Radiological Approaches. Journal of Personalized Medicine. 2023; 13(7):1029. https://doi.org/10.3390/jpm13071029
Chicago/Turabian StyleAggeletopoulou, Ioanna, Efthymios P. Tsounis, Athanasia Mouzaki, and Christos Triantos. 2023. "Creeping Fat in Crohn’s Disease—Surgical, Histological, and Radiological Approaches" Journal of Personalized Medicine 13, no. 7: 1029. https://doi.org/10.3390/jpm13071029
APA StyleAggeletopoulou, I., Tsounis, E. P., Mouzaki, A., & Triantos, C. (2023). Creeping Fat in Crohn’s Disease—Surgical, Histological, and Radiological Approaches. Journal of Personalized Medicine, 13(7), 1029. https://doi.org/10.3390/jpm13071029








