Independent Prognostic and Predictive Role of Interstitial Macrophages in Kidney Biopsies of IgA Nephropathy Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histopathology and Immunohistochemistry
2.3. Statistical Analyses
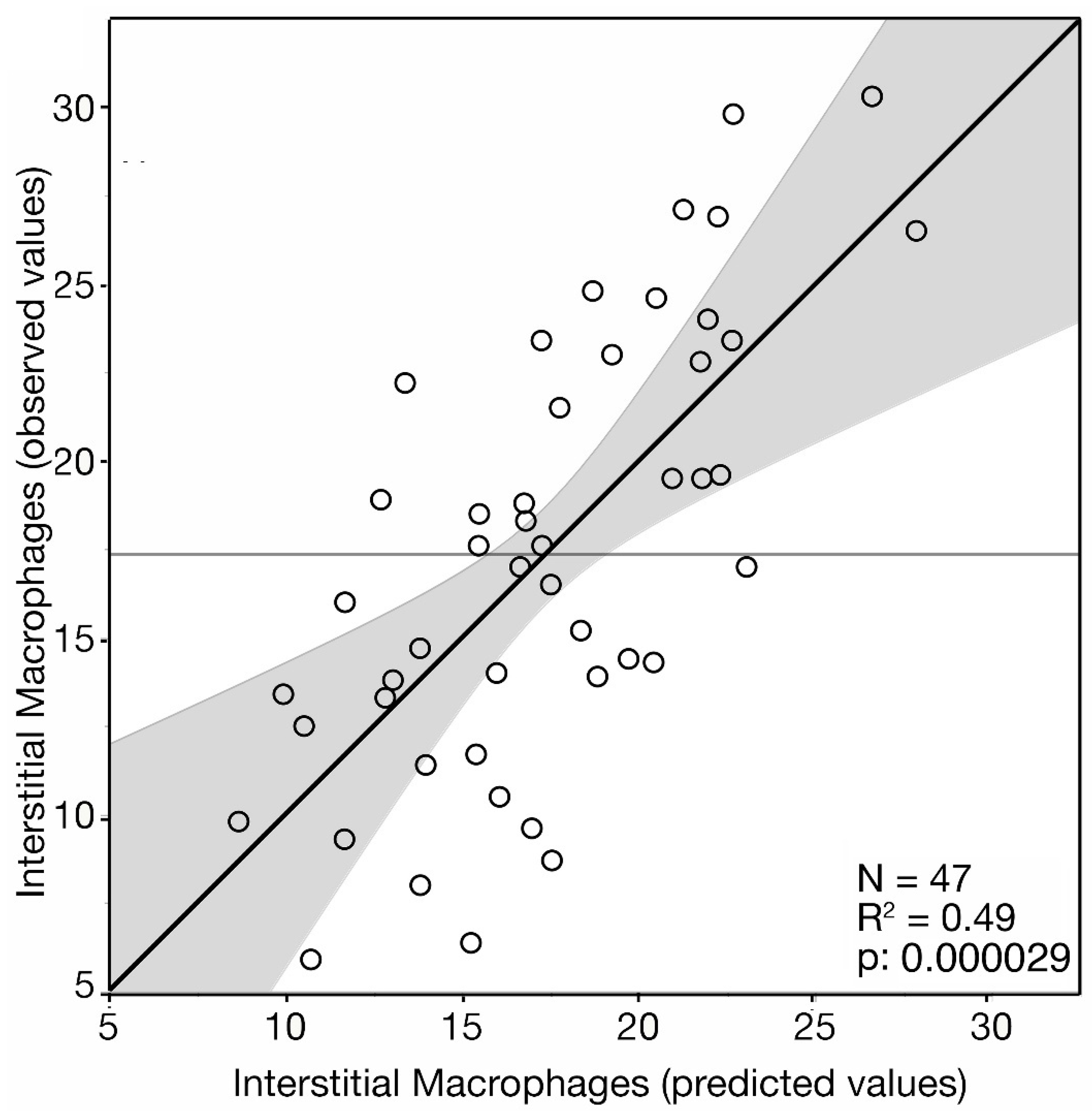
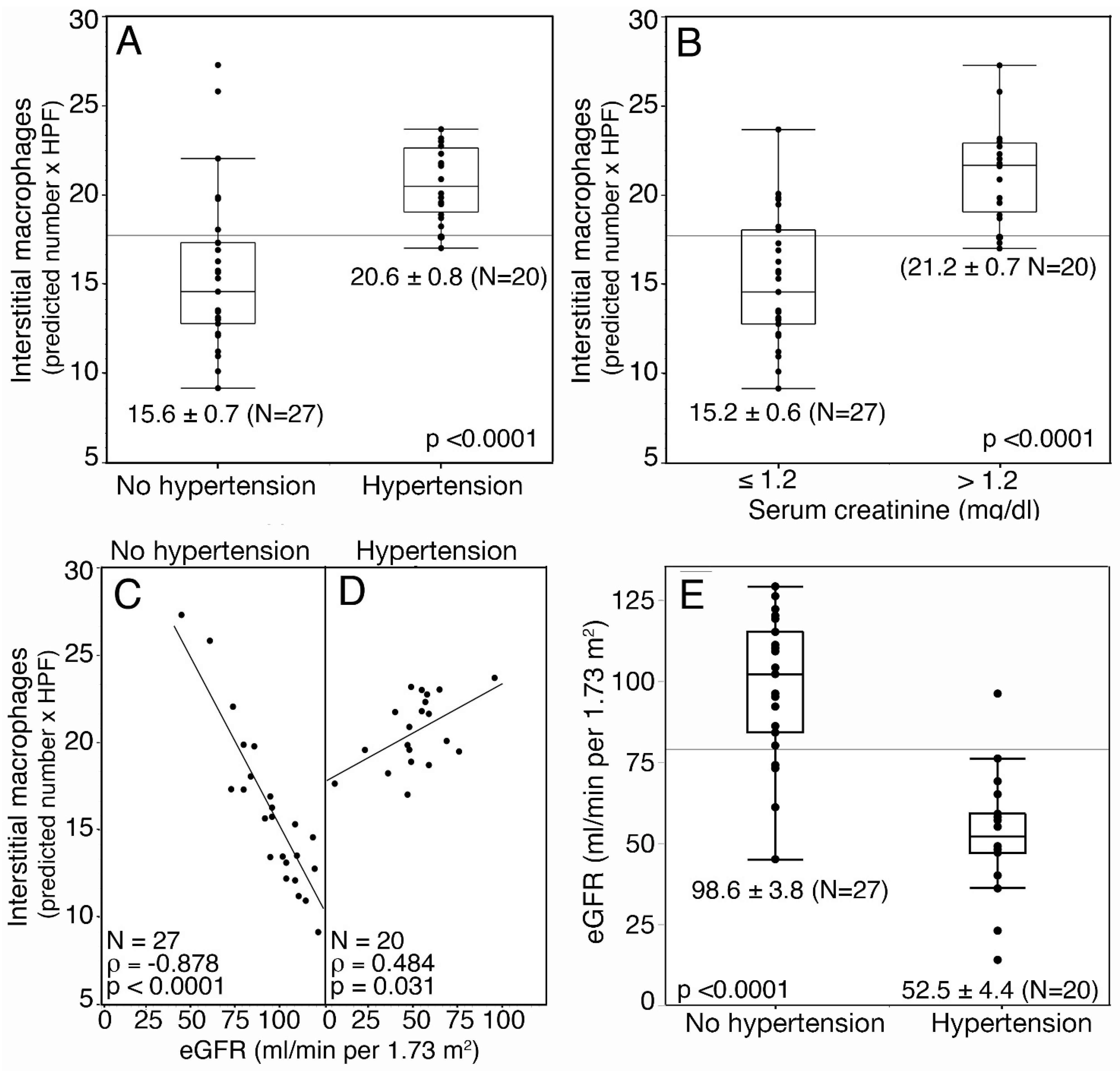
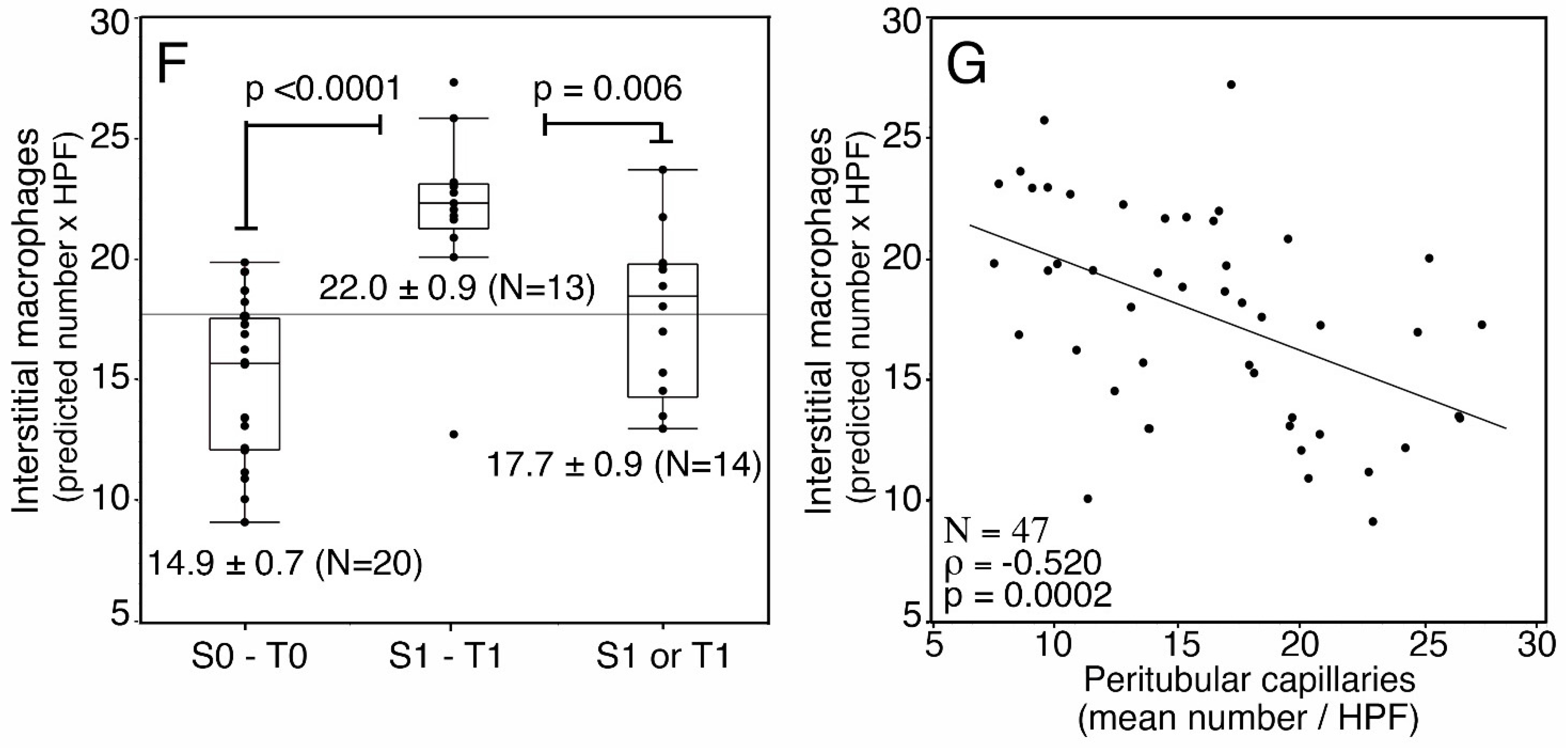
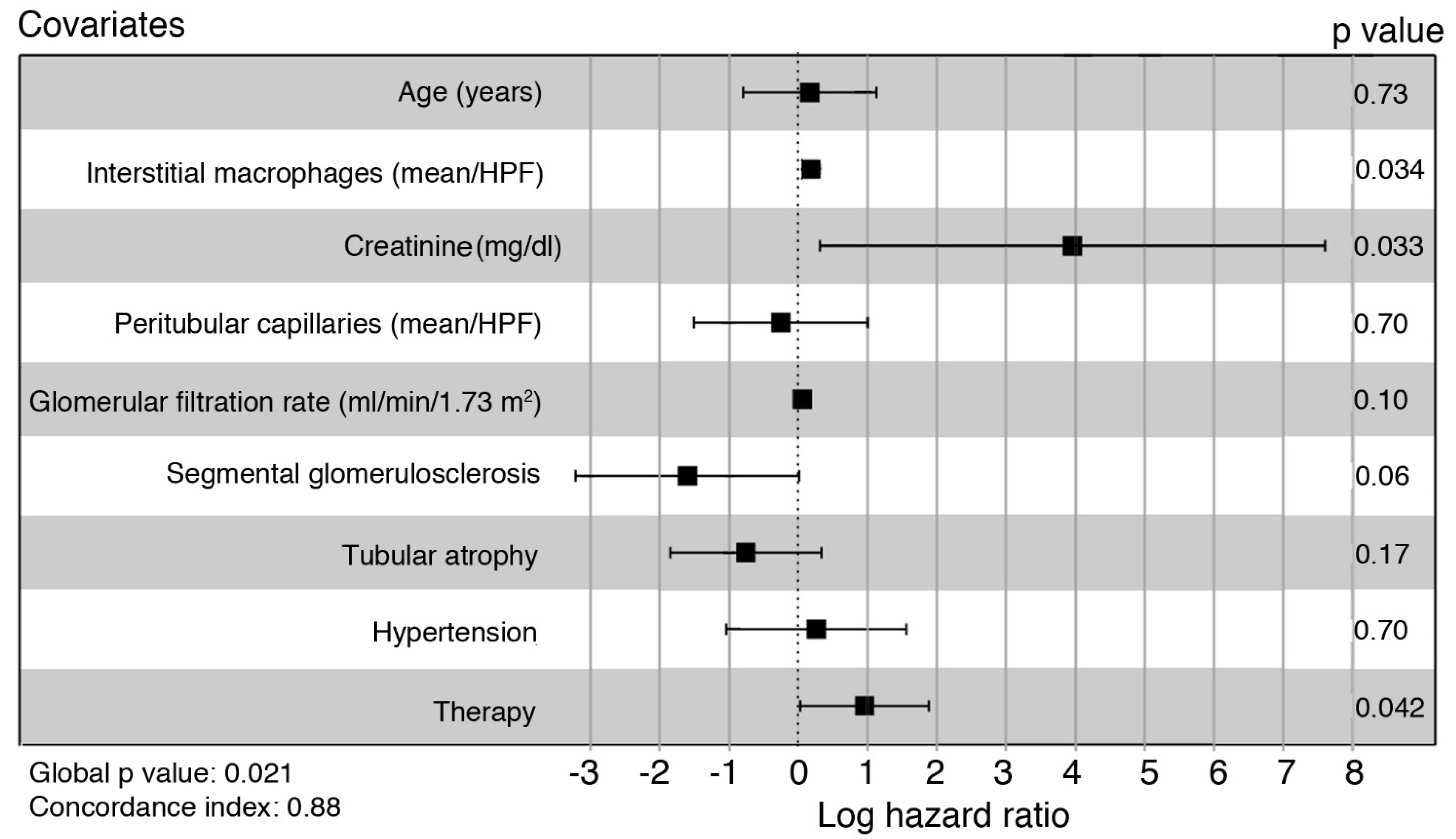
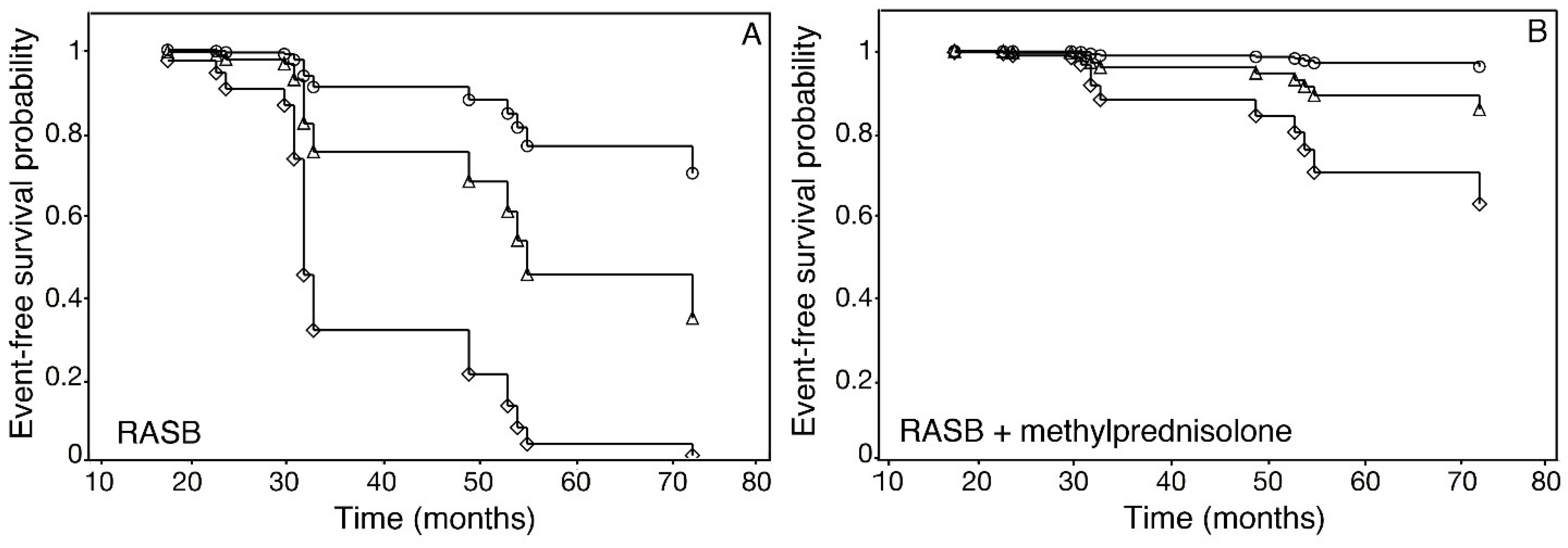
3. Results
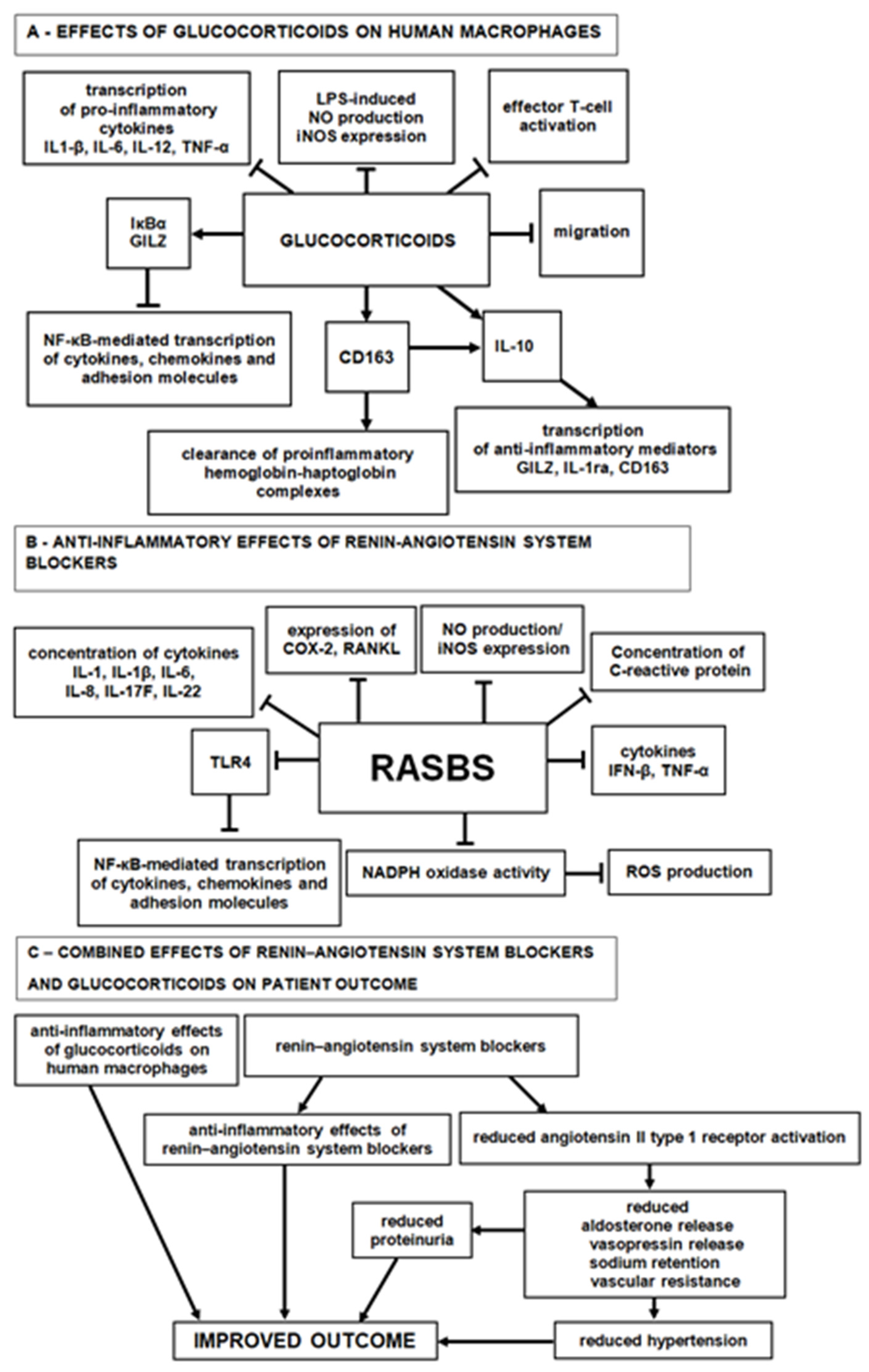
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Barratt, J. Is IgA nephropathy the same disease in different parts of the world? Semin. Immunopathol. 2021, 43, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Li, X.K. The Role of Immune Modulation in Pathogenesis of IgA Nephropathy. Front. Med. 2020, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; D’Amico, G. Factors predicting progression of IgA nephropathies. J. Nephrol. 2005, 18, 503–512. [Google Scholar]
- Huang, L.; Guo, F.L.; Zhou, J.; Zhao, Y.J. IgA nephropathy factors that predict and accelerate progression to end-stage renal disease. Cell Biochem. Biophys. 2014, 68, 443–447. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef]
- Shao, X.; Li, B.; Cao, L.; Liang, L.; Yang, J.; Wang, Y.; Feng, S.; Wang, C.; Weng, C.; Shen, X.; et al. Evaluation of crescent formation as a predictive marker in immunoglobulin A nephropathy: A systematic review and meta-analysis. Oncotarget 2017, 11, 46436–46448. [Google Scholar] [CrossRef]
- Ştefan, G.; Ismail, G.; Stancu, S.; Zugravu, A.; Andronesi, A.; Mandache, E.; Mircescu, G. Validation study of Oxford Classification of IgA Nephropathy: The significance of extracapillary hypercellularity and mesangial IgG immunostaining. Pathol. Int. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Kaihan, A.B.; Yasuda, Y.; Imaizumi, T.; Inagaki, K.; Ozeki, T.; Hishida, M.; Katsuno, T.; Tsuboi, N.; Maruyama, S. Clinical impact of endocapillary proliferation with modified cutoff points in IgA nephropathy patients. PLoS ONE 2019, 14, e0214414. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, J.; Tay, Y.C.; Yung, T.; Rangan, G.K.; Harris, D.C. Significance of CD25 positive cells and macrophages in noncrescentic IgA nephropathy. Ren. Fail. 2006, 28, 229–235. [Google Scholar] [CrossRef]
