Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women
Abstract
1. Introduction
2. Patients and Methods
2.1. Setting and Study Design
2.2. Patients
2.3. Study Protocol and Follow-Up
2.4. Clinical Outcome Measurements
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Recruited Patients
3.2. Study Outcomes
3.3. Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, A.A.; et al. ART in Europe, 2017: Results generated from European registries by ESHRE. Hum. Reprod. Open 2021, 2021, hoab026. [Google Scholar] [PubMed]
- Zhang, T.; Li, Z.; Ren, X.; Huang, B.; Zhu, G.; Yang, W.; Jin, L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Med. Baltim. 2018, 97, e9689. [Google Scholar] [CrossRef] [PubMed]
- Blesa, D.; Ruiz-Alonso, M.; Simon, C. Clinical management of endometrial receptivity. Semin. Reprod. Med. 2014, 32, 410–413. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, X.Y.; Chan, C. The association between endometrial thickness and pregnancy outcome in gonadotropin-stimulated intrauterine insemination cycles. Reprod. Biol. Endocrinol. 2019, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, P.; Bai, H.; Shi, W.; Shi, J. Endometrial thickness as a predictor of ectopic pregnancy in 1125 in vitro fertilization-embryo transfer cycles: A matched case-control study. Arch. Gynecol. Obstet. 2019, 300, 1797–1803. [Google Scholar] [CrossRef]
- Groenewoud, E.R.; Cohlen, B.J.; Al-Oraiby, A.; Brinkhuis, E.A.; Broekmans, F.J.M.; de Bruin, J.P.; van Dool, G.; Fleisher, K.; Friederich, J.; Goddijn, M.; et al. Influence of endometrial thickness on pregnancy rates in modified natural cycle frozen-thawed embryo transfer. Acta Obstet. Gynecol. Scand. 2018, 97, 808–815. [Google Scholar] [CrossRef]
- Zolghadri, J.; Haghbin, H.; Dadras, N.; Behdin, S. Vagifem is superior to vaginal Premarin in induction of endometrial thickness in the frozen-thawed cycle patients with refractory endometria: A randomized clinical trial. Iran. J. Reprod. Med. 2014, 12, 415–420. [Google Scholar]
- Wang, X.; Liu, L.; Mou, S.; Zhao, H.; Fang, J.; Xiang, Y.; Zhao, T.; Sha, T.; Ding, J.; Hao, C. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J. Cell. Biochem. 2019, 120, 7403–7411. [Google Scholar] [CrossRef]
- Hajishafiha, M.; Dehghan, M.; Kiarang, N.; Sadegh-Asadi, N.; Shayegh, S.N.; Ghasemi-Rad, M. Combined letrozole and clomiphene versus letrozole and clomiphene alone in infertile patients with polycystic ovary syndrome. Drug Des. Dev. Ther. 2013, 7, 1427. [Google Scholar]
- Al-Omari, W.; Sulaiman, W.; Al-Hadithi, N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2004, 85, 289–291. [Google Scholar] [CrossRef]
- Gadipudi, A.; Dasari, P.; Sagili, H. Tamoxifen for ovulation induction in infertile PCOS women who did not conceive with 3 or more cycles of clomiphene citrate: A prospective clinical study. Fertil. Sci. Res. 2017, 4, 22. [Google Scholar]
- Balen, A.H. Ovulation induction in the management of anovulatory polycystic ovary syndrome. Mol. Cell. Endocrinol. 2013, 373, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Behnoud, N.; Farzaneh, F.; Ershadi, S. The effect of clomiphene citrate versus letrozole on pregnancy rate in women with polycystic ovary syndrome: A randomized clinical trial. Crescent J. Med. Biol. Sci. 2019, 6, 335–340. [Google Scholar]
- Scaglione, F.; Donde, S.; Hassan, T.A.; Jannini, E.A. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: Pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin. Ther. 2017, 39, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Jerzak, M.; Kniotek, M.; Mrozek, J.; Gorski, A.; Baranowski, W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil. Steril. 2008, 90, 1848–1853. [Google Scholar] [CrossRef]
- Groenewoud, E.R.; Cantineau, A.E.; Kollen, B.J.; Macklon, N.S.; Cohlen, B.J. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 255–261. [Google Scholar] [CrossRef]
