The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serum SIRT1, Ghrelin, and Leptin Assay
2.3. Anti-Hypothalamus Autoantibody ELISA Protocols
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
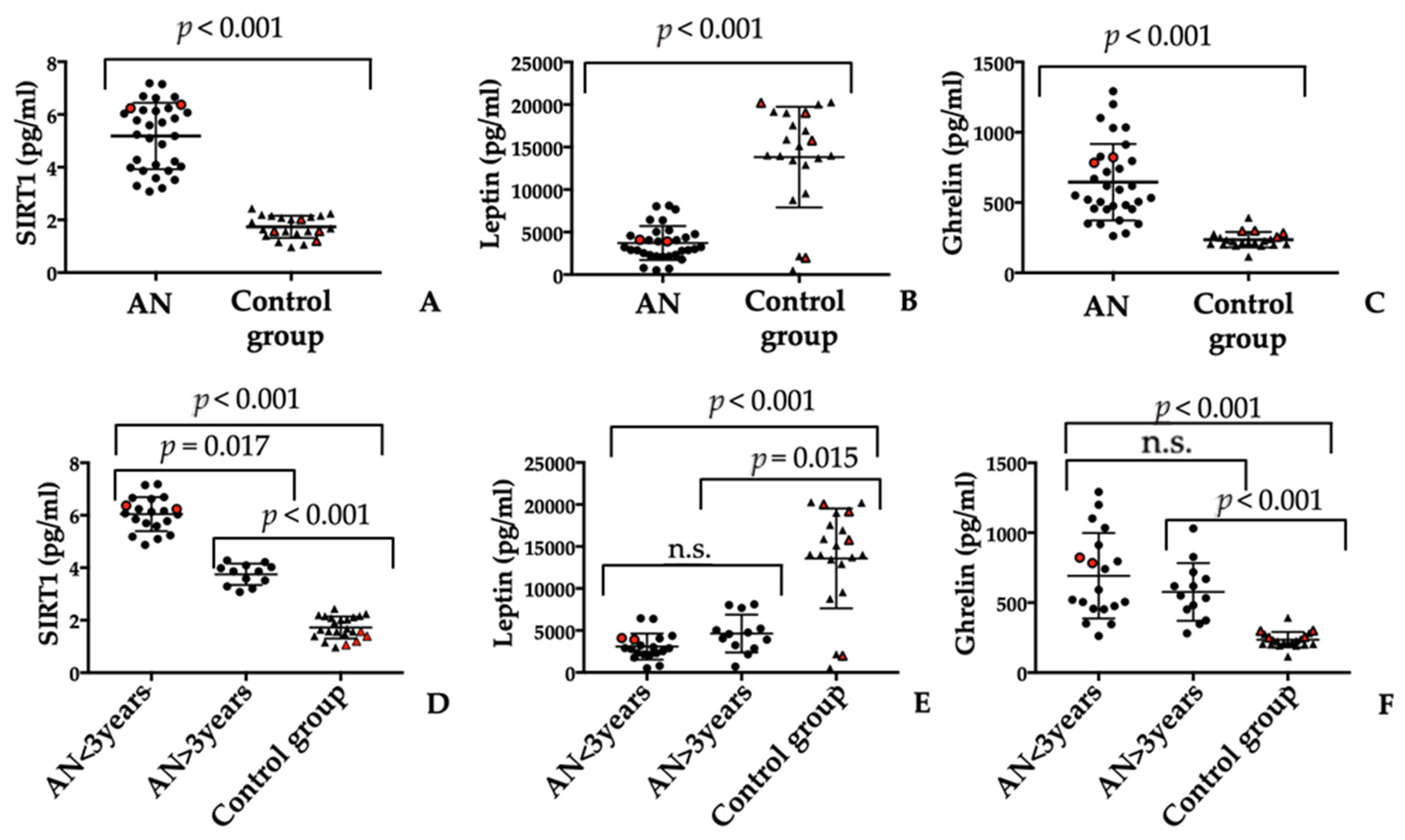
3.2. Serum SIRT1 Levels Are Increased in Patients with AN
3.3. Serum Leptin and Ghrelin Molecules Evaluation
3.4. Anti-Hypothalamic Autoantibodies Can Be Detected in Serum from Patients with AN
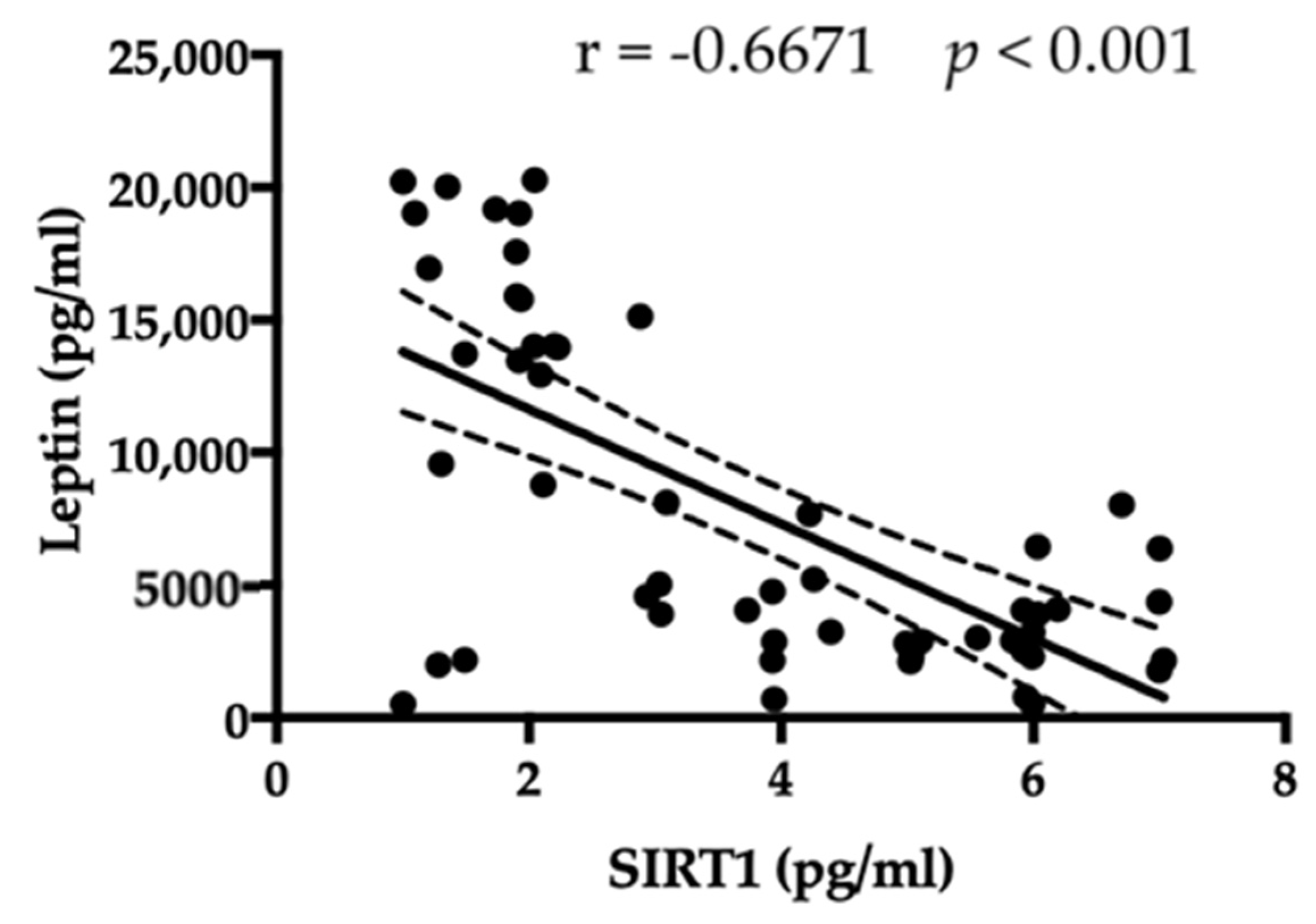
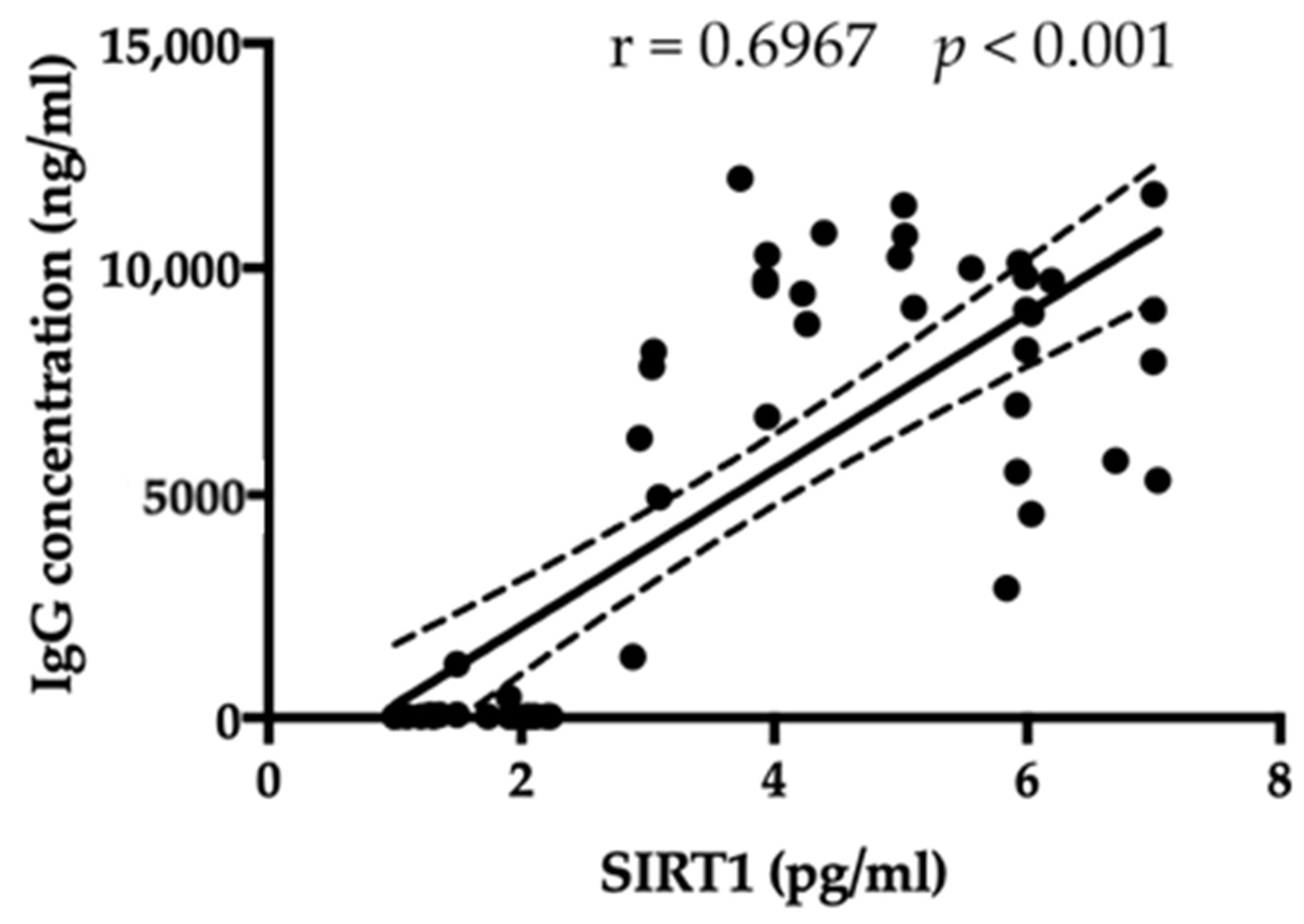
3.5. SIRT1 Relationship with Leptin, Ghrelin, BMI, and Antibody Anti-Hypothalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hubel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Solstrand Dahlberg, L.; Wiemerslage, L.; Swenne, I.; Larsen, A.; Stark, J.; Rask-Andersen, M.; Salonen-Ros, H.; Larsson, E.M.; Schiöth, H.B.; Brooks, S.J. Adolescents newly diagnosed with eating disorders have structural differences in brain regions linked with eating disorder symptoms. Nord. J. Psychiatry 2017, 71, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Shaw, H.E. Role of body dissatisfaction in the onset and maintenance of eating pathology: A synthesis of research findings. J. Psychosom. Res. 2002, 53, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Lao-Kaim, N.P.; Fonville, L.; Giampietro, V.P.; Williams, S.C.; Simmons, A.; Tchanturia, K. Aberrant function of learning and cognitive control networks underlie inefficient cognitive flexibility in anorexia nervosa: A cross-sectional fMRI study. PLoS ONE 2015, 10, e0124027. [Google Scholar] [CrossRef]
- Harrington, B.C.; Jimerson, M.; Haxton, C.; Jimerson, D.C. Initial evaluation, diagnosis, and treatment of anorexia nervosa and bulimia nervosa. Am. Fam. Physician 2015, 91, 46–52. [Google Scholar] [PubMed]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Gibson, D.; Mehler, P.S. Anorexia Nervosa and the Immune System-A Narrative Review. J. Clin. Med. 2019, 8, 1915. [Google Scholar] [CrossRef]
