Long-Term Care and Follow-Up in Laryngeal Cancer Patients: A Multicenter Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Recurrent Disease Versus Secondary Primary Tumor
2.3. Statistical Methods
3. Results
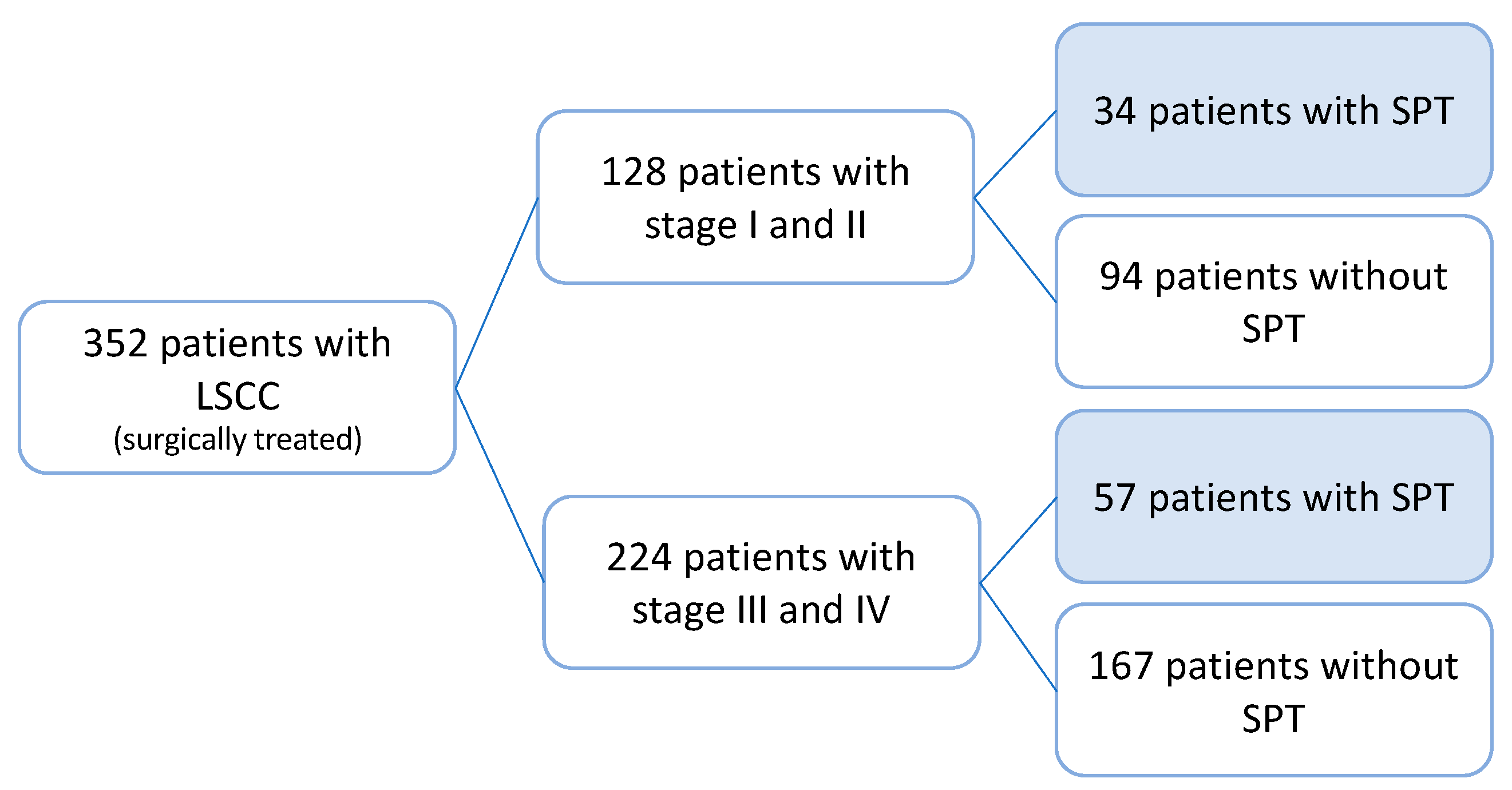
3.1. Study Cohort
3.2. Occurrence of Secondary Primary Tumor
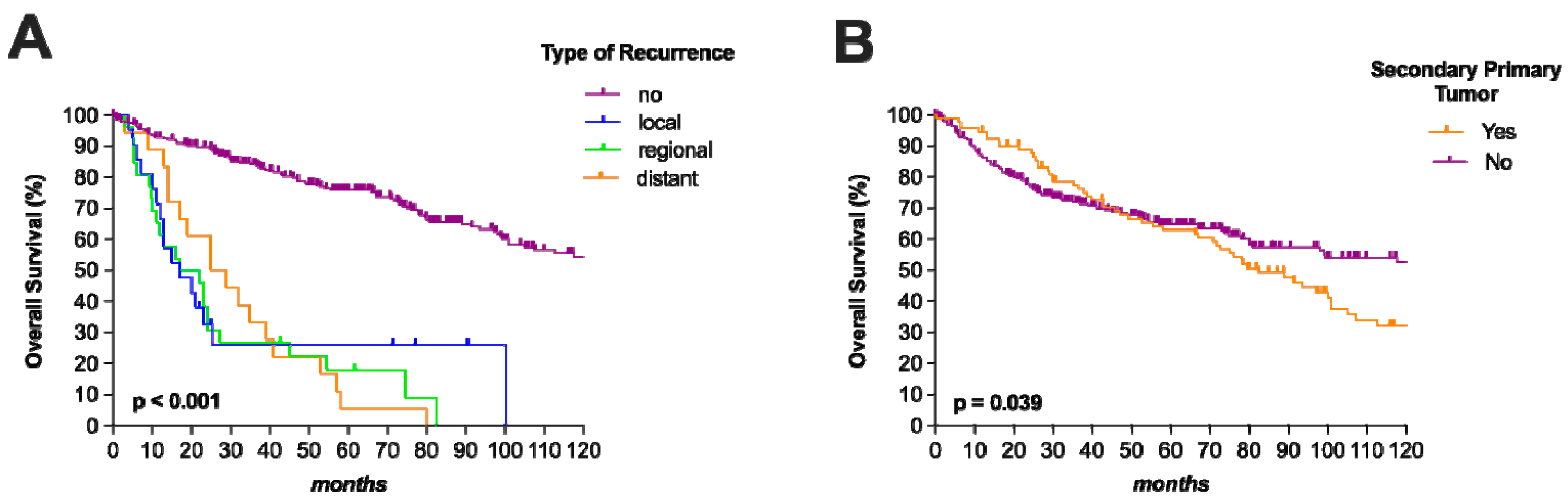
3.3. Tumor Recurrence
3.4. Oncological Outcome
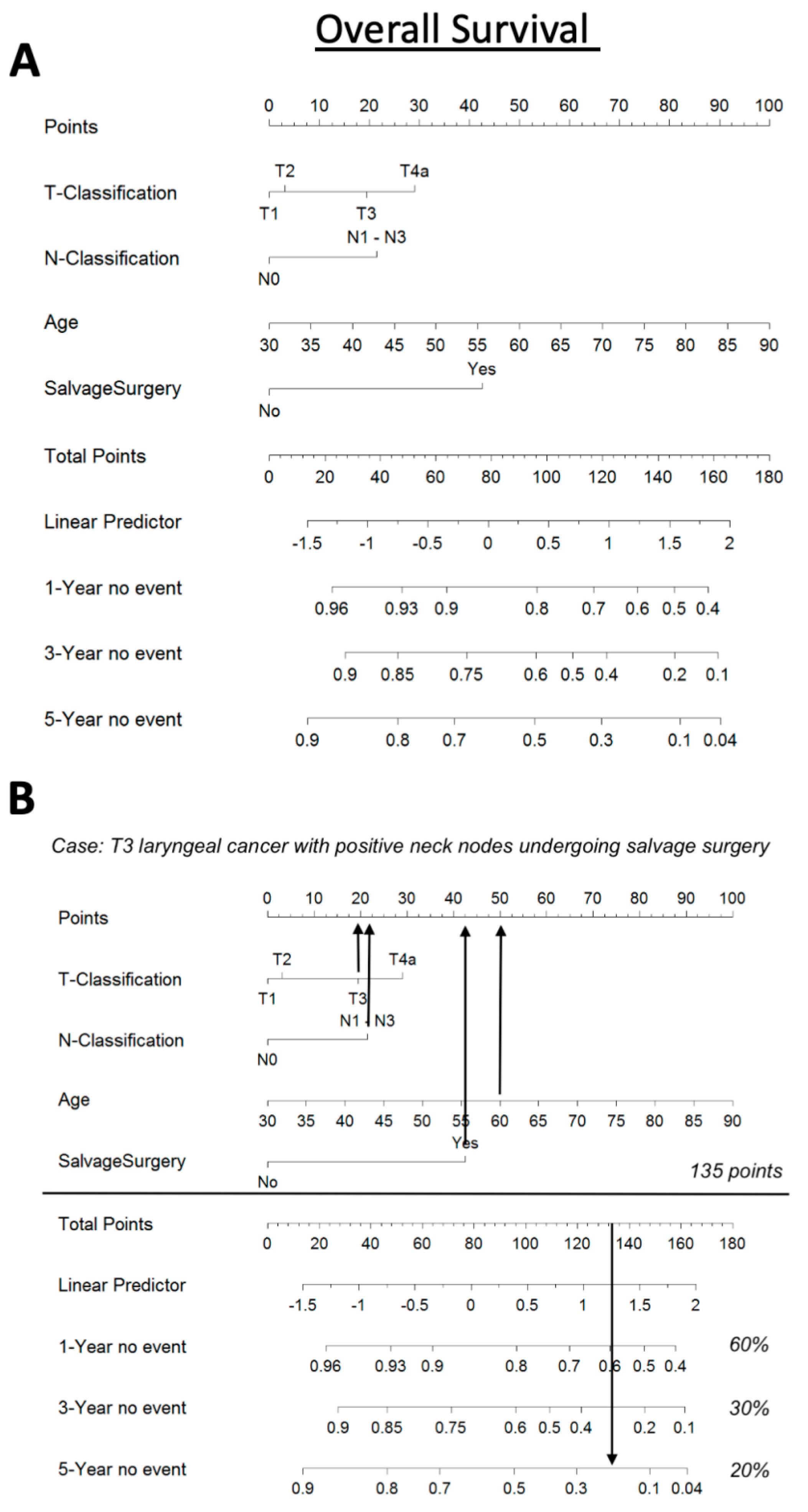
3.5. Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wushouer, A.; Li, W.; Zhang, M.; Lei, D.; Pan, X. Comparison of treatment modalities for selected advanced laryngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2021, 279, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Grasl, S.; Schmid, E.; Heiduschka, G.; Brunner, M.; Marijic, B.; Grasl, M.C.; Faisal, M.; Erovic, B.M.; Janik, S. A New Classification System to Predict Functional Outcome after Laryngectomy and Laryngopharyngectomy. Cancers 2021, 13, 1474. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, G.; Sano, D.; Arai, Y.; Hatano, T.; Takahashi, H.; Kitani, Y.; Takada, K.; Wada, T.; Oridate, N. The incidence of newly diagnosed secondary cancer; sub-analysis the prospective study of the second-look procedure for transoral surgery in patients with T1 and T2 head and neck cancer. Int. J. Clin. Oncol. 