REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Timeline
2.2. Power Analysis
2.3. Study Timeline
2.4. Population
2.5. Inclusion and Exclusion Criteria
2.6. Motor Assessment
2.7. Functional Dysmetria
2.8. Timed Up and Go Test
2.9. Sit-To-Stand Test
2.10. Quality of Life Assessment—Fibromyalgia Impact Questionnaire
2.11. REAC Technology
2.12. REAC Treatments
2.13. Neuro Psycho Physical Optimization
2.14. Study Replicability
2.15. Statistical Analysis
Statistics
3. Results
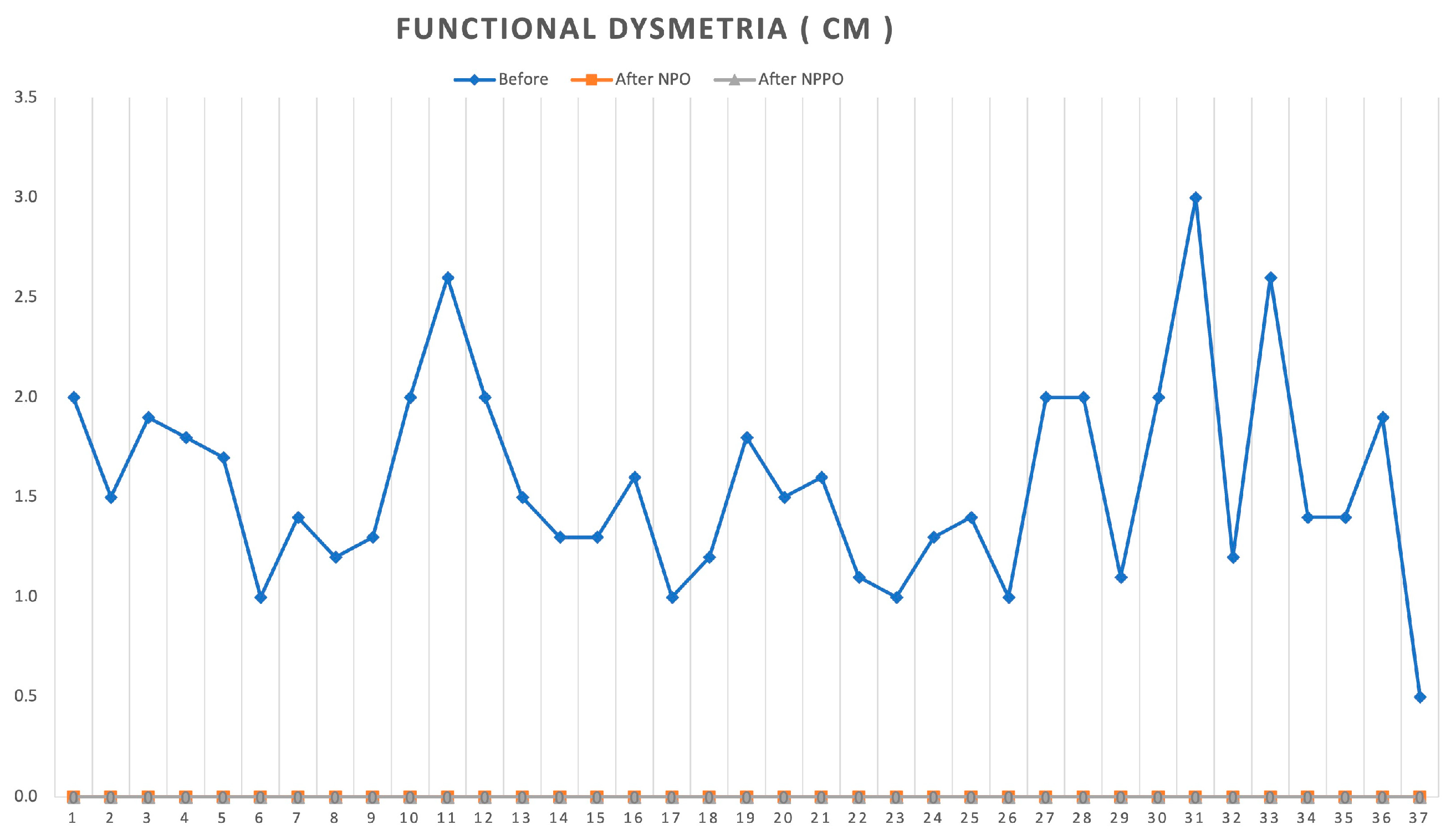
3.1. Functional Dysmetria Results
3.2. Timed Up and Go and Sit-To-Stand Results
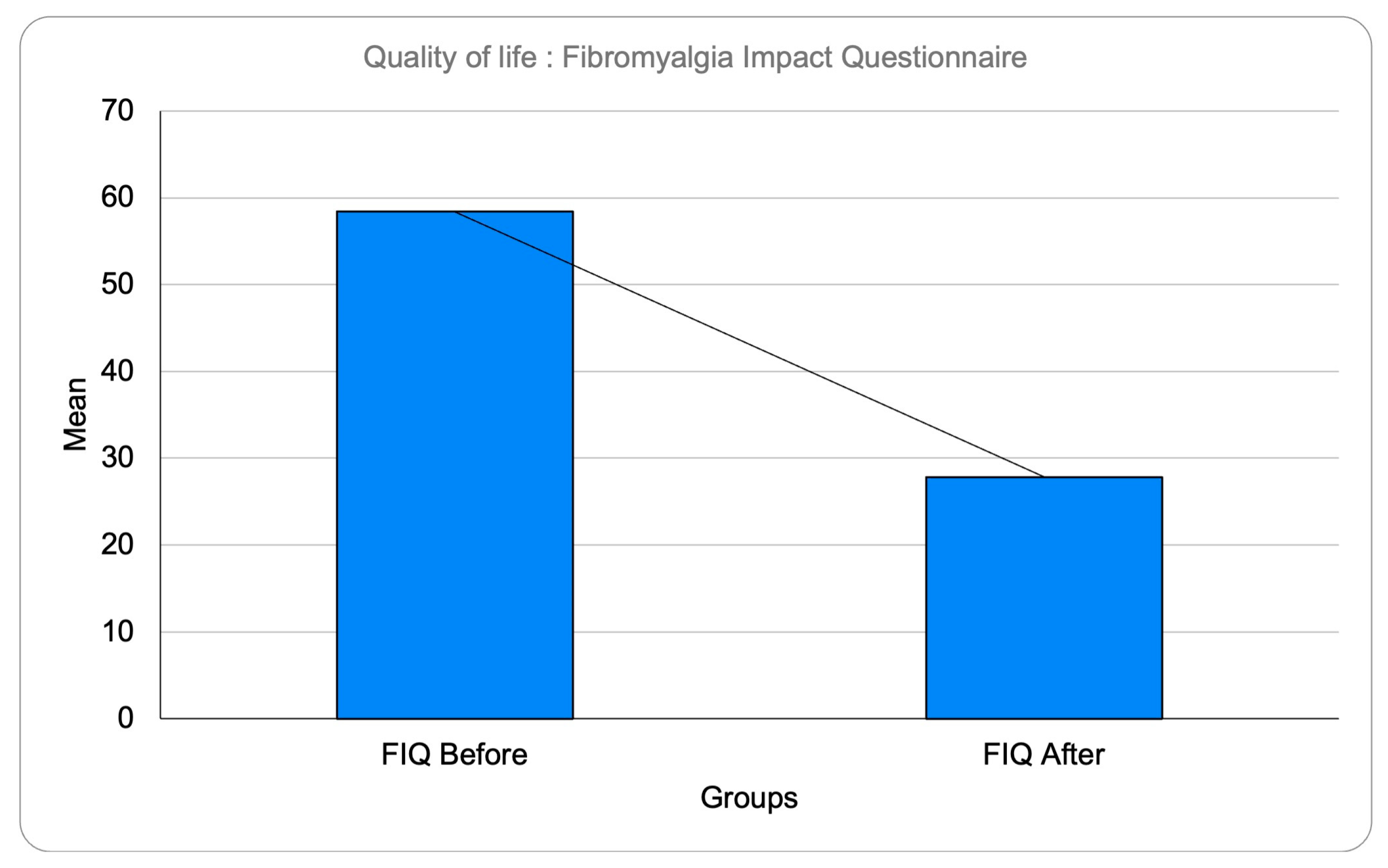
3.3. Fibromyalgia Impact Questionnaire Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clauw, D.J.; Arnold, L.M.; McCarberg, B.H. The science of fibromyalgia. Mayo Clin. Proc. 2011, 86, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Singh, L.; Kaur, A.; Bhatti, M.S.; Bhatti, R. Possible Molecular Mediators Involved and Mechanistic Insight into Fibromyalgia and Associated Co-morbidities. Neurochem. Res. 2019, 44, 1517–1532. [Google Scholar] [CrossRef]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Becker, S.; Schweinhardt, P. Dysfunctional Neurotransmitter Systems in Fibromyalgia, Their Role in Central Stress Circuitry and Pharmacological Actions on These Systems. Pain. Res. Treat. 2012, 2012, 741746. [Google Scholar] [CrossRef]
- Ovrom, E.A.; Mostert, K.A.; Khakhkhar, S.; McKee, D.P.; Yang, P.; Her, Y.F. A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia. Biomedicines 2023, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Björkander, S.; Ernberg, M.; Bileviciute-Ljungar, I. Reduced immune system responsiveness in fibromyalgia—A pilot study. Clin. Immunol. Commun. 2022, 2, 46–53. [Google Scholar] [CrossRef]
- Gupta, A.; Silman, A.J.; Ray, D.; Morriss, R.; Dickens, C.; MacFarlane, G.J.; Chiu, Y.H.; Nicholl, B.; McBeth, J. The role of psychosocial factors in predicting the onset of chronic widespread pain: Results from a prospective population-based study. Rheumatology 2006, 46, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E. Elevated excitatory neurotransmitter levels in the fibromyalgia brain. Arthritis Res. Ther. 2010, 12, 141. [Google Scholar] [CrossRef]
- Al-Nimer, M.S.M.; Mohammad, T.A.M.; Alsakeni, R.A. Serum levels of serotonin as a biomarker of newly diagnosed fibromyalgia in women: Its relation to the platelet indices. J. Res. Med. Sci. 2018, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, S.M. Fibromyalgia Syndrome: A Metabolic Approach Grounded in Biochemistry for the Remission of Symptoms. Front. Med. 2017, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef]
- Wood, P.B.; Holman, A.J. An elephant among us: The role of dopamine in the pathophysiology of fibromyalgia. J. Rheumatol. 2009, 36, 221–224. [Google Scholar] [CrossRef]
- Martinez-Lavin, M.; Vidal, M.; Barbosa, R.E.; Pineda, C.; Casanova, J.M.; Nava, A. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study [ISRCTN70707830]. BMC Musculoskelet. Disord. 2002, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, A.; Geri, T.; De Luca, S.; Maselli, F.; Rossettini, G.; Sulli, A.; Schenone, A.; Testa, M. Neuropathic pain and symptoms of potential small-fiber neuropathy in fibromyalgic patients: A national on-line survey. Jt. Bone Spine 2021, 88, 105153. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain. 2019, 15, 1744806918819944. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Jaaniste, T.; Champion, D. Chronic Widespread Pain and Fibromyalgia Syndrome: Life-Course Risk Markers in Young People. Pain. Res. Manag. 2019, 2019, 6584753. [Google Scholar] [CrossRef]
- Das, S.; Taylor, K.; Kozubek, J.; Sardell, J.; Gardner, S. Genetic risk factors for ME/CFS identified using combinatorial analysis. J. Transl. Med. 2022, 20, 598. [Google Scholar] [CrossRef]
- Martinez-Lavin, M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res. Ther. 2007, 9, 216. [Google Scholar] [CrossRef]