- Ikezumi, Y.; Suzuki, T.; Karasawa, T.; Kaneko, U.; Yamada, T.; Hasegawa, H.; Nagata, M.; Saitoh, A. Glomerular epithelial cell phenotype in diffuse mesangial sclerosis: A report of 2 cases with markedly increased urinary podocyte excretion. Hum. Pathol. 2014, 45, 1778–1783. [Google Scholar] [CrossRef]
- Barros Silva, G.E.; Costa, R.S.; Ravinal, R.C.; Ramalho, L.N.; Reis, M.A.; Moyses-Neto, M.; Romao, E.A.; Coimbra, T.M.; Dantas, M. Renal macrophage infiltration is associated with a poor outcome in IgA nephropathy. Clinics 2012, 67, 697–703. [Google Scholar] [CrossRef]
- Hu, W.; Lin, J.; Lian, X.; Yu, F.; Liu, W.; Wu, Y.; Fang, X.; Liang, X.; Hao, W. M2a and M2b macrophages predominate in kidney tissues and M2 subpopulations were associated with the severity of disease of IgAN patients. Clin. Immunol. 2019, 205, 8–15. [Google Scholar] [CrossRef]
- Pawluczyk, I.Z.A.; Soares, M.S.F.; Barratt, W.A.; Brown, J.R.; Bhachu, J.S.; Selvaskandan, H.; Zeng, Y.; Sarania, R.; Molyneux, K.; Roberts, I.S.D.; et al. Macrophage interactions with collecting duct epithelial cells are capable of driving tubulointerstitial inflammation and fibrosis in immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2020, 35, 1865–1877. [Google Scholar] [CrossRef]
- Viola, P.; Centurione, L.; Felaco, P.; Lattanzio, G.; D’Antuono, T.; Liberatore, M.; Di Pietro, R.; Ranelletti, F.O.; Bonomini, M.; Aiello, F.B. Prognostic value of morphologic and morphometric analyses in IgA nephropathy biopsies. Transl. Med. Commun. 2016, 1, 7. [Google Scholar] [CrossRef]
- Pfenning, M.B.; Schmitz, J.; Scheffner, I.; Schulte, K.; Khalifa, A.; Tezval, H.; Weidemann, A.; Kulschewski, A.; Kunzendorf, U.; Dietrich, S.; et al. High Macrophage Densities in Native Kidney Biopsies Correlate With Renal Dysfunction and Promote ESRD. Kidney. Int. Rep. 2022, 29, 341–356. [Google Scholar] [CrossRef]
- Gonzalez Guerrico, A.M.; Lieske, J.; Klee, G.; Kumar, S.; Lopez-Baez, V.; Wright, A.M.; Bobart, S.; Shevell, D.; Maldonado, M.; Troost, J.P.; et al. Nephrotic Syndrome Study Network Consortium (NEPTUNE). Urinary CD80 Discriminates Among Glomerular Disease Types and Reflects Disease Activity. Kidney Int. Rep. 2020, 14, 2021–2031. [Google Scholar] [CrossRef]
- Prodjosudjadi, W.; Gerritsma, J.S.; van Es, L.A.; Daha, M.R.; Bruijn, J.A. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: An immunohistochemical analysis. Clin. Nephrol. 1995, 44, 148–155. [Google Scholar]
