Abstract
Personalized care models are dominating modern medicine. These models are rooted in teaching future physicians the skill set to keep up with innovation. In orthopedic surgery and neurosurgery, education is increasingly influenced by augmented reality, simulation, navigation, robotics, and in some cases, artificial intelligence. The postpandemic learning environment has also changed, emphasizing online learning and skill- and competency-based teaching models incorporating clinical and bench-top research. Attempts to improve work–life balance and minimize physician burnout have led to work-hour restrictions in postgraduate training programs. These restrictions have made it particularly challenging for orthopedic and neurosurgery residents to acquire the knowledge and skill set to meet the requirements for certification. The fast-paced flow of information and the rapid implementation of innovation require higher efficiencies in the modern postgraduate training environment. However, what is taught typically lags several years behind. Examples include minimally invasive tissue-sparing techniques through tubular small-bladed retractor systems, robotic and navigation, endoscopic, patient-specific implants made possible by advances in imaging technology and 3D printing, and regenerative strategies. Currently, the traditional roles of mentee and mentor are being redefined. The future orthopedic surgeons and neurosurgeons involved in personalized surgical pain management will need to be versed in several disciplines ranging from bioengineering, basic research, computer, social and health sciences, clinical study, trial design, public health policy development, and economic accountability. Solutions to the fast-paced innovation cycle in orthopedic surgery and neurosurgery include adaptive learning skills to seize opportunities for innovation with execution and implementation by facilitating translational research and clinical program development across traditional boundaries between clinical and nonclinical specialties. Preparing the future generation of surgeons to have the aptitude to keep up with the rapid technological advances is challenging for postgraduate residency programs and accreditation agencies. However, implementing clinical protocol change when the entrepreneur–investigator surgeon substantiates it with high-grade clinical evidence is at the heart of personalized surgical pain management.
1. Introduction
Postgraduate medical education is evolving rapidly, driven by technological advancements, changes in healthcare delivery models, and shifting societal and cultural trends [1]. Traditional classroom-based learning is replaced with competency-based education [2,3,4,5,6,7,8], which focuses on developing specific skills and abilities to prepare surgical residents for today’s more complex healthcare environment. Postgraduate medical education is increasingly taking place online [9,10,11]. This trend also applies to practicing surgeons who have long since graduated. Now, online courses and training programs [12,13] sponsored by specialty organizations [14,15] and industry vendors [16] can be accessed from anywhere in the world, making education more accessible and convenient. The COVID-19 pandemic further solidified the use of online programs in postgraduate education [17,18,19,20]. Moreover, there is a growing emphasis on interprofessional collaboration to provide more comprehensive patient care. This collaborative patient-centered approach to healthcare is fueled by medical technology advancements, such as telemedicine, virtual reality [19,21,22,23,24], artificial intelligence applications [25,26,27] with wearable devices for skill-based simulations [28,29,30], electronic health records [31,32,33], and translational knowledge integration—all of which increase the demands on the next generation of orthopedic surgeons and neurosurgeons who now must be versed in the clinical application of these technologies to be effective in the delivery of the best possible care to patients in an increasingly cost-constrained environment characterized by an imbalance in clinical innovation and resource commitment. The authors of this editorialized perspective article came together to highlight the ongoing challenges in their educational programs and how the underlying changes in orthopedic and neurosurgery postgraduate training may impact personalized interventional and surgical pain management specialty care delivery in the future.
2. Shifting Trends
The international team of authors of this perspective article—many of whom are directors of postgraduate education programs in orthopedic surgery and neurosurgery—identified the shift toward competency-based training as the most significant trend altering the curriculum and culture of their programs [2,3,4,5,6,7,8]. Nowadays, proof of proficiency in specific skills is required before a trainee can progress to the next level of training [2]. This trend is playing out globally regardless of cultural differences and geographical boundaries. A quick straw poll among the authors also revealed that critical thinking with the application of a broad knowledge base rooted in evidence-based practice is also commonplace. There are, however, a few differences that are worth pointing out.
In Europe, trainees have more opportunities to undertake research projects and present at conferences with time allotted for these activities in specific rotations [18,19,23,29,30,34,35,36]. However, residency programs tend to be longer when compared to the United States, where such provisions are mandated but, in reality, uncommon. In 2020 a multinational survey by the European Association of Neurological Surgeons (EANS) reported a significant decline in surgical exposure during training from the 1970s to 2019 [37,38]. However, the reported results were doubted, and a whitewash of the data was anticipated by others [39]. It has to be assumed that this trend will be further accelerated by the new wage agreement that was passed in Germany in 2020 [38]. This wage agreement now limits the number of on-call services to a maximum of four per month, not only for residents but also for attendings [40]. The European authors of this editorial suggested that introducing new technologies, such as simulation training and virtual reality, to enhance surgical education is necessary and becoming more and more feasible because of enhanced collaboration across departmental and institutional barriers to providing more diverse and comprehensive training opportunities for trainees [29].
In Latin America, participating authors reported that program development is underway to focus on improving access to postgraduate education for surgeons in under-resourced areas [41,42,43]. This expansion is often performed on a national and international basis with the development of regional networks to promote collaboration and knowledge sharing between regional institutions and with institutions in other countries. Several of the coauthors of this perspective worked on improving postgraduate education in Orthopedics and Neurosurgery in that way. Several authors are corresponding foreign members of their country’s respective National Medical Academies. Online resources and Zoom teleconferencing with national and international faculty are heavily employed to overcome geographical barriers and enhance training opportunities [44,45]. These activities paved the way for more structured and standardized training programs in Latin America.
Our Asian coauthors reported rapidly expanding postgraduate training programs to meet the growing demand for surgical services. In southeast Asia and China, in particular, the population growth is forecast to continue until 2050 (Figure 1). The disease burden from painful musculoskeletal and spinal conditions is substantial and will likely rise (Figure 2 and Figure 3). Therefore, greater emphasis on subspecialization in spinal surgery or joint replacement is reported employing simulation training and 3D printing application in new surgical implants. Interinstitutional and international collaboration to gain access to resources and expertise, particularly in under-resourced areas, are attempted but still limited due to the postpandemic geopolitical restrictions on travel and internet access.
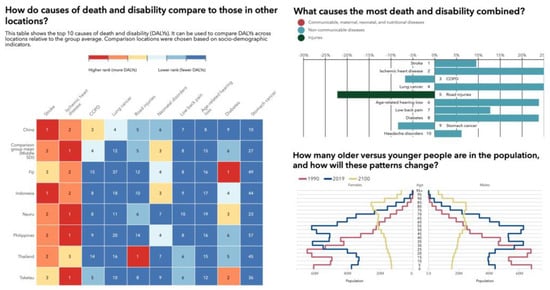
Figure 1.
Illustration of disease burden expressed in death and disability (DALYs) based on sociodemographic indicators across several locations in Southeast Asia and China relative to the group average, showing an aging population and a change in the order of the top 10 causes of death and disability (DALYs) in 2019 and percent change from 2009 to 2019 for all ages combined. In 2019, musculoskeletal conditions and low back pain were of much higher relevance to public healthcare systems than in 2009. Source: Institute for Health Metrics Evaluation. Used with permission. All rights reserved.