- Escelsior, A.; Cogorno, L.; Sukkar, S.G.; Amerio, A.; Donini, L.M.; Bellomo, M.; Iervasi, E.; Amore, M.; Saverino, D. Anti-hypothalamus autoantibodies in anorexia nervosa: A possible new mechanism in neuro-physiological derangement? Eat. Weight Disord. 2022, 27, 2481–2496. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Anorexia nervosa and autoimmune comorbidities: A bidirectional route? CNS Neurosci. Ther. 2022, 28, 1921–1929. [Google Scholar] [CrossRef]
- Marcos, A.; Varela, P.; Santacruz, I.; Munoz-Velez, A.; Morande, G. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur. J. Clin. Nutr. 1993, 47, 787–793. [Google Scholar]
- Caldiroli, A.; La Tegola, D.; Affaticati, L.M.; Manzo, F.; Cella, F.; Scalia, A.; Capuzzi, E.; Nicastro, M.; Colmegna, F.; Buoli, M.; et al. Clinical and Peripheral Biomarkers in Female Patients Affected by Anorexia: Does the Neutrophil/Lymphocyte Ratio (NLR) Affect Severity? Nutrients 2023, 15, 1133. [Google Scholar] [CrossRef]
- Silla, K.E.J.; Brigham, K.S.; Goldstein, M.; Misra, M.; Singhal, V. Clinical, biochemical, and hematological characteristics of community-dwelling adolescent and young adult males with anorexia nervosa. Int. J. Eat. Disord. 2021, 54, 2213–2217. [Google Scholar] [CrossRef]
- Dalton, B.; Leppanen, J.; Campbell, I.C.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav. Immun. 2020, 85, 88–95. [Google Scholar] [CrossRef]
- Amerio, A.; Escelsior, A.; Martino, E.; Strangio, A.; Giacomini, C.; Montagna, E.; Agiglia, A.; Bellomo, M.; Sukkar, S.G.; Saverino, D. Dysfunction of inflammatory pathways and their relationship with anti-hypothalamic autoantibodies in patients with anorexia nervosa. Nutrients 2023, 15, 2199. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Hallman, J.; Oreland, L.; Af Klinteberg, B.; Grenbäck, E.; Hulting, A.L.; Hökfelt, T. Autoantibodies against αmsh, acth, and lhrh in anorexia and bulimia nervosa patients. Proc. Natl. Acad. Sci. USA 2002, 99, 17155–17160. [Google Scholar] [CrossRef]