- Van Houdenhove, B.; Egle, U.; Luyten, P. The role of life stress in fibromyalgia. Curr. Rheumatol. Rep. 2005, 7, 365–370. [Google Scholar] [CrossRef]
- Yepez, D.; Grandes, X.A.; Talanki Manjunatha, R.; Habib, S.; Sangaraju, S.L. Fibromyalgia and Depression: A Literature Review of Their Shared Aspects. Cureus 2022, 14, e24909. [Google Scholar] [CrossRef]
- Henao-Perez, M.; Lopez-Medina, D.C.; Arboleda, A.; Bedoya Monsalve, S.; Zea, J.A. Patients With Fibromyalgia, Depression, and/or Anxiety and Sex Differences. Am. J. Mens. Health 2022, 16, 15579883221110351. [Google Scholar] [CrossRef]
- Viceconti, A.; Geri, T.; De Luca, S.; Maselli, F.; Rossettini, G.; Testa, M. Body perception distortions correlate with neuropathic features in Italian fibromyalgic patients: Findings from a self-administered online survey. Musculoskelet. Sci. Pract. 2022, 60, 102570. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, A.; Camerone, E.M.; Luzzi, D.; Pentassuglia, D.; Pardini, M.; Ristori, D.; Rossettini, G.; Gallace, A.; Longo, M.R.; Testa, M. Explicit and Implicit Own’s Body and Space Perception in Painful Musculoskeletal Disorders and Rheumatic Diseases: A Systematic Scoping Review. Front. Hum. Neurosci. 2020, 14, 83. [Google Scholar] [CrossRef]
- Zippo, A.G.; Rinaldi, S.; Pellegata, G.; Caramenti, G.C.; Valente, M.; Fontani, V.; Biella, G.E. Electrophysiological effects of non-invasive Radio Electric Asymmetric Conveyor (REAC) on thalamocortical neural activities and perturbed experimental conditions. Sci. Rep. 2015, 5, 18200. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Mura, M.; Castagna, A.; Fontani, V. Long-lasting changes in brain activation induced by a single REAC technology pulse in Wi-Fi bands. Randomized double-blind fMRI qualitative study. Sci. Rep. 2014, 4, 5668. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Castagna, A. Brain activity modification produced by a single radioelectric asymmetric brain stimulation pulse: A new tool for neuropsychiatric treatments. Preliminary fMRI study. Neuropsychiatr. Dis. Treat. 2011, 7, 649–654. [Google Scholar] [CrossRef]
- Mura, M.; Castagna, A.; Fontani, V.; Rinaldi, S. Preliminary pilot fMRI study of neuropostural optimization with a noninvasive asymmetric radioelectric brain stimulation protocol in functional dysmetria. Neuropsychiatr. Dis. Treat. 2012, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Elio, C.; Fontani, V.; Rinaldi, S.; Gasbarro, V. REAC-induced endogenous bioelectric currents in the treatment of venous ulcers: A three-arm randomized controlled prospective study. Acta Dermatovenerol. Alp. Pannonica Et. Adriat. 2020, 29, 109–113. [Google Scholar] [CrossRef]
- Sanna Passino, E.; Rocca, S.; Caggiu, S.; Columbano, N.; Castagna, A.; Fontani, V.; Rinaldi, S. REAC regenerative treatment efficacy in experimental chondral lesions: A pilot study on ovine animal model. Clin. Interv. Aging 2017, 12, 1471–1479. [Google Scholar] [CrossRef]
- Rinaldi, S.; Rinaldi, C.; Fontani, V. Regenerative Radio Electric Asymmetric Conveyer Treatment in Generalized Cerebral and Cerebellar Atrophy to Improve Motor Control: A Case Report. Cureus 2022, 14, e28245. [Google Scholar] [CrossRef]
- Rinaldi, A.; Maioli, M.; Marins Martins, M.C.; de Castro, P.C.F.; de Oliveira Silva, N.A.P.; de Mattos, J.A.V.; Fontani, V.; Rinaldi, S. REAC Non-invasive Neurobiological Stimulation for Mitigating the Impact of Internalizing Disorders in Autism Spectrum Disorder. Adv. Neurodev. Disord. 2021, 5, 446–456. [Google Scholar] [CrossRef]
- Rinaldi, A.; Rinaldi, C.; Coelho Pereira, J.A.; Lotti Margotti, M.; Bittencourt, M.N.; Barcessat, A.R.P.; Fontani, V.; Rinaldi, S. Radio electric asymmetric conveyer neuromodulation in depression, anxiety, and stress. Neuropsychiatr. Dis. Treat. 2019, 15, 469–480. [Google Scholar] [CrossRef]