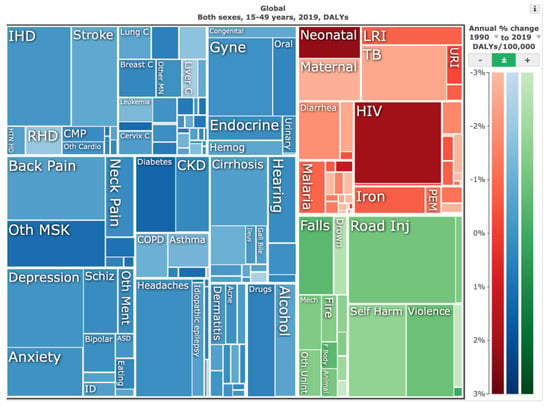
Figure 2.
Illustrative tree map of causes and global disease burden expressed in years lived with disability (YLDs) for both genders ages 15 to 49 years in 2019. YLDs for low back pain was 8.19% (6.3–10.19%), other musculoskeletal diseases 7.57% (5.54–10.08%), osteoarthritis 4.12% (2.41–7.65%), neck pain 2.12% (1.36–3.22%), falls 2.59% (2.28–2.97%), road injury 1.51% (1.37–1.67%), exposure to mechanical forces 0.88% (0.69–1.14%), interpersonal violence 0.49% (0.42%–0.56%), and other unintentional injuries 0.37% (0.3–0.47%). Source: Institute for Health Metrics Evaluation. Used with permission. All rights reserved.
Figure 3.
Infographic on shifting emphasis in orthopedic and neurosurgery residency program.
In the United States, a similar shift toward competency-based training, with milestones and objective assessments to track trainee progress, was introduced just as in Europe, with an increasing emphasis on patient safety and quality improvement and a greater focus on teamwork and communication skills [2,4,5,6,8].
Surgical education is increasingly technology-dominated by virtual and augmented reality simulators. Newer training pathways have emerged outside the traditional employment models in academia and industry. This trend is also occurring in European countries. For example, nearly 20% of German and Swiss medical school graduates do not start a postgraduate specialty training program but venture into the private sector for job opportunities, suggesting a particular frustration with low job prospects in comparison to the time investment needed to become a licensed physician [46,47]. The changing culture of postgraduate education in orthopedics and neurosurgery is characterized by a greater focus on competency-based training, increased use of technology, and greater collaboration between institutions and countries to provide more diverse and comprehensive training opportunities.
3. The Residents’ Perspective
Orthopedic and neurosurgery residents have been polled regarding their experiences with and perceptions of the modern learning environment [48]. Most respondents viewed the simulation and virtual reality technology as positive and opined that it improved their education [49]. However, a subset was concerned about losing autonomy and thought it could diminish the hands-on experience [50]. Previous reports suggest that many residents strongly desire better communication with the attending teaching surgeon with more detailed feedback about their performance, emphasizing the need for a more individualized learning experience. This notion differs from the current technology implementation trend in the postgraduate training process [51,52,53,54]. Being effectively trained and prepared for the real-world working scenarios of orthopedic surgeons and neurosurgeons required that adequate training took precedence over the desire for reduced work hours. However, work–life balance issues are relevant, making a case for more efficient learning scenarios. Hence, residents place value on attending physician mentorship, particularly when applied in hands-on patient encounters. One-on-one teaching experiences are still at the top of the list [55,56].
4. The Mentors Perspective
Mentoring is an integral part of any orthopedic and neurosurgery residency program [57]. Academic orthopedic surgeons and key opinion leaders play a crucial role in shaping the professional development of the residents they teach [58]. Conversely, mentors can enhance their knowledge and skills through mentoring, which often gives them insights into new techniques, procedures, and technologies. It may even force them to stay on top of the latest research and changes in up-to-date clinical practice protocols, thus improving the mentor’s professional development. Ideally, this interchange expands the mentor surgeon’s professional network by connecting with other mentors in the residency program and beyond, directly and indirectly, through coaching their resident mentees, potentially striking up new collaborations and opportunities for both the mentor and mentee [51]. As a result, teaching surgeons may find mentoring a rewarding experience with a sense of accomplishment and fulfillment in knowing that they played a role in their resident mentees’ success. Career advancement opportunities may open up for the mentor surgeon as they build a reputation as a respected and knowledgeable leader. As a result, mentor surgeons may be invited for speaking engagements, leadership positions, and other opportunities that can enhance their careers [57].
5. Work-Hour Restrictions
Work-hour restrictions have had a significant impact on surgeon training. Work hours for resident doctors in many countries are now mandated. One of the primary goals of work-hour restrictions was to improve resident well-being and reduce burnout and fatigue and improve quality of life [56,59]. Gone are the days were surgeons in training would learn their craft in a few years with higher surgical case volumes in routine practice or during the after-hours call. Today, residents have reduced exposure to patient care and surgical cases. Independent problem-solving and technical skills are harder to acquire because of fewer opportunities to perform surgeries. Hence, senior residents and attending staff physicians have to take on a greater supervisory role in the operating room, which can benefit some trainees but also limits their autonomy and independence. The reduction of resident work hours has also changed the clinical learning environment with the need for greater efficiencies in the learning process [60,61,62]. Where mentorship relationships between attending and resident trainee surgeons used to dominate hands-on skill acquisition, postgraduate programs are now forced to look increasingly at replacing these personal and interactive teaching scenarios [63] with more sophisticated didactic sessions and virtual surgical simulation models [5,30]. While there is no doubt that these new digital educational experiences can be helpful and should be implemented, more information is needed on whether these means of training the next generation of orthopedic surgeons and neurosurgeons are as effective as traditional methods [28]. Many program director authors of this perspective article are worried that virtual reality cannot fully replicate the clinical setting and that some trainees may feel they are missing out on a valuable hands-on experience due to work-hour restrictions.
High-quality postgraduate surgical training, at least in part, is influenced by the opportunity to perform surgeries and acquire experience and skills under the guidance of another proficient surgeon. Limiting patient encounters and surgical training opportunities by law may prompt the need to lower graduation and certification standards or increase the length of the residency to continue to provide the expected high-quality care to patients. To comply with the new wage agreement in Germany, for example, the number of residents eventually has to be increased. On 5 April 2023, a 16% resident work hour reduction was demanded by their union to be contractually mandated for the hospitals in the Kanton Zurich in Switzerland, where surgical residents are expected to work around 40 h a week [64]. Over 20 years ago, Reulen and März delineated that an annual surgery volume of 2100 neurosurgical operations allows training appropriately 7–8 residents [65]. However, the number of surgical procedures in residency programs performed per resident cannot be proportionally increased simultaneously. Unless the respective departments are expanded, residents and attendings will have fewer patient care encounters and less exposure to surgical procedures. Extending the duration of residency programs is openly discussed and has been implemented in some programs to ensure that residents are adequately prepared for independent practice [66]. However, longer training times may deter medical school graduates from becoming orthopedic surgeons or neurosurgeons. However, at this point, it is unclear whether or not this dynamic will impact the certification [67] and credentialing process [68]. Additional unintended consequences may arise from the German 2020 wage law. For example, reducing the earning potential for those surgeons who are used to more than four on-call services per month may drive them outside academic residency programs to look for more lucrative employment opportunities. Additional unintended consequences are possible.