2021, 26, 59–65. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Networks. Head and Neck Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 20 February 2022).
- Sturgis, E.M.; Miller, R.H. Second primary malignancies in the head and neck cancer patient. Ann. Otol. Rhinol. Laryngol. 1995, 104, 946–954. [Google Scholar] [CrossRef]
- Wagenfeld, D.J.; Harwood, A.R.; Bryce, D.P.; van Nostrand, A.W.; DeBoer, G. Second primary respiratory tract malignancies in glottic carcinoma. Cancer 1980, 46, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fisher, S.G.; Mohideen, N.; Emami, B. Second primary cancers in patients with laryngeal cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 427–435. [Google Scholar] [CrossRef]
- Amin, M.; Edge, S.; Greene, F.; Byrd, D.; Brookland, R.; Washington, M.; Gershenwald, J.; Compton, C.; Hess, K.; Sullivan, D. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017. [Google Scholar]
- Warren, S. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am. J. Cancer 1932, 16, 1358–1414. [Google Scholar]
- Hong, W.K.; Lippman, S.M.; Itri, L.M.; Karp, D.D.; Lee, J.S.; Byers, R.M.; Schantz, S.P.; Kramer, A.M.; Lotan, R.; Peters, L.J.; et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1990, 323, 795–801. [Google Scholar] [CrossRef]
- George, G.; Banipal, R.P.S.; Sachdeva, J.; Jeyaraj, P.; MK, M. Incidence and Clinical Behaviour of Patients with Second Primary Cancers-Single Institution 17 Years Observation. Canc Therapy Oncol. Int. J. 2017, 3, 555605. [Google Scholar] [CrossRef]
- Rott, T.; Luzar, B.; Sorli, J. Bronchopulmonary changes after laryngeal cancer treatment--differentiation between metastatic laryngeal and second primary cancer. Acta Otolaryngol. Suppl. 1997, 527, 167–169. [Google Scholar] [CrossRef]
- Leong, P.P.; Rezai, B.; Koch, W.M.; Reed, A.; Eisele, D.; Lee, D.J.; Sidransky, D.; Jen, J.; Westra, W.H. Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J. Natl. Cancer Inst. 1998, 90, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Ostrovnaya, I.; Olshen, A.B.; Seshan, V.E.; Orlow, I.; Albertson, D.G.; Begg, C.B. A metastasis or a second independent cancer? Evaluating the clonal origin of tumors using array copy number data. Stat. Med. 2010, 29, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Package ‘Hmisc’. Available online: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf (accessed on 1 May 2023).