- Grandaliano, G.; Gesualdo, L.; Ranieri, E.; Monno, R.; Montinaro, V.; Marra, F.; Schena, F.P. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: A pathogenetic role in interstitial monocytes recruitment. J. Am. Soc. Nephrol. 1996, 7, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, Z.; Wang, R.; Yan, L.; Sun, S.; Lu, L.; Ren, Z.; Zhang, Q. Chemokine (C-C motif) receptor 2 is associated with the pathological grade and inflammatory response in IgAN children. BMC Nephrol. 2022, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.D.; Rossini, M.; Manno, C.; Mattace-Raso, F.; D’Altri, C.; Ranieri, E.; Pontrelli, P.; Grandaliano, G.; Gesualdo, L.; Schena, F.P. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008, 73, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lv, L.L.; Wu, W.J.; Li, Z.L.; Chen, J.; Ni, H.F.; Zhou, L.T.; Tang, T.T.; Wang, F.M.; Wang, B.; et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. Am. J. Pathol. 2018, 188, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Bonnefoy, A.; Genest, D.S.; Quadri, J.; Rioux, J.P.; Troyanov, S. Clinical Use of Complement, Inflammation, and Fibrosis Biomarkers in Autoimmune Glomerulonephritis. Kidney Int. Rep. 2020, 23, 1690–1699. [Google Scholar] [CrossRef]
- Han, S.Y.; Jeong, K.H.; Ihm, C.G.; Kang, Y.S.; Cha, D.R. Serum interferon-γ and urinary monocyte chemoattractant peptide-1 are important factors in the pathogenesis of immunoglobulin A nephropathy. Kidney Res. Clin. Pract. 2021, 40, 69–76. [Google Scholar] [CrossRef]
- Zhai, Y.; Long, X.; Gao, J.; Yao, X.; Wang, X.; Zhao, Z. Elevated Endostatin Expression Is Regulated by the pIgA Immune Complex and Associated with Disease Severity of IgA Nephropathy. Kidney Blood Press. Res. 2021, 46, 31–40. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Roth, J.; Barczyk-Kahlert, K. More Than Suppression: Glucocorticoid Action on Monocytes and Macrophages. Front. Immunol. 2019, 10, 2028. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Aiello, F.B.; Furian, L.; Della Barbera, M.; Marino, S.; Seveso, M.; Cardillo, M.; Pierobon, E.S.; Cozzi, E.; Rigotti, P.; Valente, M. Glomerulitis and endothelial cell enlargement in C4d+ and C4d- acute rejections of renal transplant patients. Hum. Pathol. 2012, 43, 2157–2166. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Suzuki, J.; Sakai, N.; Etoh, S.; Murai, H.; Nozawa, R.; Suzuki, H. Efficacy of prednisolone and mizoribine therapy for diffuse IgA nephropathy. Am. J. Nephrol. 2004, 24, 147–153. [Google Scholar] [CrossRef]