As a result, the surgeon shortages experienced today may become more pronounced as a significant portion of currently practicing surgeons in these subspecialties are over the age of 55 and are thinking about retiring. Patient care could also be negatively impacted. Trainee surgeons may have less time to devote to each patient, which could hollow out the entire postgraduate training experience. There is some evidence to suggest that work-hour restrictions may lead to more errors and complications [61,68,69]. Hence, patient safety may suffer from reduced hands-on experience and longer training duration. The debate on these controversial issues will likely continue and further research is needed to identify the most effective postgraduate training models for the future.
6. Examples of Slow Adoption in Postgraduate Training
Delayed innovation implementation in orthopedics and neurosurgery has led to the slower adoption and integration of new technologies, procedures, or practices. For example, minimally invasive surgery (MIS) techniques employing various versions of small tubular and bladed retractor systems have been developed and proven effective in many orthopedic and neurosurgical procedures. Their widespread adoption and implementation in postgraduate training programs were initially slow. MIS procedures gained popularity in the early 2000s. Adoption delays were related to the learning curve of new surgical approaches, the need for specialized training, and the initial capital equipment costs required. It also took a few years until higher-grade clinical evidence emerged, proving their safety and effectiveness compared to traditional surgical techniques. Over time and with more experience, training programs have adapted to include MIS techniques because of the overwhelming benefits of reduced scarring, faster recovery, and improved patient outcomes that are being realized. Endoscopic spine surgery is a similar MIS example where adoption and integration into orthopedic and neurosurgical postgraduate training programs have been even slower. Both the retractor-based and endoscopic techniques emerged around the same time in the 1990s. However, endoscopic spinal decompression surgery is much harder to learn as it requires a higher skill level. The MIS retractor-based surgeries are minimized tissue-sparing versions of traditional translaminar open surgery and, therefore, easier understood by traditionally trained spine surgeons. Endoscopic decompression, mainly through the transforaminal approach, requires becoming accustomed to new surgical access unfamiliar to most spine surgeons and mastering eye–hand coordination integrating hand maneuvers with direct visualization of the surgical site on the video screen. Advances in imaging technology and 3D printing have allowed for the creation of patient-specific implants in orthopedic surgery. These implants are custom-designed to match the patient’s anatomy, resulting in better fit and improved outcomes. However, the implementation of patient-specific implants has been relatively slow due to the need for specialized software, equipment, and additional regulatory considerations. Therefore, it has not permeated postgraduate training programs. Robotics-assisted surgery is another such technology application, which has the potential to enhance precision and accuracy in orthopedic surgery and neurosurgery. However, the high costs associated with acquiring and maintaining robotic systems, as well as the need for specialized training, have contributed to the delayed implementation in many institutions. The added time required to learn the technology is difficult to carve out of an already packed postgraduate residency training program schedule. Regenerative strategies have been in preclinical and clinical trials for over two decades. Autologous chondrocyte harvesting followed by in vitro expansion and reimplantation has been practiced since the 1990s. However, these tissue engineering and cellular therapies did not find widespread use because of the many challenges related to regulatory approval and protocol standardization. Moreover, the limited clinical evidence is weaker than that of total joint replacement. As the benefits and effectiveness of these innovations become more established and the barriers to implementation are addressed, their integration into orthopedic surgery practices is expected to increase, ultimately improving patient outcomes and advancing the field.
7. Resident Clinical Research and Solutions
Program directors of this article worry about whether clinical research is still a priority of orthopedic and neurosurgery residents. In the United States, the Accreditation Council for Graduate Medical Education (ACGME) requires that orthopedic and neurosurgery residency programs provide structured educational experiences in research, including training in research methodology and data analysis. The ACGME also mandates that residents complete at least one research project during training and that each resident be provided with at least 60 days of protected time for research. While the pandemic has affected clinical research activities, recent studies found that residents and researchers have adapted to the situation by using virtual platforms to conduct meetings, research activities, and data analysis [20,70]. One particular study found that 83% of resident research thesis projects were published on average approximately 7 years from the start of their residency training. The graduate adjusted H-index was associated with increased success and decreased time to publication, while a lower journal impact factor was associated with taking significantly less time to reach publication. Coming out of the COVID-19 pandemic, clinical research activities are expected to extend from the virtual to in-person interactive platforms on the benchtop and clinical levels.
Research rotations may also provide residents with the skill set to quickly understand and adopt new technologies. Preparing the future generation of surgeons to have the aptitude to keep up with the rapid technological advances may be challenging for postgraduate residency programs but critical to solving the lag problem between innovation and implementation. Another solution to the fast-paced innovation cycle in orthopedic surgery and neurosurgery includes adaptive learning skills to seize opportunities for innovation with execution and implementation by facilitating translational research and clinical program development across traditional boundaries between clinical and nonclinical specialties.
8. Impact of Transformative Technologies and Targeted Care Models
The emerging technologies likely to impact orthopedic and neurosurgery residency programs within the next five years include robotics, augmented reality, 3D printing, artificial intelligence, nanotechnology, and regenerative technologies with stem cells and their respective stimulatory and growth factors. Robotic surgery has improved accuracy in total joint replacement component placement [71,72,73]. Navigation is another add-on technology with similar goals of improving patient outcomes by reducing inaccuracies in the surgical approaches and techniques thought to be the source of higher complication and revision surgery rates [74,75,76,77,78]. Depending on the application, navigation and augmented reality (AR) may be utilized separately or together [79,80,81,82]. Both technologies aim at more accurately navigating complex structures during surgery. Artificial intelligence (AI) may improve diagnostic accuracy and treatment plans based on large amounts of patient data to determine which painful or tumorous conditions may benefit from intervention and which not—a break with traditional laboratory or imaged-based medical necessity criteria for surgery [26,83,84,85]. AI could also assist surgeons during surgery, providing real-time feedback and guidance [86,87,88]. Three-dimensional printing will further facilitate and simplify manufacturing-customized implants and prosthetics [89,90,91]. The academic research conducted within postgraduate residency programs could show whether it cost-effectively improves clinical outcomes and lowers complication and revision rates [90]. Alternatively, 3D printing may also be used to create models of a patient’s anatomy to assist training programs in planning, teaching, and simulating complex surgeries. Nanotechnology may be applied in the targeted delivery of drugs and other therapeutic agents to treat affected tissues directly [92,93]. Regenerative technologies with the utilization of stem cells [94] and their respective growth factors have the potential to play a significant role in the future of orthopedic surgery and neurosurgery with nerve, cartilage, and bone regeneration to help with bone defects, spinal cord injuries, and peripheral nerve damage [95]. Tissue engineering technologies in conjunction with 3D printing [95] could play into regenerative strategies by producing newly formed replacement tissues. Stem cells may also play a role in chronic pain management [95,96,97]. These trends highlight the need for postgraduate training programs to incorporate education on these new technologies that provide the basis for more personalized and targeted care models aimed at the structural correlate causing the patient’s pain. Interventional and surgical pain management that incorporates these emerging technologies will likely improve clinical outcomes while reducing costs via lower complication and reoperation rates. On the other hand, the question is how these transformative technologies and targeted care models can be adopted and deployed in a cost-responsible and affordable, yet financially enabling, way.