- Obid, R.; Redlich, M.; Tomeh, C. The Treatment of Laryngeal Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Dockerty, M.B.; Baggenstoss, A.H. Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer 1961, 14, 231–237. [Google Scholar] [CrossRef]
- Argiris, A.; Brockstein, B.E.; Haraf, D.J.; Stenson, K.M.; Mittal, B.B.; Kies, M.S.; Rosen, F.R.; Jovanovic, B.; Vokes, E.E. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin. Cancer Res. 2004, 10, 1956–1962. [Google Scholar] [CrossRef]
- Garavello, W.; Ciardo, A.; Spreafico, R.; Gaini, R.M. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 762–766. [Google Scholar] [CrossRef]
- Bertolini, F.; Trudu, L.; Banchelli, F.; Schipilliti, F.; Napolitano, M.; Alberici, M.P.; Depenni, R.; D’Angelo, E.; Mattioli, F.; Rubino, L.; et al. Second primary tumors in head and neck cancer patients: The importance of a “tailored” surveillance. Oral Dis. 2020, 27, 1412–1420. [Google Scholar] [CrossRef]
- Haughey, B.H.; Gates, G.A.; Arfken, C.L.; Harvey, J. Meta-analysis of second malignant tumors in head and neck cancer: The case for an endoscopic screening protocol. Ann. Otol. Rhinol. Laryngol. 1992, 101, 105–112. [Google Scholar] [CrossRef]
- Fantini, M.; Crosetti, E.; Affaniti, R.; Sprio, A.E.; Bertotto, I.; Succo, G. Preoperative prognostic factors for functional and clinical outcomes after open partial horizontal laryngectomies. Head Neck 2021, 43, 3459–3467. [Google Scholar] [CrossRef]
- Sievert, M.; Goncalves, M.; Binder, B.; Mueller, S.K.; Rupp, R.; Koch, M.; Durr, S.; Traxdorf, M.; Hecht, M.; Iro, H.; et al. Salvage laryngectomy after primary radio- and radiochemotherapy: A retrospective study. HNO 2021, 69, 47–52. [Google Scholar] [CrossRef]
- Sabharwal, R.; Mahendra, A.; Moon, N.J.; Gupta, P.; Jain, A.; Gupta, S. Genetically altered fields in head and neck cancer and second field tumor. South Asian J. Cancer 2014, 3, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Singh, C.A.; Thakar, A.; Kakkar, A.; Sikka, K.; Kumar, R.; Sharma, S.C. Prevalence of Synchronous ESCN in Head and Neck Cancer: A Single-Institution Perspective. Laryngoscope 2021, 131, E807–E814. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Rodrigo, J.P.; Silver, C.E.; Haigentz, M., Jr.; Bishop, J.A.; Strojan, P.; Hartl, D.M.; Bradley, P.J.; Mendenhall, W.M.; Suarez, C.; et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck 2016, 38 (Suppl. S1), E2374–E2385. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Goto, T.; Nakagomi, T.; Hirotsu, Y.; Oyama, T.; Amemiya, K.; Mochizuki, H.; Omata, M. Discrimination Between Primary Lung Cancer and Lung Metastases by Genomic Profiling. JTO Clin. Res. Rep. 2021, 2, 100255. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hirotsu, Y.; Mochizuki, H.; Nakagomi, T.; Shikata, D.; Yokoyama, Y.; Oyama, T.; Amemiya, K.; Okimoto, K.; Omata, M. Mutational analysis of multiple lung cancers: Discrimination between primary and metastatic lung cancers by genomic profile. Oncotarget 2017, 8, 31133–31143. [Google Scholar] [CrossRef]