- Rinaldi, S.; Calza, L.; Giardino, L.; Biella, G.E.; Zippo, A.G.; Fontani, V. Radio electric asymmetric conveyer: A novel neuromodulation technology in Alzheimer’s and other neurodegenerative diseases. Front. Psychiatry 2015, 6, 22. [Google Scholar] [CrossRef]
- Pinheiro Barcessat, A.R.; Nolli Bittencourt, M.; Goes Goncalves, R.; Goncalves de Oliveira Cruz, A.V.; Coelho Pereira, J.A.; Bechelli, F.A.; Rinaldi, A. REAC Neuromodulation Treatments in Depression, Anxiety and Stress. A Comparative Retrospective Study. Psychol. Res. Behav. Manag. 2020, 13, 1247–1256. [Google Scholar] [CrossRef]
- Olivieri, E.B.; Vecchiato, C.; Ignaccolo, N.; Mannu, P.; Castagna, A.; Aravagli, L.; Fontani, V.; Rinaldi, S. Radioelectric brain stimulation in the treatment of generalized anxiety disorder with comorbid major depression in a psychiatric hospital: A pilot study. Neuropsychiatr. Dis. Treat. 2011, 7, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Olazaran, J.; Gonzalez, B.; Lopez-Alvarez, J.; Castagna, A.; Osa-Ruiz, E.; Herrero-Cano, V.; Aguera-Ortiz, L.; Rinaldi, S.; Martinez-Martin, P. Motor effects of REAC in advanced Alzheimer’s disease: Results from a pilot trial. J. Alzheimers Dis. 2013, 36, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Mannu, P.; Rinaldi, S.; Fontani, V.; Castagna, A.; Margotti, M.L. Noninvasive brain stimulation by radioelectric asymmetric conveyor in the treatment of agoraphobia: Open-label, naturalistic study. Patient Prefer. Adherence 2011, 5, 575–580. [Google Scholar] [CrossRef] [PubMed]
- (ICH), I.C.f.H.o.T.R.f.P.f.H.U. ICH Guideline E8 (R1): General Considerations for Clinical Trials. ICH. 2019. Available online: https://database.ich.org/sites/default/files/E8_R1_Guideline.pdf (accessed on 6 March 2023).
- Collado-Mateo, D.; Dominguez-Munoz, F.J.; Adsuar, J.C.; Merellano-Navarro, E.; Olivares, P.R.; Gusi, N. Reliability of the Timed up and Go Test in Fibromyalgia. Rehabil. Nurs. 2018, 43, 35–39. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Adsuar, J.C.; Dominguez-Munoz, F.J.; Olivares, P.R.; Gusi, N. Impact of Fibromyalgia in the Sit-to-Stand-to-Sit Performance Compared With Healthy Controls. PMR 2017, 9, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Anastasopoulos, K.; Kastrinis, A.; Dimitriadis, Z. What is most appropriate number of repetitions of the sit-to-stand test in older adults: A reliability study. J. Frailty Sarcopenia Falls 2020, 5, 109–113. [Google Scholar] [CrossRef]
- Ataullah, A.H.M.; Naqvi, I.A. Cerebellar Dysfunction. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Fontani, V.; Rinaldi, A.; Rinaldi, C.; Araldi, L.; Azzara, A.; Carta, A.M.; Casale, N.; Castagna, A.; Del Medico, M.; Di Stasio, M.; et al. Long-Lasting Efficacy of Radio Electric Asymmetric Conveyer Neuromodulation Treatment on Functional Dysmetria, an Adaptive Motor Behavior. Cureus 2022, 14, e25768. [Google Scholar] [CrossRef]
- Lee, J.; Muzio, M.R. Neuroanatomy, Extrapyramidal System. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Deviaterikova, A.; Kasatkin, V.; Malykh, S. The Role of the Cerebellum in Visual-Spatial Memory in Pediatric Posterior Fossa Tumor Survivors. Cerebellum 2023, 22, 1–7. [Google Scholar] [CrossRef]
- Chin, P.W.; Augustine, G.J. The cerebellum and anxiety. Front. Cell. Neurosci. 2023, 17, 1130505. [Google Scholar] [CrossRef] [PubMed]
- Manni, E.; Petrosini, L. A century of cerebellar somatotopy: A debated representation. Nat. Rev. Neurosci. 2004, 5, 241–249. [Google Scholar] [CrossRef]
- Hussain, T.; Sanchez, K.; Crayton, J.; Saha, D.; Jeter, C.; Lu, Y.; Abba, M.; Seo, R.; Noebels, J.L.; Fonken, L.; et al. WWOX P47T partial loss-of-function mutation induces epilepsy, progressive neuroinflammation, and cerebellar degeneration in mice phenocopying human SCAR12. Prog. Neurobiol. 2023, 223, 102425. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Ran, N.; Zhong, Y.; Yang, F.; Sun, H. A family with mental disorder as the first symptom finally confirmed with Gerstmann-Sträussler-Scheinker disease with P102L mutation in PRNP gene—Case report. Prion 2023, 17, 37–43. [Google Scholar] [CrossRef]