- Gorwood, P.; Blanchet-Collet, C.; Chartrel, N.; Duclos, J.; Dechelotte, P.; Hanachi, M.; Fetissov, S.; Godart, N.; Melchior, J.C.; Ramoz, N.; et al. New Insights in Anorexia Nervosa. Front. Neurosci. 2016, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Lipp, M.; Vogelgsang, J.; Vukovich, R.; Zindler, T.; Luedecke, D.; Gingele, S.; Malchow, B.; Frieling, H.; Kühn, S.; et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: A narrative review and proposed diagnostic approach. Brain Behav. Immun. Health 2020, 9, 100154. [Google Scholar] [CrossRef]
- Hyla-Klekot, L.; Wolny, A.; Janas-Kozik, M.; Koszutski, T. Anorexia nervosa and juvenile lupus erythematosus in a 16-year-old female patient—Common disease origin or random coincidence? Cent. Eur. J. Immunol. 2021, 46, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tokatly Latzer, I.; Lerner-Geva, L.; Stein, D.; Weiss, B.; Pinhas-Hamiel, O. Disordered eating behaviors in adolescents with celiac disease. Eat. Weight Disord. 2020, 25, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ilzarbe, L.; Fàbrega, M.; Quintero, R.; Bastidas, A.; Pintor, L.; García-Campayo, J.; Gomollón, F.; Ilzarbe, D. Inflammatory bowel disease and eating disorders: A systematized review of comorbidity. J Psychosom. Res. 2017, 102, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, C.; Luton, J.P. Anorexie mentale et maladie de Crohn: Intrications et difficultés diagnostiques. Trois cas [Anorexia nervosa and Crohn disease: Diagnostic intricacies and difficulties. 3 cases]. Presse Med. 2002, 31, 312–315. [Google Scholar]
- Pehlivantürk Kızılkan, M.; Kanbur, N.; Akgül, S.; Alikaşifoğlu, A. An adolescent boy with comorbid anorexia nervosa and Hashimoto thyroiditis. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 92–95. [Google Scholar] [CrossRef]
- Smalls-Mantey, A.; Steinglass, J.; Primack, M.; Clark-Hamilton, J.; Bongiovi, M. Hypothyroidism due to Hashimoto’s thyroiditis masked by anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.S.; Katz, M. Eating disorders and connective tissue disease. Etiologic and treatment considerations. Psychosomatics 1992, 33, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Bruning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Canto, C.; Menzies, K.J.; Auwerx, J. NAD(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Bordone, L.; Guarente, L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E.; Supinski, A.M.; Bonkowski, M.S.; Donmez, G.; Guarente, L.P. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009, 23, 2812–2817. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Shao, N.; Heller, E.; Feng, J.; Neve, R.; Kim, H.D.; Call, T.; Magazu, S.; Shen, L.; Nestler, E.J. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J. Neurosci. 2015, 35, 3100–3111. [Google Scholar] [CrossRef]
- Libert, S.; Guarente, L. Metabolic and neuropsychiatric effects of calorie restriction and sirtuins. Annu. Rev. Physiol. 2013, 75, 669–684. [Google Scholar] [CrossRef]
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef]
- Kishi, T.; Yoshimura, R.; Kitajima, T.; Okochi, T.; Okumura, T.; Tsunoka, T.; Yamanouchi, Y.; Kinoshita, Y.; Kawashima, K.; Fukuo, Y.; et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. J. Affect. Disord. 2010, 126, 167–173. [Google Scholar] [CrossRef]
- Kovanen, L.; Donner, K.; Partonen, T. SIRT1 polymorphisms associate with seasonal weight variation, depressive disorders, and diastolic blood pressure in the general population. PLoS ONE 2015, 10, e0141001. [Google Scholar] [CrossRef]
- Avraham, Y.; Van Heukelom, B.; Zolotarev, O.; Magen, I.; Vorobiev, L.; Zwas, D.R. Insulin normalized brain metabolic status on a Model of Anorexia Nervosa in Mice. Physiol. Behav. 2022, 249, 113738. [Google Scholar] [CrossRef]
- Mariani, S.; di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarnicola, A.; Genco, A.; et al. Inverse association of circulating SIRT1 and adiposity: A study on underweight, normal weight, and obese patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Robinette, T.M.; Nicholatos, J.W.; Francisco, A.B.; Brooks, K.E.; Diao, R.Y.; Sorbi, S.; Ricca, V.; Nacmias, B.; Brieño-Enríquez, M.A.; Libert, S. SIRT1 accelerates the progression of activity-based anorexia. Nat. Commun. 2020, 11, 2814. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef]