- Holland, J.M.; Arsanjani, A.; Liem, B.J.; Hoffelt, S.C.; Cohen, J.I.; Stevens, K.R., Jr. Second malignancies in early-stage laryngeal carcinoma patients treated with radiotherapy. J. Laryngol. Otol. 2002, 116, 190–193. [Google Scholar] [CrossRef]
- Jayaprakash, V.; Cheng, C.; Reid, M.; Dexter, E.U.; Nwogu, C.E.; Hicks, W.; Sullivan, M.; Demmy, T.L.; Yendamuri, S. Previous head and neck cancers portend poor prognoses in lung cancer patients. Ann. Thorac. Surg. 2011, 92, 1056–1060; discussion 1060–1051. [Google Scholar] [CrossRef]
- Morgan, R.L.; Eguchi, M.M.; McDermott, J.; Mueller, A.C.; Amini, A.; Goddard, J.A.; Trivedi, P.S.; Karam, S.D. Comparative effectiveness of posttreatment imaging modalities for Medicare patients with advanced head and neck cancer. Cancer 2021, 127, 535–543. [Google Scholar] [CrossRef]
- De Felice, F.; Musio, D.; Tombolini, V. Follow-up in head and neck cancer: A management dilemma. Adv. Otolaryngol. 2015, 2015, 703450. [Google Scholar] [CrossRef]
- Ho, A.S.; Tsao, G.J.; Chen, F.W.; Shen, T.; Kaplan, M.J.; Colevas, A.D.; Fischbein, N.J.; Quon, A.; Le, Q.T.; Pinto, H.A.; et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer 2013, 119, 1349–1356. [Google Scholar] [CrossRef]
- Heineman, T.E.; Kuan, E.C.; St John, M.A. When should surveillance imaging be performed after treatment for head and neck cancer? Laryngoscope 2017, 127, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Dunsky, K.A.; Wehrmann, D.J.; Osman, M.M.; Thornberry, B.M.; Varvares, M.A. PET-CT and the detection of the asymptomatic recurrence or second primary lesions in the treated head and neck cancer patient. Laryngoscope 2013, 123, 2161–2164. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.M.; Wang, H.H.; Lee, C.T.; Tai, C.M.; Tseng, C.H.; Chen, C.C.; Tsai, Y.N.; Chen, T.H.; Hsu, M.H.; Wang, C.C.; et al. A nationwide population-based study to access the risk of metachronous esophageal cancers in head and neck cancer survivors. Sci. Rep. 2020, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-term effects of radiation exposure among adult survivors of childhood cancer: Results from the childhood cancer survivor study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Patel, S.G.; Chu, P.Y.; Matsuo, J.M.; Singh, B.; Wong, R.J.; Kraus, D.H.; Shaha, A.R.; Shah, J.P.; Boyle, J.O. Second primary malignancy of the aerodigestive tract in patients treated for cancer of the oral cavity and larynx. Head Neck 2005, 27, 1042–1048. [Google Scholar] [CrossRef]
- Chu, P.Y.; Chang, S.Y.; Huang, J.L.; Tai, S.K. Different patterns of second primary malignancy in patients with squamous cell carcinoma of larynx and hypopharynx. Am. J. Otolaryngol. 2010, 31, 168–174. [Google Scholar] [CrossRef]
- Marzic, D.; Marijic, B.; Braut, T.; Janik, S.; Avirovic, M.; Hadzisejdic, I.; Tudor, F.; Radobuljac, K.; Coklo, M.; Erovic, B.M. IMP3 Protein Overexpression Is Linked to Unfavorable Outcome in Laryngeal Squamous Cell Carcinoma. Cancers 2021, 13, 4306. [Google Scholar] [CrossRef]