- Rasouli, O.; Fors, E.A.; Borchgrevink, P.C.; Ohberg, F.; Stensdotter, A.K. Gross and fine motor function in fibromyalgia and chronic fatigue syndrome. J. Pain. Res. 2017, 10, 303–309. [Google Scholar] [CrossRef]
- Doerr, J.M.; Fischer, S.; Nater, U.M.; Strahler, J. Influence of stress systems and physical activity on different dimensions of fatigue in female fibromyalgia patients. J. Psychosom. Res. 2017, 93, 55–61. [Google Scholar] [CrossRef]
- Souza, M.M.; Ansai, J.H.; da Silva, D.C.P.; Rossi, P.G.; Takahashi, A.C.M.; de Andrade, L.P. Can timed up and go subtasks predict functional decline in older adults with cognitive impairment? Dement. Neuropsychol. 2022, 16, 466–474. [Google Scholar] [CrossRef]
- Chou, S.J.; Tung, H.H.; Peng, L.N.; Chen, L.K. Timed Up and Go test and gastrointestinal disorders among hospitalized older adults with fall risk. Arch. Gerontol. Geriatr. 2023, 107, 104918. [Google Scholar] [CrossRef]
- Kataoka, Y.; Saito, Y.; Takeda, R.; Ishida, T.; Tadano, S.; Suzuki, T.; Nakamura, K.; Nakata, A.; Osuka, S.; Yamada, S.; et al. Evaluation of Lower-Limb Kinematics during Timed Up and Go (TUG) Test in Subjects with Locomotive Syndrome (LS) Using Wearable Gait Sensors (H-Gait System). Sensors (Basel) 2023, 23, 687. [Google Scholar] [CrossRef]
- Neto, I.V.S.; Diniz, J.S.; Alves, V.P.; Ventura Oliveira, A.R.; Barbosa, M.P.S.; da Silva Prado, C.R.; Alencar, J.A.; Vilaca, E.S.K.H.C.; Silva, C.R.; Lissemerki Ferreira, G.M.; et al. Field-Based Estimates of Muscle Quality Index Determine Timed-Up-and-Go Test Performance in Obese Older Women. Clin. Interv. Aging 2023, 18, 293–303. [Google Scholar] [CrossRef]
- Tulipani, L.J.; Meyer, B.; Fox, S.; Solomon, A.J.; McGinnis, R.S. The Sit-to-Stand Transition as a Biomarker for Impairment: Comparison of Instrumented 30-Second Chair Stand Test and Daily Life Transitions in Multiple Sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Lim, J.Y.; Assantachai, P.; Tanaka, T.; Kim, M.; Lee, S.Y.; Lim, W.S.; Arai, H. Five-repetition sit-to-stand test: End with the fifth stand or sit? Geriatr. Gerontol. Int. 2022, 22, 362–364. [Google Scholar] [CrossRef]
- Kacmaz, K.S.; Unver, B.; Karatosun, V. The Reliability and Validity of the Lie-to-Sit-to-Stand-to-Walk Transfer (LSSWT) Test in Knee Osteoarthritis. Indian. J. Orthop. 2023, 57, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Huynh, T.L.T.; Jones, C.D.; Feasel, C.D.; Jeng, B.; Motl, R.W. Validity of the 30-Second Sit-to-Stand test as a measure of lower extremity function in persons with multiple sclerosis: Preliminary evidence. Mult. Scler. Relat. Disord. 2023, 71, 104552. [Google Scholar] [CrossRef]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The fibromyalgia impact questionnaire: Development and validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar] [PubMed]
- Lupi, J.B.; Carvalho de Abreu, D.C.; Ferreira, M.C.; Oliveira, R.D.R.; Chaves, T.C. Brazilian Portuguese version of the Revised Fibromyalgia Impact Questionnaire (FIQR-Br): Cross-cultural validation, reliability, and construct and structural validation. Disabil. Rehabil. 2017, 39, 1650–1663. [Google Scholar] [CrossRef]
- Lee, M. Clinimetrics: The Revised Fibromyalgia Impact Questionnaire. J. Physiother. 2021, 67, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Bushmakin, A.G.; Cappelleri, J.C.; Chandran, A.B.; Zlateva, G. Evaluation of the fibromyalgia impact questionnaire at baseline as a predictor for time to pain improvement in two clinical trials of pregabalin. Int. J. Clin. Pract. 2013, 67, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aranda, A.; Feliu-Soler, A.; Mist, S.D.; Jones, K.D.; Lopez-Del-Hoyo, Y.; Olivan-Arevalo, R.; Kratz, A.; Williams, D.A.; Luciano, J.V. Subgrouping a Large U.S. Sample of Patients with Fibromyalgia Using the Fibromyalgia Impact Questionnaire-Revised. Int. J. Environ. Res. Public. Health 2020, 18, 247. [Google Scholar] [CrossRef]