- Solak, Y.; Afsar, B.; Vaziri, N.D.; Aslan, G.; Yalcin, C.E.; Covic, A.; Kanbay, M. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res. 2016, 39, 567–573. [Google Scholar] [CrossRef]
- Robles, N.R.; Cerezo, I.; Hernandez-Gallego, R. Renin-angiotensin system blocking drugs. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 14–33. [Google Scholar] [CrossRef]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Immunomodulatory Activity of the Most Commonly Used Antihypertensive Drugs-Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers. Int. J. Mol. Sci. 2022, 4, 1772. [Google Scholar] [CrossRef]
- Risdon, R.A.; Sloper, J.C.; De Wardener, H.E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968, 17, 363–366. [Google Scholar] [CrossRef]
- D’Amico, G.; Ferrario, F.; Rastaldi, M.P. Tubulointerstitial damage in glomerular diseases: Its role in the progression of renal damage. Am. J. Kidney Dis. 1995, 26, 124–132. [Google Scholar] [CrossRef]
- Hruby, Z.; Smolska, D.; Filipowski, H.; Rabczyński, J.; Cieślar, E.; Kopeć, W.; Dulawa, J. The importance of tubulointerstitial injury in the early phase of primary glomerular disease. J. Intern. Med. 1998, 243, 215–222. [Google Scholar] [CrossRef]
- Morii, T.; Fujita, H.; Narita, T.; Koshimura, J.; Shimotomai, T.; Fujishima, H.; Yoshioka, N.; Imai, H.; Kakei, M.; Ito, S. Increased urinary excretion of monocyte chemoattractant protein-1 in proteinuric renal diseases. Ren. Fail. 2003, 25, 439–444. [Google Scholar] [CrossRef]
- Murali, N.S.; Ackerman, A.W.; Croatt, A.J.; Cheng, J.; Grande, J.P.; Sutor, S.L.; Bram, R.J.; Bren, G.D.; Badley, A.D.; Alam, J.; et al. Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: Implications for chronic kidney disease. Am. J. Physiol. Renal Physiol. 2007, 292, F837–F844. [Google Scholar] [CrossRef]
- Burton, C.J.; Combe, C.; Walls, J.; Harris, K.P. Secretion of chemokines and cytokines by human tubular epithelial cells in response to proteins. Nephrol. Dial. Transplant. 1999, 14, 2628–2633. [Google Scholar] [CrossRef]