9. Discussion
Emerging technologies will probably change the surgical indications for many painful chronic degenerative conditions and thus impact the array of surgical techniques taught in academic orthopedic and neurosurgery postgraduate residency programs as traditional methods are increasingly replaced by modern less burdensome and more targeted procedures. They may form the basis for future sound stewardship principles in public healthcare systems. This expected significant impact on the training of the next generation of orthopedic surgeons and neurosurgeons will require specialized knowledge of these advanced technologies and new skills as these technologies become more widely used in clinical practice. Thus, they will become part of the standard postgraduate residency training curriculum. Moreover, many of these technologies require collaboration between orthopedics, engineering, biology, neurosurgery, and many other disciplines. Hence, postgraduate residency programs, both on the clinical and research sides, need to adapt to this more multidisciplinary training approach by facilitating collaborations with healthcare professionals and researchers from different fields. The growing emphasis on regenerative medicine and other advanced technologies also requires the next-generation orthopedic surgeon and neurosurgeon to be more versed in basic and clinical research. Benchtop research skills and basic concepts of clinical trial design and execution, research methodology, data analysis, and other skills necessary for conducting clinical trials and other research studies, including skills in applying for and securing research funding, will have to be taught at a more sophisticated level. Finally, postgraduate training programs in orthopedic surgery and neurosurgery must nurture a culture of innovation consistent with common societal values of community advancement. The onus of research into new ways to treat painful conditions must fall on more than just scientists or those with specialized training outside the clinical arena. Instead, the key features of innovation must be ingrained in our training programs. One place to start is quality control and monitoring, where regulations are much more forgiving for the institution of new ideas in the care protocols surrounding patients. However, this type of innovation cannot be the sole purview of clinician educators. If left to quality measures alone, we would have efficient protocols but no new treatments. The fast-moving and constantly evolving nature of orthopedics and neurosurgery and the emergence of advanced technologies are likely to accelerate the innovation cycle. For this reason, the next generation of orthopedic surgeons and neurosurgeons will require the skills to stay up to date with the latest developments in their fields.
Residents have a demanding and often rigorous training schedule that can leave little time for personal pursuits. Balancing clinical duties, research, family obligations, and test preparation can be challenging and require excellent time-management and prioritization skills. The amount of time orthopedic residents have to accomplish these tasks can vary depending on various factors, including the specific residency program, the number of hours worked per week, and the resident’s work pace and efficiency. However, most residency programs struggle with allotting enough time for research and academic pursuits outside clinical duties. Still, the amount of time available may be limited. Program directors need to be prepared to teach some of these skills and, in some cases, act not just as mentors but as life coaches to whom resident trainees look up for advice and guidance on navigating the complex and rigorous orthopedic and neurosurgery training curriculum.
The future orthopedic surgeon and neurosurgeon involved in personalized surgical pain management will need to be versed in several disciplines, such as bioengineering, computers, basic research, clinical research including trial designs, epidemiological research, public health policy development, and economic accountability. Adaptive learning skills are needed to seize opportunities for innovation with execution and implementation by facilitating translational research and clinical program development across traditional boundaries between clinical and nonclinical specialties. Preparing the future generation of surgeons to have the aptitude to keep up with rapid technological advances is challenging for postgraduate residency programs and accreditation agencies. However, implementing clinical protocol change when the entrepreneur–investigator surgeon substantiates it with high-grade clinical evidence is at the heart of personalized surgical pain management.
10. Conclusions
The objectives in the orthopedic and neurosurgical residency core curriculum programs are changing mainly in response to the changes in healthcare delivery models and the rapid emergence of new technologies. Teaching programs are shifting emphasis to more skill- and competency-based teaching methods. Work-hour restrictions and attention to work–life balance issues may improve the mental health of residents by lowering burnout rates but necessitate higher efficiencies in teaching methodologies. Advanced AR simulation and 3D modeling techniques may be helpful, [98] but one-on-one interaction with their mentors in a postgraduate residency training program remains crucial. Residents should work closely with their program directors and mentors to establish a schedule that allows for efficient use of their time while still meeting the demands of their clinical and academic responsibilities. [99] They should also prioritize their tasks and delegate obligations where possible to ensure they can complete their duties without sacrificing their personal or professional goals. Ultimately, the ability to balance these various demands will vary from individual to individual and depend on multiple factors, including their work ethic, time-management skills, and support systems. Residency programs have to teach the next generation of orthopedic surgeons and neurosurgeons the life-long learning skills to position themselves effectively in the future personalized care models of surgical pain management.
Author Contributions
Conceptualization, K.-U.L., B.W.B., M.P.L., P.A.W., J.M.O., R.A.A.V. and A.E.T.; methodology, K.-U.L., B.W.B., M.P.L., J.F.R.L., R.K.A.F., I.A., P.S.T.d.C. and A.Y.; software, K.-U.L., I.A., B.W.B.; validation, K.-U.L., J.C.E., Z.-M.L., B.W.B., M.P.L., P.A.W., J.M.O., A.E.T., Á.D., R.A.A.V., R.R., I.A., M.A., H.Y., X.Z., J.F.R.L., R.K.A.F., M.G.P., P.S.T.d.C., H.D., K.T.L., J.M., H.-S.K., N.M., A.Y. and P.N.; formal analysis, K.-U.L., B.W.B., M.P.L., P.A.W., J.M.O., A.E.T., Á.D., R.A.A.V., R.R., I.A., M.A., P.S.T.d.C., N.M. and A.Y.; investigation, K.-U.L., B.W.B., M.P.L., P.A.W., J.M.O., A.E.T., Á.D., R.A.A.V., R.R., I.A., M.A., P.S.T.d.C., N.M. and A.Y.; resources, K.-U.L., B.W.B., M.P.L., P.A.W., J.M.O., A.E.T., Á.D., R.A.A.V., R.R., I.A., M.A., H.Y., X.Z., J.F.R.L., R.K.A.F., M.G.P., P.S.T.d.C., H.D., K.T.L., J.M., H.-S.K., N.M., A.Y. and P.N.; data curation, K.-U.L., B.W.B., Á.D., R.A.A.V., J.F.R.L., R.K.A.F. and P.S.T.d.C.; writing—original draft preparation K.-U.L., J.C.E., Z.-M.L., B.W.B., M.P.L., P.A.W., Á.D., M.A., J.F.R.L., R.K.A.F., P.S.T.d.C., H.D. and A.Y.; writing—review and editing, K.-U.L., J.C.E., Z.-M.L., B.W.B., M.P.L., P.A.W., J.M.O., A.E.T., Á.D., R.A.A.V., R.R., I.A., M.A., H.Y., X.Z., J.F.R.L., R.K.A.F., M.G.P., P.S.T.d.C., H.D., K.T.L., J.M., H.-S.K., N.M., A.Y. and P.N.; visualization, K.-U.L., B.W.B., M.P.L., R.K.A.F., P.S.T.d.C. and A.Y.; supervision, K.-U.L., B.W.B., M.P.L., R.K.A.F., P.S.T.d.C. and A.Y.; project administration, K.-U.L., B.W.B., M.P.L., R.K.A.F., P.S.T.d.C. and A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Hence, this perspective article not involving any humans did not require Institutional Review board approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are publicly available.