- Pellegata, G.; Caracci, S.; Medaglini, S. Radio Electric Asymmetric Conveyer Neurobiological Treatments in Non-Specific Neck Pain: A Retrospective Study. J. Pain. Res. 2020, 13, 2451–2459. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, S.; Aravagli, L.; Mannu, P.; Castagna, A.; Margotti, M.L. Noninvasive radioelectric asymmetric brain stimulation in the treatment of stress-related pain and physical problems: Psychometric evaluation in a randomized, single-blind placebo-controlled, naturalistic study. Int. J. Gen. Med. 2011, 4, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Mannu, P.; Rinaldi, S.; Fontani, V.; Castagna, A.; Margotti, M.L. Radio electric treatment vs. Es-Citalopram in the treatment of panic disorders associated with major depression: An open-label, naturalistic study. Acupunct. Electrother. Res. 2009, 34, 135–149. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Moretti, E.; Rosettani, B.; Aravagli, L.; Sarago, G.; Collodel, G. A new approach on stress-related depression and anxiety: Neuro-Psycho- Physical-Optimization with Radio Electric Asymmetric-Conveyer. Indian. J. Med. Res. 2010, 132, 189–194. [Google Scholar]
- Pinheiro Barcessat, A.R.; Nolli Bittencourt, M.; Duarte Ferreira, L.; de Souza Neri, E.; Coelho Pereira, J.A.; Bechelli, F.; Rinaldi, A. REAC Cervicobrachial Neuromodulation Treatment of Depression, Anxiety, and Stress During the COVID-19 Pandemic. Psychol. Res. Behav. Manag. 2020, 13, 929–937. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, S.; Castagna, A.; Margotti, M.L. Noninvasive radioelectric asymmetric conveyor brain stimulation treatment improves balance in individuals over 65 suffering from neurological diseases: Pilot study. Ther. Clin. Risk Manag. 2012, 8, 73–78. [Google Scholar] [CrossRef]
- Mannu, P.; Rinaldi, S.; Fontani, V.; Castagna, A. Radio electric asymmetric brain stimulation in the treatment of behavioral and psychiatric symptoms in Alzheimer disease. Clin. Interv. Aging 2011, 6, 207–211. [Google Scholar] [CrossRef]
- Olazaran, J.; Gonzalez, B.; Osa-Ruiz, E.; Felipe-Ruiz, S.; Boyano, I.; Fontani, V.; Castagna, A.; Mendoza, C.; Zea, M.A.; Frades, B.; et al. Motor effects of radio electric asymmetric conveyer in Alzheimer’s disease: Results from a cross-over trial. J. Alzheimers Dis. 2014, 42, 325–332. [Google Scholar] [CrossRef]
- Lorenzini, L.; Giuliani, A.; Sivilia, S.; Baldassarro, V.A.; Fernandez, M.; Lotti Margotti, M.; Giardino, L.; Fontani, V.; Rinaldi, S.; Calza, L. REAC technology modifies pathological neuroinflammation and motor behaviour in an Alzheimer’s disease mouse model. Sci. Rep. 2016, 6, 35719. [Google Scholar] [CrossRef]
- Castagna, A.; Rinaldi, S.; Fontani, V.; Aravagli, L.; Mannu, P.; Margotti, M.L. Does osteoarthritis of the knee also have a psychogenic component? Psycho-emotional treatment with a radio-electric device vs. intra-articular injection of sodium hyaluronate: An open-label, naturalistic study. Acupunct. Electrother. Res. 2010, 35, 1–16. [Google Scholar] [CrossRef]
- Castagna, A.; Rinaldi, S.; Fontani, V.; Mannu, P.; Margotti, M.L. Comparison of two treatments for coxarthrosis: Local hyperthermia versus radio electric asymmetrical brain stimulation. Clin. Interv. Aging 2011, 6, 201–206. [Google Scholar] [CrossRef]
- Collodel, G.; Fioravanti, A.; Pascarelli, N.A.; Lamboglia, A.; Fontani, V.; Maioli, M.; Santaniello, S.; Pigliaru, G.; Castagna, A.; Moretti, E.; et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to IL-1beta. A biochemical and morphological study. Clin. Interv. Aging 2013, 8, 309–316. [Google Scholar] [CrossRef]
- Barcessat, A.R.P.; Bittencourt, M.N.; Pereira, J.A.C.; Castagna, A.; Fontani, V.; Rinaldi, S. REAC neurobiological treatments in acute post-traumatic knee medial collateral ligament lesion. Heliyon 2020, 6, e04539. [Google Scholar] [CrossRef]
- Fontani, V.; Castagna, A.; Rinaldi, S. The Reparative Effects of Radio Electric Asymmetric Conveyer Technology on Facial Injuries: A Report of Two Cases. Cureus 2022, 14, e26273. [Google Scholar] [CrossRef]