- Zoja, C.; Benigni, A.; Remuzzi, G. Protein overload activates proximal tubular cells to release vasoactive and inflammatory mediators. Exp. Nephrol. 1999, 7, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol. Dial. Transplant. 2011, 26, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Afsar, R.E.; Dagel, T.; Kaya, E.; Erus, S.; Ortiz, A.; Covic, A.; Kanbay, M. Capillary rarefaction from the kidney point of view. Clin. Kidney J. 2018, 11, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Suarez-Martinez, A.D.; Bagher, P.; Gonzalez, A.; Liu, R.; Murfee, W.L.; Mohandas, R. Microvascular dysfunction and kidney disease: Challenges and opportunities? Microcirculation 2021, 28, e12661. [Google Scholar] [CrossRef]
- Bábíčková, J.; Klinkhammer, B.M.; Buhl, E.M.; Djudjaj, S.; Hoss, M.; Heymann, F.; Tacke, F.; Floege, J.; Becker, J.U.; Boor, P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017, 91, 70–85. [Google Scholar] [CrossRef]
- Ohashi, R.; Kitamura, H.; Yamanaka, N. Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J. Am. Soc. Nephrol. 2000, 11, 47–56. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Dimke, H.; Sparks, M.A.; Thomson, B.R.; Frische, S.; Coffman, T.M.; Quaggin, S.E. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015, 26, 1027–1038. [Google Scholar] [CrossRef]
- Namikoshi, T.; Satoh, M.; Horike, H.; Fujimoto, S.; Arakawa, S.; Sasaki, T.; Kashihara, N. Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron Physiol. 2006, 102, 9–16. [Google Scholar] [CrossRef]
- Feng, S.; Huang, N.; Xue, M.; Zhang, P.; Zhong, Z.; Guo, Q.; Li, Z. Association between urinary VEGFA and renal pathology of IgA nephropathy patients. J. Clin. Lab. Anal. 2021, 35, e23995. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Zhu, L.; Shi, S.F.; Liu, L.J.; Lv, J.C.; Zhang, H. Elevated soluble VEGF receptor sFlt-1 correlates with endothelial injury in IgA nephropathy. PLoS ONE 2014, 9, e101779. [Google Scholar] [CrossRef]
- Tanabe, K.; Sato, Y.; Wada, J. Endogenous Antiangiogenic Factors in Chronic Kidney Disease: Potential Biomarkers of Progression. Int. J. Mol. Sci. 2018, 19, 1859. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase production from macrophages–A perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp. Physiol. 2016, 101, 1327–1337. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Franco, M.; Johnson, R.J. Impaired pressure natriuresis is associated with interstitial inflammation in salt-sensitive hypertension. Curr. Opin. Nephrol. Hypertens. 2013, 22, 37–44. [Google Scholar] [CrossRef]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 12, 3146. [Google Scholar] [CrossRef]