Acknowledgments
The authors acknowledge the peer-review and consensus work that was carried out by the authors of the respective endorsing surgeon societies who championed the review and approval process through their respective committees.
Conflicts of Interest
There was no formal funding by private, government or commercial funders. The participating clinical centers (Center For Advanced Spine Care of Southern Arizona—Tucson, Arizona, Department of Orthopaedic Surgery—The University of Arizona, Desert Institute of Spine Care—Phoenix, Arizona, Department of Orthopaedics, Fundación Universitaria Sanitas—Bogotá, D.C., Colombia, Department of Neurosurgery and Orthopaedics at Hospital Universitário Gaffre e Guinle, Universidade Federal do Estado do Rio de Janeiro—Rio de Janeiro, Brazil, Department of Orthopaedic Surgery, The First Affiliated Hospital of Soochow University, The American-British Cowdray Medical Center, Good Doctor Teun Teun Spine Hospital, Endoscopic Spine Clinic, Santiago, Chile, Department of Neurosurgery, Nanoori Hospital Gangnam Hospital, West China Hospital Sichuan University, The First Affiliated Hospital of Soochow University, supported with their internal resources the design and conduction of this study. They aided in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results. The authors declare no conflict of interest, and there was no personal circumstance or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. This research was not compiled to enrich anyone. It was merely intended to highlight challenges in postgraduate education and how they may impact the use of personalized care protocols in orthopedic surgery and neurosurgery.
References
- O’Brien, B.C.; Forrest, K.; Wijnen-Meijer, M.; ten Cate, O. A global view of structures and trends in medical education. In Understanding Medical Education: Evidence, Theory, and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 7–22. [Google Scholar]
- Alman, B.A.; Ferguson, P.; Kraemer, W.; Nousiainen, M.T.; Reznick, R.K. 49: Competency-based education: A new model for teaching orthopaedics. Instr. Course Lect. 2013, 62, 565–570. [Google Scholar]
- Harris, K.A.; Nousiainen, M.T.; Reznick, R. Competency-based resident education—The Canadian perspective. Surgery 2020, 167, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Nousiainen, M.; McQueen, S.; Hall, J.; Kraemer, W.; Ferguson, P.; Marsh, J.; Reznick, R.; Reed, M.; Sonnadara, R. Resident education in orthopaedic trauma: The future role of competency-based medical education. Bone Jt. J. 2016, 98, 1320–1325. [Google Scholar] [CrossRef]
- Nousiainen, M.T.; McQueen, S.A.; Ferguson, P.; Alman, B.; Kraemer, W.; Safir, O.; Reznick, R.; Sonnadara, R. Simulation for teaching orthopaedic residents in a competency-based curriculum: Do the benefits justify the increased costs? Clin. Orthop. Relat. Res. 2016, 474, 935–944. [Google Scholar] [CrossRef]
- Nousiainen, M.T.; Mironova, P.; Hynes, M.; Glover Takahashi, S.; Reznick, R.; Kraemer, W.; Alman, B.; Ferguson, P.; CBC Planning Committee. Eight-year outcomes of a competency-based residency training program in orthopedic surgery. Med. Teach. 2018, 40, 1042–1054. [Google Scholar] [CrossRef]
- Sonnadara, R.R.; Mui, C.; McQueen, S.; Mironova, P.; Nousiainen, M.; Safir, O.; Kraemer, W.; Ferguson, P.; Alman, B.; Reznick, R. Reflections on competency-based education and training for surgical residents. J. Surg. Educ. 2014, 71, 151–158. [Google Scholar] [CrossRef]
- Van Heest, A.E.; Armstrong, A.D.; Bednar, M.S.; Carpenter, J.E.; Garvin, K.L.; Harrast, J.J.; Martin, D.F.; Murray, P.M.; Peabody, T.D.; Saltzman, C.L. American Board of Orthopaedic Surgery’s Initiatives toward Competency-Based Education. JBJS Open Access 2022, 7, e21. [Google Scholar] [CrossRef]
- Choules, A. The use of elearning in medical education: A review of the current situation. Postgrad. Med. J. 2007, 83, 212–216. [Google Scholar] [CrossRef]
- Harden, R. Trends and the future of postgraduate medical education. Emerg. Med. J. 2006, 23, 798–802. [Google Scholar] [CrossRef]
- Teo, A. The current state of medical education in Japan: A system under reform. Med. Educ. 2007, 41, 302–308. [Google Scholar] [CrossRef]
- Bhashyam, A.R.; Dyer, G.S. “Virtual” boot camp: Orthopaedic intern education in the time of COVID-19 and beyond. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, e735–e743. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, M.P.; Lewis, K.O.; Berger, G. The role of electronic learning in orthopaedic graduate medical training: A consensus from leaders in orthopaedic training programs. JAAOS-J. Am. Acad. Orthop. Surg. 2021, 29, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Mun, F.; Suresh, K.V.; Pollak, A.N.; Morris, C.D. Professional Society Opportunities and Involvement for Early-Career Orthopaedic Surgeons. JAAOS-J. Am. Acad. Orthop. Surg. 2022, 31, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Clough, M. The Internet and the Orthopaedic Surgeon. In The Internet for Orthopaedists; Springer: Cham, Switzerland, 2003; pp. 50–62. [Google Scholar]
- Rogers, M.J.; Zeidan, M.; Flinders, Z.S.; Presson, A.P.; Burks, R. Educational resource utilization by current orthopaedic surgical residents: A nation-wide survey. JAAOS Glob. Res. Rev. 2019, 3, e041. [Google Scholar] [CrossRef]
- Chhabra, H.S.; Bagaraia, V.; Keny, S.; Kalidindi, K.K.V.; Mallepally, A.; Dhillon, M.S.; Malhotra, R.; Rajasekharan, S. COVID-19: Current knowledge and best practices for orthopaedic surgeons. Indian J. Orthop. 2020, 54, 411–425. [Google Scholar] [CrossRef]
- Dattani, R.; Morgan, C.; Li, L.; Bennett-Brown, K.; Wharton, R.M. The impact of COVID-19 on the future of orthopaedic training in the UK. Acta Orthop. 2020, 91, 627–632. [Google Scholar] [CrossRef]
- Hardie, J.; Green, G.; Bor, R.; Brennan, P. Cutting edge selection: Learning from high reliability organisations for virtual recruitment in surgery during the COVID-19 pandemic. Ann. R. Coll. Surg. Engl. 2021, 103, 385–389. [Google Scholar] [CrossRef]
- Kogan, M.; Klein, S.E.; Hannon, C.P.; Nolte, M.T. Orthopaedic education during the COVID-19 pandemic. J. Am. Acad. Orthop. Surg. 2020, 28, e456–e464. [Google Scholar] [CrossRef]
- Aïm, F.; Lonjon, G.; Hannouche, D.; Nizard, R. Effectiveness of virtual reality training in orthopaedic surgery. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 224–232. [Google Scholar] [CrossRef]
- Berton, A.; Longo, U.G.; Candela, V.; Fioravanti, S.; Giannone, L.; Arcangeli, V.; Alciati, V.; Berton, C.; Facchinetti, G.; Marchetti, A. Virtual reality, augmented reality, gamification, and telerehabilitation: Psychological impact on orthopedic patients’ rehabilitation. J. Clin. Med. 2020, 9, 2567. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Jain, V.K.; Vaishya, R. Virtual postgraduate orthopaedic practical examination: A pilot model. Postgrad. Med. J. 2021, 97, 650–654. [Google Scholar] [PubMed]
- Kamarudin, M.F.B.; Zary, N. Augmented Reality, Virtual Reality and Mixed Reality in Medical Education: A Comparative Web of Science Scoping Review. 2019. Available online: https://www.semanticscholar.org/paper/Augmented-Reality%2C-Virtual-Reality-and-Mixed-in-A-Kamarudin-Zary/dba7a6a58f96f6ffffe18975c1e106a8d5467c1c (accessed on 15 April 2023).