- Fontani, V.; Coelho Pereira, J.A.; Carrera Bittencourt, M.; Rinaldi, S. Radio Electric Asymmetric Conveyer (REAC) Reparative Effects on Pressure Ulcer (PU) and Burn Injury (BI): A Report of Two Cases. Cureus 2022, 14, e27060. [Google Scholar] [CrossRef]
- Fontani, V.; Coelho Pereira, J.A.; Rinaldi, S. Radio Electric Asymmetric Conveyer Tissue Reparative Treatment on Post-surgical Breast Skin Necrosis. A Report of Four Cases. Cureus 2022, 14, e25666. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Cruciani, S.; Necas, A.; Fontani, V.; Corda, G.; Santaniello, S.; Rinaldi, A.; Barcessat, A.; Necasova, A.; et al. Antisenescence effect of REAC biomodulation to counteract the evolution of myelodysplastic syndrome. Physiol. Res. 2022, 71, 539–549. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Pigliaru, G.; Santaniello, S.; Basoli, V.; Castagna, A.; Fontani, V.; Ventura, C. REAC technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci. Rep. 2016, 6, 28682. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Lotti Margotti, M.; Bagella, L.; Fontani, V.; Ventura, C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014, 36, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Gualini, S.; Fontani, V.; Ventura, C. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: A new tool for improving tissue regeneration. Cell Transplant. 2012, 21, 1225–1233. [Google Scholar] [CrossRef]
- Rinaldi, S.; Maioli, M.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Gualini, S.; Margotti, M.L.; Carta, A.; Fontani, V.; Ventura, C. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: An in vitro beta-galactosidase study. Clin. Interv. Aging 2012, 7, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Gualini, S.; Cavallini, C.; Fontani, V.; Ventura, C. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013, 22, 1227–1235. [Google Scholar] [CrossRef]
- Goncalves de Oliveira Cruz, A.V.; Goes Goncalves, R.; Nunes, L.; Douglas Quaresma de Oliveira, J.; Lima Monteiro, E.S.; Soares Eneias, I.; Guilherme Lima, T.C.; Duarte Ferreira, L.; Souza Neri, E.; da Cunha Pena, J.L.; et al. Neuro Postural Optimization Neuromodulation Treatment of Radio Electric Asymmetric Conveyer Technology on Stress and Quality of Life in Institutionalized Children in a Capital City of the Brazilian Amazon. Cureus 2022, 14, e26550. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.G.; Brun, A.B.S.; Manffra, E.F. Effects of the radio electric asymmetric conveyer (REAC) on motor disorders: An integrative review. Front. Med. Technol. 2023, 5, 1122245. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Aravagli, L.; Margotti, M.L. Psychological and symptomatic stress-related disorders with radio-electric treatment: Psychometric evaluation. Stress Health 2010, 26, 350–358. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Aravagli, L.; Mannu, P.; Castagna, A.; Margotti, M.L.; Rosettani, B. Stress-related psycho-physiological disorders: Randomized single blind placebo controlled naturalistic study of psychometric evaluation using a radio electric asymmetric treatment. Health Qual. Life Outcomes 2011, 9, 54. [Google Scholar] [CrossRef]
- Fontani, V.; Aravagli, L.; Margotti, M.L.; Castagna, A.; Mannu, P.; Rinaldi, S. Neuropsychophysical optimization by REAC technology in the treatment of: Sense of stress and confusion. Psychometric evaluation in a randomized, single blind, sham-controlled naturalistic study. Patient Prefer. Adherence 2012, 6, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Martins, M.C.M.; Maioli, M.; Rinaldi, S.; Fontani, V. REAC Noninvasive Neurobiological Stimulation in Autism Spectrum Disorder for Alleviating Stress Impact. Adv. Neurodev. Disord. 2022, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- World Medical. A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Menzies, V.; Lyon, D.E.; Archer, K.J.; Zhou, Q.; Brumelle, J.; Jones, K.H.; Gao, G.; York, T.P.; Jackson-Cook, C. Epigenetic alterations and an increased frequency of micronuclei in women with fibromyalgia. Nurs. Res. Pract. 2013, 2013, 795784. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, C.; Mohazab, N.; Redmond, D.; Yacyshyn, E.; Clifford, A.; Russell, A.S.; Osman, M.S.; Cohen Tervaert, J.W. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia: PR3-versus MPO-ANCA-associated vasculitis, an exploratory cross-sectional study. Lancet Reg. Health Am. 2023, 20, 100460. [Google Scholar] [CrossRef]