- Kida, Y. Peritubular Capillary Rarefaction: An Underappreciated Regulator of CKD Progression. Int. J. Mol. Sci. 2020, 21, 8255. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Pacilio, M.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. Hypertension and Prehypertension and Prediction of Development of Decreased Estimated GFR in the General Population: A Meta-analysis of Cohort Studies. Am. J. Kidney Dis. 2016, 67, 89–97. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, H.; Xu, X.; Zhou, Z.; Su, C.; Jia, N.; Liu, Y.; Hou, F.F. Intensity of Macrophage Infiltration in Glomeruli Predicts Response to Immunosuppressive Therapy in Patients with IgA Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 3187–3196. [Google Scholar] [CrossRef]
- Tesar, V.; Troyanov, S.; Bellur, S.; Verhave, J.C.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Cattran, D.; Coppo, R. VALIGA study of the ERA-EDTA Immunonephrology Working Group. Corticosteroids in IgA Nephropathy: A Retrospective Analysis from the VALIGA Study. J. Am. Soc. Nephrol. 2015, 26, 2248–2258. [Google Scholar] [CrossRef]
- Coppo, R.; Troyanov, S.; Bellur, S.; Cattran, D.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Morando, L.; Camilla, R.; Tesar, V.; et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014, 86, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Itami, S.; Moriyama, T.; Miyabe, Y.; Karasawa, K.; Nitta, K.A. Novel Scoring System Based on Oxford Classification Indicating Steroid Therapy Use for IgA Nephropathy. Kidney Int. Rep. 2021, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Nakanishi, K.; Ito, S.; Saito, M.; Sako, M.; Ishikura, K.; Hataya, H.; Honda, M.; Iijima, K.; Yoshikawa, N. Japanese Pediatric IgA Nephropathy Treatment Study Group. Long-term results of a randomized controlled trial in childhood IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Bellur, S.S.; Lepeytre, F.; Vorobyeva, O.; Troyanov, S.; Cook, H.T.; Roberts, I.S. International IgA Nephropathy Working Group. Evidence from the Oxford Classification cohort supports the clinical value of subclassification of focal segmental glomerulosclerosis in IgA nephropathy. Kidney Int. 2017, 91, 235–243. [Google Scholar] [CrossRef]