- Marijic, B.; Braut, T.; Babarovic, E.; Krstulja, M.; Marzic, D.; Avirovic, M.; Kujundzic, M.; Hadzisejdic, I. Nuclear EGFR Expression Is Associated With Poor Survival in Laryngeal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 576–584. [Google Scholar] [CrossRef]

| Characteristics | No. of Patients (%) |
|---|---|
| Sex | 352 (100.0) |
| Female | 24 (6.8) |
| Male | 328 (93.2) |
| Age | |
| Mean ± SD | 62.5 ± 9.2 |
| Nicotin | |
| No | 84 (23.9) |
| Yes | 268 (76.1) |
| Alcohol | |
| No | 162 (46.0) |
| Yes | 190 (54.0) |
| T-classification | |
| T1 | 81 (23.0) |
| T2 | 53 (15.1) |
| T3 | 129 (36.6) |
| T4a | 89 (25.3) |
| N-classification | |
| N0 | 281 (79.8) |
| N1 | 28 (8.0) |
| N2 | 40 (11.4) |
| N3 | 3 (0.9) |
| Tumor Stage | |
| Stage I | 81 (23.0) |
| Stage II | 47 (13.4) |
| Stage III | 109 (31.0) |
| Stage IVa | 115 (32.7) |
| Grading | |
| Gx | 19 (5.4) |
| G1 | 78 (22.2) |
| G2 | 204 (58.0) |
| G3 | 51 (14.5) |
| Surgical margins | |
| R0 | 324 (92.0) |
| R1 | 25 (7.1) |
| R2 | 3 (0.9) |
| Type of Surgery | |
| Partial laryngectomy | 102 (29.0) |
| Total laryngectomy | 191 (54.3) |
| Partial laryngopharyngectomy | 49 (13.9) |
| Total laryngopharyngectomy | 10 (2.8) |
| Salvage Surgery | |
| No | 308 (87.5) |
| Yes | 44 (12.5) |
| PORT | |
| No | 172 (48.9) |
| Yes | 180 (51.1) |
| Outcome | No. of Patients (%) |
|---|---|
| Survival | |
| Alive | 188 (53.4) |
| Dead | 164 (46.6) |
| Cause of death | |
| Primary cancer | 62 (17.6) |
| Second cancer | 45 (12.8) |
| Other cause | 57 (16.2) |
| Recurrence | |
| No | 287 (81.5) |
| Yes | 65 (18.5) |
| Type of recurrence | |
| Local | 21 (6.0) |
| Regional | 26 (7.4) |
| Distant | 18 (5.1) |
| Second cancer | |
| No | 261 (74.1) |
| Yes | 91 (25.9) |
| Localization of second cancer | |
| Head and neck area | 21 (6.0) |
| Lung | 29 (8.2) |
| Other than HNC | 70 (19.9) |
| Second Head and Neck Cancer | Second Lung Cancer | |||||
|---|---|---|---|---|---|---|
| CHARACTERISTICS | No | Yes | p | No | Yes | p |
| No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | |||
| Gender | ||||||
| Male | 307 (93.6) | 21 (6.4) | 300 (91.5) | 28 (8.5) | ||
| Female | 24 (100.0) | 0 (0.0) | 0.201 | 23 (95.8) | 1 (4.2) | 0.707 |
| Age | ||||||
| <62.7 | 158 (91.3) | 15 (8.7) | 161 (93.1) | 12 (6.9) | ||
| ≥62.7 | 173 (96.6) | 6 (3.4) | 0.043 | 162 (90.5) | 17 (9.5) | 0.441 |
| T-classification | ||||||
| T1 | 79 (97.5) | 2 (2.5) | 77 (95.1) | 4 (4.9) | ||
| T2 | 50 (94.3) | 3 (5.7) | 48 (90.6) | 5 (9.4) | ||
| T3 | 117 (90.3) | 12 (9.3) | 120 (93.0) | 9 (7.0) | ||
| T4a | 85 (95.5) | 4 (4.5) | 0.197 | 78 (87.6) | 11 (12.4) | 0.316 |
| N-classification | ||||||
| N0 | 267 (95.0) | 14 (5.0) | 259 (92.2) | 22 (7.8) | ||
| N1 | 23 (82.1) | 5 (17.9) | 25 (89.3) | 3 (10.7) | ||
| N2 | 38 (95.0) | 2 (5.0) | 36 (90.0) | 4 (10.0) | ||
| N3 | 3 (100.0) | 0 (0.0) | 0.050 | 3 (100.0) | 0 (0.0) | 0.868 |