- Pehlivan, M.J.; Miskovic-Wheatley, J.; Le, A.; Maloney, D.; National Eating Disorders Research Consortium; Touyz, S.; Maguire, S. Models of care for eating disorders: Findings from a rapid review. J. Eat. Disord. 2022, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A. Tests based on EDF statistics. In Goodness-of-Fit Techniques; D’Agostino, R.B., Stephens, M.A., Eds.; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Naoi, M.; Riederer, P.; Maruyama, W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: Genetic and environmental factors involved in type A MAO expression. J. Neural Transm. 2016, 123, 91–106. [Google Scholar] [CrossRef]
- Quiñones, M.; Martínez-Grobas, E.; Fernø, J.; Pérez-Lois, R.; Seoane, L.M.; Al Massadi, O. Hypothalamic Actions of SIRT1 and SIRT6 on Energy Balance. Int. J. Mol. Sci. 2021, 22, 1430. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Acres, M.J.; Heath, J.J.; Morris, J.A. Anorexia nervosa, autoimmunity and the hygiene hypothesis. Med. Hypotheses 2012, 78, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Pando, R.; Phillip, M. Nutritional catch-up growth. World Rev. Nutr. Diet. 2013, 106, 83–89. [Google Scholar]
- Stroe-Kunold, E.; Buckert, M.; Friederich, H.C.; Wesche, D.; Kopf, S.; Herzog, W.; Wild, B. Time course of leptin in patients with anorexia nervosa during inpatient treatment: Longitudinal relationships to BMI and psychological factors. PLoS ONE 2016, 11, e0166843. [Google Scholar] [CrossRef] [PubMed]
- Haas, V.; Onur, S.; Paul, T.; Nutzinger, D.O.; Bosy-Westphal, A.; Hauer, M.; Brabant, G.; Klein, H.; Müller, M.J. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am. J. Clin. Nutr. 2005, 81, 889–896. [Google Scholar] [CrossRef]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ouyang, T.; Li, X.; Chen, Y.; Ke, S.; Chen, J.; Liu, Y.; Zeng, F.; Chen, Y.; Long, J.; et al. Serum sirtuin-1 a potential marker in the diagnosis of rheumatoid arthritis. Autoimmunity 2023, 56, 2181234. [Google Scholar] [CrossRef] [PubMed]