- Haas, M.; Verhave, J.C.; Liu, Z.H.; Alpers, C.E.; Barratt, J.; Becker, J.U.; Cattran, D.; Cook, H.T.; Coppo, R.; Feehally, J. Multicenter Study of the Predictive Value of Crescents in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 691–701. [Google Scholar] [CrossRef]
- Coppo, R. Towards a personalized treatment for IgA nephropathy considering pathology and pathogenesis. Nephrol. Dial. Transplant. 2019, 34, 1832–1838. [Google Scholar] [CrossRef]
- Hilhorst, M.; Anders, H.J. IgA Nephropathy Needs a Diagnostic Marker of Immunologic Activity to Select the Right Patients for Immunotherapies. J. Am. Soc. Nephrol. 2021, 32, 2982–2984. [Google Scholar] [CrossRef]
- Lv, J.; Wong, M.G.; Hladunewich, M.A.; Jha, V.; Hooi, L.S.; Monaghan, H.; Zhao, M.; Barbour, S.; Jardine, M.J.; Reich, H.N.; et al. TESTING Study Group. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2022, 327, 1888–1898. [Google Scholar] [CrossRef]


| Interstitial Macrophages | Peritubular Capillaries | |||||
|---|---|---|---|---|---|---|
| Variables | n | Median (IQR) 1 | Wilcoxon p | Median (IQR) 1 | Wilcoxon p | |
| hypertension | absent | 27 | 14.7 (10.1–20.6) | 0.0087 | 17.9 (13.1–20.8) | 0.054 |
| present | 20 | 19.6 (17.1–23.7) | 14.4 (9.8–17.5) | |||
| proteinuria | <0.5 g/die | 5 | 9.8 (9.2–13.9) | 0.014 | 16.9 (13.4–21.7) | 0.52 |
| >0.5 g/die | 42 | 18.0 (13.9–23.1) | 15.9 (10.8–19.8) | |||
| mesangial hypercellularity | M0 | 36 | 16.8 (11.5–22.7) | 0.22 | 17.1 (10.7–20.3) | 0.35 |
| M1 | 11 | 18.3 (14.7–23.4) | 13.6 (11.6–15.4) | |||
| segmental glomerulosclerosis | S0 | 26 | 15.6 (10.3–18.6) | 0.0102 | 17.8 (11.5–21.3) | 0.17 |
| S1 | 21 | 19.6 (14.1–25.6) | 14.6 (10.2–17.7) | |||
| tubular atrophy | T0 | 28 | 14.2 (10.7–17.6) | <0.0001 | 17.8 (13.7–20.7) | 0.067 |
| T1 | 19 | 22.2 (18.5–26.5) | 13.1 (9.8–17.2) | |||
| fibrosis | absent | 35 | 15.2 (11.7–19.6) | 0.0108 | 17.0 (11.6–20.8) | 0.18 |
| present | 12 | 21.75 (17.4–26.1) | 14.6 (10.3–17.0) | |||
| Interstitial macrophages | Peritubular capillaries | |||||
| Variables | n | Spearman r | p | Spearman r | p | |
| creatinine mg/dL | 47 | 0.5008 | 0.0003 | 0.2199665 | 0.14 | |
| eGFR (mL/min per 1.73/m2) | 47 | −0.5200 | 0.0002 | −0.2756926 | 0.061 | |
| glomerular macrophages (mean/glomerulus) | 47 | 0.3022 | 0.0390 | −0.2196074 | 0.14 | |
| interstitial macrophages (mean/HPF) | 47 | −0.3685 | 0.0108 | |||
| Variables | n | RR 1 | CI 2 | p 3 | |
|---|---|---|---|---|---|