- Staud, R.; Spaeth, M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008, 13, 12–17. [Google Scholar] [CrossRef]
- Müller, M.; Wüthrich, F.; Federspiel, A.; Wiest, R.; Egloff, N.; Reichenbach, S.; Exadaktylos, A.; Jüni, P.; Curatolo, M.; Walther, S. Altered central pain processing in fibromyalgia-A multimodal neuroimaging case-control study using arterial spin labelling. PLoS ONE 2021, 16, e0235879. [Google Scholar] [CrossRef]
- Minhas, D.; Murphy, A.; Clauw, D.J. Fibromyalgia and centralized pain in the rheumatoid arthritis patient. Curr. Opin. Rheumatol. 2023, 35, 170–174. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Cassisi, G.; Sarzi-Puttini, P.; Casale, R.; Cazzola, M.; Boccassini, L.; Atzeni, F.; Stisi, S. Pain in fibromyalgia and related conditions. Reumatismo 2014, 66, 72–86. [Google Scholar] [CrossRef]
- Barjola, P.; Peláez, I.; Ferrera, D.; González-Gutiérrez, J.L.; Velasco, L.; Peñacoba-Puente, C.; López-López, A.; Fernandes-Magalhaes, R.; Mercado, F. Electrophysiological indices of pain expectation abnormalities in fibromyalgia patients. Front. Hum. Neurosci. 2022, 16, 943976. [Google Scholar] [CrossRef]
- Aicha, B.T.; Ines, C.; Olfa, S.; Selma, B.; Leila, R.; Rawdha, T.; Ines, M.; Leila, A. Central Sensitization in Spondyloarthritis: The crossroads between disease activity, health-related quality of life and Fibromyalgia. Curr. Rheumatol. Rev. 2023. [Google Scholar] [CrossRef]
- González-Vives, S.; Díaz-Marsá, M.; De la Vega, I.; Palomares, N.; Vázquez, S.; López-Villatoro, J.M.; Palomo, T.; Carrasco, J.L. Hypothalamic-pituitary axis response to a 0.25-MG dexamethasone test in women with fibromyalgia. Stress 2020, 23, 284–289. [Google Scholar] [CrossRef]
- Beiner, E.; Lucas, V.; Reichert, J.; Buhai, D.V.; Jesinghaus, M.; Vock, S.; Drusko, A.; Baumeister, D.; Eich, W.; Friederich, H.C.; et al. Stress biomarkers in individuals with fibromyalgia syndrome: A systematic review with meta-analysis. Pain 2022, 19, 10–1097. [Google Scholar] [CrossRef]
- Tak, L.M.; Cleare, A.J.; Ormel, J.; Manoharan, A.; Kok, I.C.; Wessely, S.; Rosmalen, J.G. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol. Psychol. 2011, 87, 183–194. [Google Scholar] [CrossRef]
- Stankiewicz, A.M.; Swiergiel, A.H.; Lisowski, P. Epigenetics of stress adaptations in the brain. Brain Res. Bull. 2013, 98, 76–92. [Google Scholar] [CrossRef]
- Polli, A.; Hendrix, J.; Ickmans, K.; Bakusic, J.; Ghosh, M.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Nijs, J.; Godderis, L. Genetic and epigenetic regulation of Catechol-O-methyltransferase in relation to inflammation in chronic fatigue syndrome and Fibromyalgia. J. Transl. Med. 2022, 20, 487. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Sahagun, D.; Schliebs, R.; Pallas, M. Editorial: Epigenetic Mechanisms Regulating Neural Plasticity. Front. Cell. Neurosci. 2019, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Ghosh, M.; Bakusic, J.; Ickmans, K.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Godderis, L.; Nijs, J. DNA Methylation and Brain-Derived Neurotrophic Factor Expression Account for Symptoms and Widespread Hyperalgesia in Patients With Chronic Fatigue Syndrome and Comorbid Fibromyalgia. Arthritis Rheumatol. 2020, 72, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Gerra, M.C.; Carnevali, D.; Pedersen, I.S.; Donnini, C.; Manfredini, M.; González-Villar, A.; Triñanes, Y.; Pidal-Miranda, M.; Arendt-Nielsen, L.; Carrillo-de-la-Peña, M.T. DNA methylation changes in genes involved in inflammation and depression in fibromyalgia: A pilot study. Scand. J. Pain. 2021, 21, 372–383. [Google Scholar] [CrossRef]