- Silén, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Pigni, M.; Albanese, N.D.; Falbo, M.; Di Guardo, S.; Brasola, E.; Biso, F.; Nacinovich, R. Eating Disorders in Children and Adolescent Males: A Peculiar Psychopathological Profile. Int. J. Environ. Res. Public Health 2022, 19, 11449. [Google Scholar] [CrossRef] [PubMed]


| Anorexia Nervosa (N = 32) | Control Group (N = 22) | p-Value | |
|---|---|---|---|
| Gender (male/female) | 2/30 | 4/18 | - |
| Age (years) | 24.77 ± 9.72 | 27.17 ± 8.61 | 0.448 |
| Body Mass Index (kg/m2) | 15.55 ± 1.98 | 20.57 ± 4.00 | <0.001 |
| Serum markers | |||
| SIRT-1 (pg/mL) | 5.18 ± 1.26 | 1.74 ± 0.42 | <0.001 |
| Leptin (pg/mL) | 3715 ± 1994 | 13,831 ± 5917 | <0.001 |
| Ghrelin (pg/mL) | 645.3 ± 271.9 | 234.5 ± 54.04 | <0.001 |
| IgG autoantibody to hypothalamic cells (ng/mL) | 8476 ± 2267 | 167.5 ± 368.4 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amerio, A.; Escelsior, A.; Martino, E.; Strangio, A.; Aguglia, A.; Marcatili, M.; Conio, B.; Sukkar, S.G.; Saverino, D. The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa. J. Pers. Med. 2023, 13, 928. https://doi.org/10.3390/jpm13060928
Amerio A, Escelsior A, Martino E, Strangio A, Aguglia A, Marcatili M, Conio B, Sukkar SG, Saverino D. The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa. Journal of Personalized Medicine. 2023; 13(6):928. https://doi.org/10.3390/jpm13060928
Chicago/Turabian StyleAmerio, Andrea, Andrea Escelsior, Eleonora Martino, Antonella Strangio, Andrea Aguglia, Matteo Marcatili, Benedetta Conio, Samir Giuseppe Sukkar, and Daniele Saverino. 2023. "The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa" Journal of Personalized Medicine 13, no. 6: 928. https://doi.org/10.3390/jpm13060928
APA StyleAmerio, A., Escelsior, A., Martino, E., Strangio, A., Aguglia, A., Marcatili, M., Conio, B., Sukkar, S. G., & Saverino, D. (2023). The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa. Journal of Personalized Medicine, 13(6), 928. https://doi.org/10.3390/jpm13060928