- Jayakumar, P.; Moore, M.L.; Bozic, K.J. Value-based healthcare: Can artificial intelligence provide value in orthopaedic surgery? Clin. Orthop. Relat. Res. 2019, 477, 1777. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.G.; Ramkumar, P.N.; Ricciardi, B.F.; Urish, K.L.; Kipper, J.; Ketonis, C. Artificial intelligence and orthopaedics: An introduction for clinicians. J. Bone Jt. Surgery. Am. 2020, 102, 830–840. [Google Scholar] [CrossRef]
- St Mart, J.-P.; Goh, E.L.; Liew, I.; Shah, Z.; Sinha, J. Artificial intelligence in orthopaedics surgery: Transforming technological innovation in patient care and surgical training. Postgrad. Med. J. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.K.; Scott, D.J.; Pedowitz, R.A.; Sweet, R.M.; Feins, R.H.; Deutsch, E.S.; Sachdeva, A.K. Best practices across surgical specialties relating to simulation-based training. Surgery 2015, 158, 1395–1402. [Google Scholar] [CrossRef]
- Milburn, J.; Khera, G.; Hornby, S.; Malone, P.; Fitzgerald, J. Introduction, availability and role of simulation in surgical education and training: Review of current evidence and recommendations from the Association of Surgeons in Training. Int. J. Surg. 2012, 10, 393–398. [Google Scholar] [CrossRef]
- Pedowitz, R.A.; Marsh, L.J. Motor skills training in orthopaedic surgery: A paradigm shift toward a simulation-based educational curriculum. JAAOS-J. Am. Acad. Orthop. Surg. 2012, 20, 407–409. [Google Scholar] [CrossRef]
- Hollenbeck, S.M.; Bomar, J.D.; Wenger, D.R.; Yaszay, B. Electronic medical record adoption: The effect on efficiency, completeness, and accuracy in an academic orthopaedic practice. J. Pediatr. Orthop. 2017, 37, 424–428. [Google Scholar] [CrossRef]
- Scott, D.J.; Labro, E.; Penrose, C.T.; Bolognesi, M.P.; Wellman, S.S.; Mather III, R.C. The impact of electronic medical record implementation on labor cost and productivity at an outpatient orthopaedic clinic. JBJS 2018, 100, 1549–1556. [Google Scholar] [CrossRef]
- Shaha, J.S.; El-Othmani, M.M.; Saleh, J.K.; Bozic, K.J.; Wright, J.; Tokish, J.M.; Shaha, S.H.; Saleh, K.J. The growing gap in electronic medical record satisfaction between clinicians and information technology professionals: Issues of most concern and suggested remediations. JBJS 2015, 97, 1979–1984. [Google Scholar] [CrossRef]
- Syed, S.; Mirza, A.H.; Ali, A. A brief comparison of orthopaedic training in English-speaking countries. Ann. R. Coll. Surg. Engl. 2009, 91, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Giddings, C.; Khera, G.; Marron, C. Improving the Future of Surgical Training and Education: Consensus Recommendations from the Association of Surgeons in Training; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 389–392. [Google Scholar]
- Fritz, T.; Stachel, N.; Braun, B.J. Evidence in surgical training—A review. Innov. Surg. Sci. 2019, 4, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Freyschlag, C.F.; Schaller, K.; Meling, T. Procedures performed during neurosurgery residency in Europe. Acta Neurochir 2020, 162, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.W.; Oertel, J.M.; Hendrix, P. Neurosurgical procedures performed during residency in Europe—Preliminary numbers and time trends. Acta Neurochir 2019, 161, 1975–1976. [Google Scholar] [CrossRef]
- Stienen, M.N.; Bartek, J., Jr.; Czabanka, M.A.; Freyschlag, C.F.; Kolias, A.; Krieg, S.M.; Moojen, W.; Renovanz, M.; Sampron, N.; Adib, S.D.; et al. Response to: Neurosurgical procedures performed during residency in Europe-preliminary numbers and time trends. Acta Neurochir 2019, 161, 1977–1979. [Google Scholar] [CrossRef]
- Tarifeinigung zum TV-Ärzte: Neue Vorgaben zur Arbeitszeit, Zusatzurlaub und Entgelterhöhungen. Available online: https://www.marburger-bund.de/bundesverband/tarifpolitik/tarifeinigung-zum-tv-aerzte-neue-vorgaben-zur-arbeitszeit-zusatzurlaub (accessed on 15 April 2023).