| Age | <34 | 15 | 1.0 | (1.5–36.3) | 0.008 |
| >34 | 15 | 5.6 | |||
| Gender | female | 6 | 1.0 | (0.3–4.7) | 0.95 |
| male | 24 | 1.1 | |||
| Hypertension | absent | 17 | 1 | (0.5–4.7) | 0.56 |
| present | 13 | 1.4 | |||
| Creatinine (mg/dL) | ≤1.2 | 17 | 1.0 | (1.0–15.5) | 0.042 |
| >1.2 | 13 | 3.4 | |||
| eGFR (ml/min per 1.73/m2) | >76 | 14 | 1.0 | (1.3–31.7) | 0.016 |
| ≤76 | 16 | 4.3 | |||
| Proteinuria g/die | ≤0.5 | 4 | 1 | (0.3–30.5) | 0.6 |
| >0.5 | 26 | 1.7 | |||
| Segmental glomerulosclerosis | S0 | 15 | 1 | (0.4–5.2) | 0.56 |
| S1 | 15 | 1.4 | |||
| Tubular atrophy | T0 | 19 | 1 | (0.7–7.3) | 0.17 |
| T1 | 11 | 2.19 | |||
| Fibrosis | absent | 23 | 1.0 | (0.2–2.6) | 0.75 |
| present | 7 | 0.8 | |||
| Interstitial macrophages/HPF | ≤19.5 | 19 | 1.0 | (1.1–10.7) | 0.038 |
| >19.5 | 11 | 3.2 | |||
| Peritubular Capillaries/HPF | >17.2 | 11 | 1.0 | (0.6–62.8) | 0.18 |
| ≤17.2 | 19 | 3.3 | |||
| Therapy | RASBs + steroids | 11 | 31 | (1.2–17.3) | 0.028 |
| RASBs | 16 | 3.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, F.B.; Ranelletti, F.O.; Liberatore, M.; Felaco, P.; De Luca, G.; Lamolinara, A.; Schena, F.P.; Bonomini, M. Independent Prognostic and Predictive Role of Interstitial Macrophages in Kidney Biopsies of IgA Nephropathy Patients. J. Pers. Med. 2023, 13, 935. https://doi.org/10.3390/jpm13060935
Aiello FB, Ranelletti FO, Liberatore M, Felaco P, De Luca G, Lamolinara A, Schena FP, Bonomini M. Independent Prognostic and Predictive Role of Interstitial Macrophages in Kidney Biopsies of IgA Nephropathy Patients. Journal of Personalized Medicine. 2023; 13(6):935. https://doi.org/10.3390/jpm13060935
Chicago/Turabian StyleAiello, Francesca Bianca, Franco Oreste Ranelletti, Marcella Liberatore, Paolo Felaco, Graziano De Luca, Alessia Lamolinara, Francesco Paolo Schena, and Mario Bonomini. 2023. "Independent Prognostic and Predictive Role of Interstitial Macrophages in Kidney Biopsies of IgA Nephropathy Patients" Journal of Personalized Medicine 13, no. 6: 935. https://doi.org/10.3390/jpm13060935
APA StyleAiello, F. B., Ranelletti, F. O., Liberatore, M., Felaco, P., De Luca, G., Lamolinara, A., Schena, F. P., & Bonomini, M. (2023). Independent Prognostic and Predictive Role of Interstitial Macrophages in Kidney Biopsies of IgA Nephropathy Patients. Journal of Personalized Medicine, 13(6), 935. https://doi.org/10.3390/jpm13060935