- Gerra, M.C.; Carnevali, D.; Ossola, P.; González-Villar, A.; Pedersen, I.S.; Triñanes, Y.; Donnini, C.; Manfredini, M.; Arendt-Nielsen, L.; Carrillo-de-la-Peña, M.T. DNA Methylation Changes in Fibromyalgia Suggest the Role of the Immune-Inflammatory Response and Central Sensitization. J. Clin. Med. 2021, 10, 4992. [Google Scholar] [CrossRef]
- Casale, R.; Sarzi-Puttini, P.; Botto, R.; Alciati, A.; Batticciotto, A.; Marotto, D.; Torta, R. Fibromyalgia and the concept of resilience. Clin. Exp. Rheumatol. 2019, 37, 105–113. [Google Scholar]
- Choy, E.H. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Tseng, A.S.; Levin, M. Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration. Anat. Rec. 2012, 295, 1541–1551. [Google Scholar] [CrossRef]
- McMillen, P.; Oudin, M.J.; Levin, M.; Payne, S.L. Beyond Neurons: Long Distance Communication in Development and Cancer. Front. Cell Dev. Biol. 2021, 9, 739024. [Google Scholar] [CrossRef]
- Mathews, J.; Levin, M. The body electric 2.0: Recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotechnol. 2018, 52, 134–144. [Google Scholar] [CrossRef]
- Levin, M. Technological Approach to Mind Everywhere: An Experimentally-Grounded Framework for Understanding Diverse Bodies and Minds. Front. Syst. Neurosci. 2022, 16, 768201. [Google Scholar] [CrossRef]
- Levin, M. The Biophysics of Regenerative Repair Suggests New Perspectives on Biological Causation. Bioessays 2020, 42, e1900146. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A.; Levin, M. Bioelectricity: A Quick Reminder of a Fast-Advancing Discipline! Bioelectricity 2020, 2, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Cervera, J.; Meseguer, S.; Levin, M.; Mafe, S. Bioelectrical model of head-tail patterning based on cell ion channels and intercellular gap junctions. Bioelectrochemistry 2020, 132, 107410. [Google Scholar] [CrossRef] [PubMed]
- Cervera, J.; Pietak, A.; Levin, M.; Mafe, S. Bioelectrical coupling in multicellular domains regulated by gap junctions: A conceptual approach. Bioelectrochemistry 2018, 123, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Casella, A.; Panitch, A.; Leach, J.K. Endogenous Electric Signaling as a Blueprint for Conductive Materials in Tissue Engineering. Bioelectricity 2021, 3, 27–41. [Google Scholar] [CrossRef] [PubMed]
| Mean ± Standard Deviation Before | Mean ± Standard Deviation After | p-Value | |
|---|---|---|---|
| TUG (time) | 12.53 ± 2.35 | 10.37 ± 1.67 | ≤0.001 |
| STS tests | 5.8 ± 1.27 | 7.5 ± 1.40 | ≤0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Barcessat, A.R.; Gonçalves, R.; Landre, C.; Brandão, L.; Nunes, L.; Feitosa, H.; Costa, L.; Silva, R.; de Lima, E.; et al. REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia. J. Pers. Med. 2023, 13, 902. https://doi.org/10.3390/jpm13060902
Silva A, Barcessat AR, Gonçalves R, Landre C, Brandão L, Nunes L, Feitosa H, Costa L, Silva R, de Lima E, et al. REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia. Journal of Personalized Medicine. 2023; 13(6):902. https://doi.org/10.3390/jpm13060902
Chicago/Turabian StyleSilva, Analízia, Ana Rita Barcessat, Rebeca Gonçalves, Cleuton Landre, Lethícia Brandão, Lucas Nunes, Hyan Feitosa, Leonardo Costa, Raquel Silva, Emanuel de Lima, and et al. 2023. "REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia" Journal of Personalized Medicine 13, no. 6: 902. https://doi.org/10.3390/jpm13060902
APA StyleSilva, A., Barcessat, A. R., Gonçalves, R., Landre, C., Brandão, L., Nunes, L., Feitosa, H., Costa, L., Silva, R., de Lima, E., Monteiro, E. S., Rinaldi, A., Fontani, V., & Rinaldi, S. (2023). REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia. Journal of Personalized Medicine, 13(6), 902. https://doi.org/10.3390/jpm13060902