- Murguia-Fuentes, R.; Husein, N.; Vega, A.; Rangel-Castilla, L.; Rotta, J.M.; Quinones-Hinojosa, A.; Guinto, G.; Esquenazi, Y. Neurosurgical residency training in Latin America: Current status, challenges, and future opportunities. World Neurosurg. 2018, 120, e1079–e1097. [Google Scholar] [CrossRef]
- Beltrán, J.Q.; Ogando-Rivas, E.; Nettel-Rueda, B.; Velasco-Campos, F.; Navarro-Olvera, J.L.; Aguado-Carrillo, G.; Soriano-Sanchez, J.A.; Alpizar-Aguirre, A.; Carrillo-Ruiz, J.D. Women in neurosurgery: First neurosurgeon in Latin America and current Mexican leaders. World Neurosurg. 2021, 150, 114–120. [Google Scholar] [CrossRef]
- Zanon, N.; Niquen-Jimenez, M.; Kim, E.E.; Zegarra, A.B.; Ramírez-Reyes, A.G.; Quiroga, D.P.; Molina, E.I.M.; Santana, N.V.; Garcia, R.M.; Rosseau, G. Progress in neurosurgery: Contributions of women neurosurgeons in Latin America. J. Clin. Neurosci. 2021, 86, 347–356. [Google Scholar] [CrossRef]
- Rasouli, J.J.; Shin, J.H.; Than, K.D.; Gibbs, W.N.; Baum, G.R.; Baaj, A.A. Virtual spine: A novel, international teleconferencing program developed to increase the accessibility of spine education during the COVID-19 pandemic. World Neurosurg. 2020, 140, e367–e372. [Google Scholar] [CrossRef]
- Hussein, A.; Bauer, I.; Cavagnaro, M.; Farhadi, D.S.; Prim, M.; Baaj, A.; Orenday, J.M. Virtual learning in Neurosurgery During the COVID-19 Pandemic, A Systematic Literature Review. Pan Arab J. Neurosurg. 2022, 17, 3–8. [Google Scholar] [CrossRef]
- Drossard, S. Structured surgical residency training in Germany: An overview of existing training programs in 10 surgical subspecialties. Innov. Surg. Sci. 2019, 4, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Görgen, J. Berufswechsel als Arzt: Viele Wählen Alternative Berufsfelder. Available online: https://www.praktischarzt.ch/arzt/berufswechsel-als-arzt-viele-waehlen-alternative-berufsfelder/ (accessed on 6 March 2023).
- Marwan, Y.; Luo, L.; Toobaie, A.; Benaroch, T.; Snell, L. Operating Room Educational Environment in Canada: Perceptions of Surgical Residents. J. Surg. Educ. 2021, 78, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, L.O.; Ponce, B.A.; Worley, J.R.; Keeney, J.A. Mentorship in Orthopedics: A National Survey of Orthopedic Surgery Residents. J. Surg. Educ. 2018, 75, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Bartoletta, J.J.; Hinchcliff, K.; Rhee, P. Learner Preferences and Perceptions of Virtual Hand Surgery Education during the COVID-19 Pandemic. J. Hand Surg. Am. 2021, 48, 405.e1–405.e8. [Google Scholar] [CrossRef] [PubMed]
- Brook, E.M.; Hu, C.H.; Li, X.; Smith, E.L.; Matzkin, E.G. The influence of mentors in orthopedic surgery. Orthopedics 2020, 43, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Flint, J.H.; Jahangir, A.A.; Browner, B.D.; Mehta, S. The value of mentorship in orthopaedic surgery resident education: The residents’ perspective. J. Bone Jt. Surg. Am. 2009, 91, 1017–1022. [Google Scholar] [CrossRef]
- Hart, R.A.; Eltorai, A.E.M.; Yanney, K.; Marsh, J.L.; Mulcahey, M.K.; Daniels, A.H. Update on Mentorship in Orthopaedic Resident Education: A Report from the American Orthopaedic Association. J. Bone Jt. Surg. Am. 2020, 102, e20. [Google Scholar] [CrossRef]
- Winfrey, S.R.; Parameswaran, P.; Gerull, K.M.; LaPorte, D.; Cipriano, C.A. Effective Mentorship of Women and Underrepresented Minorities in Orthopaedic Surgery: A Mixed-Methods Investigation. JB JS Open Access 2022, 7, e22.00053. [Google Scholar] [CrossRef]
- Choo, K.J.; Arora, V.M.; Barach, P.; Johnson, J.K.; Farnan, J.M. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J. Hosp. Med. 2014, 9, 169–175. [Google Scholar] [CrossRef]
- Fabricant, P.D.; Dy, C.J.; Dare, D.M.; Bostrom, M.P. A narrative review of surgical resident duty hour limits: Where do we go from here? J. Grad. Med. Educ. 2013, 5, 19–24. [Google Scholar] [CrossRef]
- Akhigbe, T.; Zolnourian, A.; Bulters, D. Mentoring models in neurosurgical training: Review of literature. J. Clin. Neurosci. 2017, 45, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Mulcahey, M.K.; Waterman, B.R.; Hart, R.; Daniels, A.H. The role of mentoring in the development of successful orthopaedic surgeons. JAAOS-J. Am. Acad. Orthop. Surg. 2018, 26, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Boone, S.; Tan, L.; Dyrbye, L.N.; Sotile, W.; Satele, D.; West, C.P.; Sloan, J.; Oreskovich, M.R. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch. Intern. Med. 2012, 172, 1377–1385. [Google Scholar] [CrossRef]
- Bailit, J.L.; Weisberger, A.; Knotek, J. Resident job satisfaction and quality of life before and after work hour reform. J. Reprod. Med. 2005, 50, 649–652. [Google Scholar] [PubMed]
- Jena, A.B.; Farid, M.; Blumenthal, D.; Bhattacharya, J. Association of residency work hour reform with long term quality and costs of care of US physicians: Observational study. Bmj 2019, 366, l4134. [Google Scholar] [CrossRef]
- Whang, E.E.; Perez, A.; Ito, H.; Mello, M.M.; Ashley, S.W.; Zinner, M.J. Work hours reform: Perceptions and desires of contemporary surgical residents. J. Am. Coll. Surg. 2003, 197, 624–630. [Google Scholar] [CrossRef]
- Ransom, N.A.; Gollogly, S.; Lewandrowski, K.U.; Yeung, A. Navigating the learning curve of spinal endoscopy as an established traditionally trained spine surgeon. J. Spine Surg. 2020, 6 (Suppl. 1), S197–S207. [Google Scholar] [CrossRef]
- Huber, M. Zürcher Assistenzärzte kündigen den Gesamtarbeitsvertrag. Sie wollen pro Woche acht Stunden weniger arbeiten—Aber ist das realistisch? Neue Zürcher Zeitung. 2023. Available online: https://www.nzz.ch/zuerich/belastung-von-assistenzaerzten-42-statt-50-wochenstunden-verlangt-ld.1733149?reduced=true (accessed on 3 May 2023).