| Tumor Stage | ||||||
| Stage I | 79 (97.5) | 2 (2.5) | 77 (95.1) | 4 (4.9) | ||
| Stage II | 45 (95.7) | 2 (4.3) | 42 (89.4) | 5 (10.6) | ||
| Stage III | 99 (90.8) | 10 (9.2) | 103 (94.5) | 6 (5.5) | ||
| Stage IVa | 108 (93.9) | 7 (6.1) | 0.260 | 101 (87.8) | 14 (12.2) | 0.175 |
| Smoking | ||||||
| No | 82 (97.6) | 2 (2.4) | 77 (91.7) | 7 (8.3) | ||
| Yes | 249 (92.9) | 19 (7.1) | 0.183 | 246 (91.8) | 22 (8.2) | 1.000 |
| Alcohol | ||||||
| No | 154 (95.1) | 8 (4.9) | ||||
| Yes | 177 (93.2) | 13 (6.8) | 0.505 | 175 (92.1) | 29 (8.2) | 0.847 |
| CHARACTERISTICS | TYPE OF RECURRENCE | ||||
|---|---|---|---|---|---|
| No | Local | Regional | Distant | p | |
| No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | ||
| T-classification | |||||
| T1 | 75 (92.6) | 4 (4.9) | 2 (2.5) | 0 (0.0) | |
| T2 | 44 (83.0) | 3 (5.7) | 2 (3.8) | 4 (7.5) | |
| T3 | 109 (84.5) | 5 (3.9) | 8 (6.2) | 7 (5.4) | |
| T4a | 59 (66.3) | 9 (10.1) | 14 (15.7) | 7 (7.9) | 0.002 |
| N-classification | |||||
| N0 | 240 (85.4) | 18 (6.4) | 15 (5.3) | 8 (2.8) | |
| N1 | 20 (71.4) | 1 (3.6) | 7 (25.0) | 0 (0.0) | |
| N2 | 25 (62.5) | 2 (5.0) | 4 (10.0) | 9 (22.5) | |
| N3 | 2 (66.7) | 0 (0.0) | 0 (0.0) | 1 (33.3) | <0.001 |
| Tumor Stage | |||||
| Stage I | 75 (92.6) | 4 (4.9) | 2 (2.5) | 0 (0.0) | |
| Stage II | 41 (87.2) | 3 (6.4) | 1 (2.1) | 2 (4.3) | |
| Stage III | 95 (87.2) | 4 (3.7) | 7 (6.4) | 3 (2.8) | |
| Stage IVa | 76 (66.1) | 10 (8.7) | 16 (13.9) | 13 (11.3) | <0.001 |
| Grading | |||||
| Gx | 18 (94.7) | 1 (5.3) | 0 (0.0) | 0 (0.0) | |
| G1 | 68 (87.2) | 6 (7.7) | 4 (5.1) | 0 (0.0) | |
| G2 | 159 (77.9) | 11 (5.4) | 18 (8.8) | 16 (7.8 | |
| G3 | 42 (82.4) | 3 (5.9) | 4 (7.8) | 2 (3.9) | 0.205 |
| Type of Surgery | |||||
| Partial laryngectomy | 97 (95.1) | 3 (2.9) | 2 (2.0) | 0 (0.0) | |
| Total laryngectomy | 152 (79.6) | 12 (6.3) | 16 (8.4) | 11 (5.8) | |
| Partial laryngopharyngectomy | 30 (61.2) | 5 (10.2) | 7 (14.3) | 7 (14.3) | |
| Total laryngopharyngectomy | 8 (80.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | <0.001 |
| Salvage Surgery | |||||
| No | 261 (84.7) | 11 (3.6) | 21 (6.8) | 15 (4.9) | |
| Yes | 26 (59.1) | 10 (22.7) | 5 (11.4) | 3 (6.8) | <0.001 |
| PORT | |||||
| No | 144 (83.7) | 14 (8.1) | 9 (5.2) | 5 (2.9) | |
| Yes | 143 (79.4) | 7 (3.9) | 17 (9.4) | 13 (7.2) | 0.043 |
| UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | |||||
|---|---|---|---|---|---|---|
| HR | P | 95% CI | HR | p | 95% CI | |
| Overall Survival | ||||||
| Sex (Female) | 0.643 | 0.225 | 0.32–1.31 | |||
| Age (>62.5 years) | 1.536 | 0.009 | 1.11–2.12 | 1.610 | 0.007 | 1.14–2.27 |
| T3–T4a versus T1–T2 | 2.268 | <0.001 | 1.57–3.27 | 0.703 | 0.474 | 0.27–1.85 |
| N+ versus N- | 2.141 | <0.001 | 1.52–3.01 | 1.299 | 0.205 | 0.87–1.95 |
| Stage III–IV versus I–II | 2.564 | <0.001 | 1.75–3.76 | 3.194 | 0.032 | 1.10–9.26 |
| G1 versus G2 versus G3 | 1.426 | 0.007 | 1.10–1.84 | 1.159 | 0.309 | 0.87–1.54 |