- Reulen, H.J.; März, U. 5 years’ experience with a structured operative training programme for neurosurgical residents. Acta Neurochir 1998, 140, 1197–1203. [Google Scholar] [CrossRef]
- Friedlaender, G.E. The 80-hour duty week: Rationale, early attitudes, and future questions. Clin. Orthop. Relat. Res. 2006, 449, 138–142. [Google Scholar] [CrossRef]
- Jeray, K.J.; Frick, S.L. A survey of resident perspectives on surgical case minimums and the impact on milestones, graduation, credentialing, and preparation for practice: AOA critical issues. J. Bone Jt. Surg. Am. 2014, 96, e195. [Google Scholar] [CrossRef]
- Hopmans, C.J.; den Hoed, P.T.; van der Laan, L.; van der Harst, E.; van der Elst, M.; Mannaerts, G.H.; Dawson, I.; Timman, R.; Wijnhoven, B.P.; JN, I.J. Impact of the European Working Time Directive (EWTD) on the operative experience of surgery residents. Surgery 2015, 157, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.D.; Kubiak, E.N.; Immerman, I.; Dicesare, P. The early effects of code 405 work rules on attitudes of orthopaedic residents and attending surgeons. J. Bone Jt. Surg. Am. 2005, 87, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.T.-Y.; Ling, S.K.-K.; Wong, R.M.-Y.; Ho, K.K.-W.; Chow, S.K.-H.; Cheung, L.W.-H.; Yung, P.S.-H. Impact of COVID-19 on orthopaedic clinical service, education and research in a university hospital. J. Orthop. Transl. 2020, 25, 125–127. [Google Scholar] [CrossRef]
- Sugano, N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin. Orthop. Surg. 2013, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, X.G.; Xu, Y.F.; Fan, M.X.; Zhao, J.W.; Liu, Y.J.; He, D.; Tian, W. Robotic navigation during spine surgery. Expert Rev. Med. Devices 2020, 17, 27–32. [Google Scholar] [CrossRef]
- McAfee, P.C.; Lieberman, I.H.; Theodore, N. Innovations in Robotics and Navigation, Part 2. Int. J. Spine Surg. 2022, 16 (Suppl. 2), S6–S7. [Google Scholar] [CrossRef] [PubMed]
- Mezger, U.; Jendrewski, C.; Bartels, M. Navigation in surgery. Langenbecks Arch. Surg. 2013, 398, 501–514. [Google Scholar] [CrossRef]
- Karkenny, A.J.; Mendelis, J.R.; Geller, D.S.; Gomez, J.A. The Role of Intraoperative Navigation in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e849–e858. [Google Scholar] [CrossRef]
- Kochanski, R.B.; Lombardi, J.M.; Laratta, J.L.; Lehman, R.A.; O’Toole, J.E. Image-Guided Navigation and Robotics in Spine Surgery. Neurosurgery 2019, 84, 1179–1189. [Google Scholar] [CrossRef]
- Huang, M.; Tetreault, T.A.; Vaishnav, A.; York, P.J.; Staub, B.N. The current state of navigation in robotic spine surgery. Ann. Transl. Med. 2021, 9, 86. [Google Scholar] [CrossRef]
- Hagan, M.J.; Remacle, T.; Leary, O.P.; Feler, J.; Shaaya, E.; Ali, R.; Zheng, B.; Bajaj, A.; Traupe, E.; Kraus, M.; et al. Navigation Techniques in Endoscopic Spine Surgery. Biomed. Res. Int. 2022, 2022, 8419739. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.; Mahapatra, S.; Weber-Levine, C.; Awosika, T.; Theodore, J.N.; Zakaria, H.M.; Liu, A.; Witham, T.F.; Theodore, N. Augmented Reality in Spine Surgery: A Narrative Review. HSS J. 2021, 17, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.A.; Hanna, G.; Ross, L.; Molina, C.; Urakov, T.; Johnson, P.; Kim, T.; Drazin, D. Augmented Reality in Spinal Surgery: Highlights From Augmented Reality Lectures at the Emerging Technologies Annual Meetings. Cureus 2021, 13, e19165. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, F.; Frantz, T.; Soomro, M.H.; Elprama, S.A.; Vannerom, C.; Jacobs, A.; Vandemeulebroucke, J.; Jansen, B.; Scheerlinck, T.; Duerinck, J. Augmented Reality-Assisted Neurosurgical Drain Placement (ARANED): Technical Note. Acta Neurochir Suppl. 2021, 131, 267–273. [Google Scholar]
- Yuk, F.J.; Maragkos, G.A.; Sato, K.; Steinberger, J. Current innovation in virtual and augmented reality in spine surgery. Ann. Transl. Med. 2021, 9, 94. [Google Scholar] [CrossRef]
- Panchmatia, J.R.; Visenio, M.R.; Panch, T. The role of artificial intelligence in orthopaedic surgery. Br. J. Hosp. Med. 2018, 79, 676–681. [Google Scholar] [CrossRef]
- Langerhuizen, D.W.G.; Janssen, S.J.; Mallee, W.H.; van den Bekerom, M.P.J.; Ring, D.; Kerkhoffs, G.; Jaarsma, R.L.; Doornberg, J.N. What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review. Clin. Orthop. Relat. Res. 2019, 477, 2482–2491. [Google Scholar] [CrossRef]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R., Jr.; Bihorac, A. Artificial Intelligence and Surgical Decision-making. JAMA Surg. 2020, 155, 148–158. [Google Scholar] [CrossRef]
- D’Antoni, F.; Russo, F.; Ambrosio, L.; Vollero, L.; Vadalà, G.; Merone, M.; Papalia, R.; Denaro, V. Artificial Intelligence and Computer Vision in Low Back Pain: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 10909. [Google Scholar] [CrossRef]
- Martin, R.K.; Ley, C.; Pareek, A.; Groll, A.; Tischer, T.; Seil, R. Artificial intelligence and machine learning: An introduction for orthopaedic surgeons. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 361–364. [Google Scholar] [CrossRef]
- Rohde, S.; Münnich, N. [Artificial intelligence in orthopaedic and trauma surgery imaging]. Orthopadie 2022, 51, 748–756. [Google Scholar] [PubMed]
- Cai, H.; Liu, Z.; Wei, F.; Yu, M.; Xu, N.; Li, Z. 3D Printing in Spine Surgery. Adv. Exp. Med. Biol. 2018, 1093, 345–359. [Google Scholar] [PubMed]
- Hasan, O.; Atif, M.; Jessar, M.M.; Hashmi, P. Application of 3D printing in orthopaedic surgery. A new affordable horizon for cost-conscious care. J. Pak. Med. Assoc. 2019, 69 (Suppl. 1), S46–S50. [Google Scholar]
- Zamborsky, R.; Kilian, M.; Jacko, P.; Bernadic, M.; Hudak, R. Perspectives of 3D printing technology in orthopaedic surgery. Bratisl. Lek. Listy 2019, 120, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; McHale, K.J.; Parvizi, J.; Mehta, S. Nanotechnology: Current concepts in orthopaedic surgery and future directions. Bone Jt. J. 2014, 96, 569–573. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Rajaram Manoharan, S.R.; Subramanian, S.; Moon, A. Nanotechnology in Spine Surgery: A Current Update and Critical Review of the Literature. World Neurosurg. 2019, 123, 142–155. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem. Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Thomas, C.N.; Mavrommatis, S.; Schroder, L.K.; Cole, P.A. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury 2022, 53, 977–983. [Google Scholar] [CrossRef]
- Suresh, D.; Aydin, A.; James, S.; Ahmed, K.; Dasgupta, P. The Role of Augmented Reality in Surgical Training: A Systematic Review. Surg. Innov. 2022, 2022, 15533506221140506. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, S.A.; Behzadpour, V.; McCormack, T.J.; Cline, J.A.; Willis, J.T.; Mendez, G.M.; Zackula, R.E.; Dart, B.R.; Hearon, B.F. Improving Medical Student Mentorship in Orthopaedic Surgery. Kans. J. Med. 2023, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).