| Salvage surgery (Yes) | 2.874 | <0.001 | 1.95–4.22 | 2.232 | 0.001 | 1.39–3.60 |
| PORT (No) | 0.691 | 0.024 | 0.50–0.95 | 0.831 | 0.383 | 0.55–1.26 |
| Recurrence (Yes) | 6.135 | <0.001 | 4.31–8.70 | 5.050 | <0.001 | 3.46–7.35 |
| Second cancer (Yes) | 1.397 | 0.040 | 1.02–1.92 | 1.441 | 0.039 | 1.02–2.04 |
| Cancer-specific Survival | ||||||
| Sex (Female) | 0.924 | 0.878 | 0.34–2.55 | |||
| Age (>62.5 years) | 0.883 | 0.630 | 0.54–1.46 | |||
| T3–T4a versus T1–T2 | 6.757 | <0.001 | 2.91–15.6 | 1.792 | 0.431 | 0.42–7.69 |
| N+ versus N− | 4.237 | <0.001 | 2.57–6.99 | 3.144 | <0.001 | 1.66–5.95 |
| Stage III–IV versus I–II | 9.804 | <0.001 | 3.56–27.0 | 3.144 | 0.215 | 0.51–13.2 |
| G1 versus G2 versus G3 | 1.811 | 0.004 | 1.21–2.72 | 1.102 | 0.963 | 0.61–1.68 |
| Salvage surgery (Yes) | 5.076 | <0.001 | 2.99–8.62 | 5.128 | <0.001 | 2.66–9.80 |
| PORT (No) | 0.781 | 0.339 | 0.47–1.30 | |||
| Recurrence (Yes) | 41.67 | <0.001 | 20.4–83.3 | 33.33 | <0.001 | 15.6–71.4 |
| Second cancer (Yes) | 0.362 | 0.007 | 0.17–0.76 | 0.518 | 0.098 | 0.27–1.12 |
| Recurrence-free Survival | ||||||
| Sex (Female) | 0.886 | 0.815 | 0.32–2.44 | |||
| Age (>62.5 years) | 0.886 | 0.626 | 0.54–1.44 | |||
| T3–T4a versus T1–T2 | 2.404 | 0.003 | 1.35–4.27 | 0.618 | 0.439 | 0.18–2.09 |
| N+ versus N− | 2.801 | <0.001 | 1.67–4.65 | 2.227 | 0.006 | 1.26–3.92 |
| Stage III–IV versus I–II | 2.967 | 0.001 | 1.58–5.56 | 2.959 | 0.125 | 0.74–11.7 |
| G1 versus G2 versus G3 | 1.257 | 0.258 | 0.85–1.87 | |||
| Salvage surgery (Yes) | 4.115 | <0.001 | 2.38–7.09 | 3.922 | <0.001 | 2.23–6.85 |
| PORT (No) | 0.812 | 0.405 | 0.48–1.33 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marijić, B.; Tudor, F.; Janik, S.; Grasl, S.; Frommlet, F.; Maržić, D.; Hadžisejdić, I.; Vukelić, J.; Braut, T.; Velepič, M.; et al. Long-Term Care and Follow-Up in Laryngeal Cancer Patients: A Multicenter Retrospective Analysis. J. Pers. Med. 2023, 13, 927. https://doi.org/10.3390/jpm13060927
Marijić B, Tudor F, Janik S, Grasl S, Frommlet F, Maržić D, Hadžisejdić I, Vukelić J, Braut T, Velepič M, et al. Long-Term Care and Follow-Up in Laryngeal Cancer Patients: A Multicenter Retrospective Analysis. Journal of Personalized Medicine. 2023; 13(6):927. https://doi.org/10.3390/jpm13060927
Chicago/Turabian StyleMarijić, Blažen, Filip Tudor, Stefan Janik, Stefan Grasl, Florian Frommlet, Diana Maržić, Ita Hadžisejdić, Jelena Vukelić, Tamara Braut, Marko Velepič, and et al. 2023. "Long-Term Care and Follow-Up in Laryngeal Cancer Patients: A Multicenter Retrospective Analysis" Journal of Personalized Medicine 13, no. 6: 927. https://doi.org/10.3390/jpm13060927
APA StyleMarijić, B., Tudor, F., Janik, S., Grasl, S., Frommlet, F., Maržić, D., Hadžisejdić, I., Vukelić, J., Braut, T., Velepič, M., & Erovic, B. M. (2023). Long-Term Care and Follow-Up in Laryngeal Cancer Patients: A Multicenter Retrospective Analysis. Journal of Personalized Medicine, 13(6), 927. https://doi.org/10.3390/jpm13060927