- Lane, A. “Effect of Vaginal Sildenafil on the Outcome of In Vitro Fertilization (IVF) After Multiple IVF Failures Attributed to Poor En-dometrial Development”(2002), by Geoffrey Sher and Jeffrey Fisch. In Embryo Project Encyclopedia; Arizona State University: Tempe, AZ, USA, 2020. [Google Scholar]
- Fanchin, R.; Cunha-Filho, J.S.; Schonäuer, L.M.; Righini, C.; de Ziegler, D.; Frydman, R. Luteal estradiol administration strengthens the relationship between day 3 follicle-stimulating hormone and inhibin B levels and ovarian follicular status. Fertil. Steril. 2003, 79, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Salomon, L.; Castelo-Branco, A.; Olivennes, F.; Frydman, N.; Frydman, R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum. Reprod. 2003, 18, 2698–2703. [Google Scholar] [CrossRef]
- Feng, W.; Nie, L.; Wang, X.; Yang, F.; Pan, P.; Deng, X. Effect of Oral versus Vaginal Administration of Estradiol and Dydrogesterone on the Proliferative and Secretory Transformation of Endometrium in Patients with Premature Ovarian Failure and Preparing for Assisted Reproductive Technology. Drug Des. Dev. Ther. 2021, 15, 1521. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 27–53. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A simple formula for the calculation of sample size in pilot studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam ESHRE/ASRM–Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS). Hum Reprod. 2012, 27, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Adams, J.; Mason, H.; Polson, D. Ovulatory disorders in women with polycystic ovary syndrome. Clin. Obstet. Gynecol. 1985, 12, 605–632. [Google Scholar] [CrossRef]
- Kistner, R.W. Induction of ovulation with clomiphene citrate (clomid). Obstet. Gynecol. Surv. 1965, 20, 873–900. [Google Scholar] [CrossRef]
- Mitwally, M.F.; Casper, R.F. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil. Steril. 2001, 75, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Wessman, A.; McArdle, C.R.; Achiron, R. Ultrasound in infertility. In Infertility—A Comprehensive Text, 2nd ed.; Machelle, M., Seibel, M.D., Eds.; Appleton and Lange: Stamford, CT, USA, 1996; pp. 447–492. [Google Scholar]
- Badawy, A.; State, O.; Abdelgawad, S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: A cross-over trial. Acta Obstet. Gynecol. Scand. 2007, 86, 218–222. [Google Scholar] [CrossRef]
- Mitwally, M.F.; Casper, R.F. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs 2006, 66, 2149–2160. [Google Scholar] [CrossRef]
- Reynolds, K.; Khoury, J.; Sosnowski, J.; Thie, J.; Hofmann, G. Comparison of the effect of tamoxifen on endometrial thickness in women with thin endometrium (<7 mm) undergoing ovulation induction with clomiphene citrate. Fertil. Steril. 2010, 93, 2091–2093. [Google Scholar]
- Alnemr, A.A.; Ammar, I.M.; Aboelfath, A.M.; Talaat, B. Effect of estradiol valerate on the pregnancy rate in patients receiving letrozole for induction of ovulation. Middle East Fertil. Soc. J. 2018, 23, 131–136. [Google Scholar] [CrossRef]
- Groothuis, P.G.; Dassen, H.H.; Romano, A.; Punyadeera, C. Estrogen and the endometrium: Lessons learned from gene expression profiling in rodents and human. Hum. Reprod. Update 2007, 13, 405–417. [Google Scholar] [CrossRef]
- Kortam, M.F.; Mohammad, H.F.; Mobarak, M.H.; Bazazo, A.I. The effect of estradiol valerate with and without oral sildenafil on endometrial thickness and pregnancy rates in infertile women: A RCT. Evid. Based Womens Health J. 2018, 8, 5. [Google Scholar] [CrossRef]
- Benni, J.M.; Patil, P.A. An overview on sildenafil and female infertility. Indian J. Health Sci. Biomed. Res. KLEU 2016, 9, 131. [Google Scholar] [CrossRef]
- Isong, I.; Okhormhe, Z.; Eze-Bassey, I.; Okpokam, D.C.; Usoro, C.A.O. Levels of prolactin, progesterone, estradiol, luteinizing hormone and follicle stimulating hormone in infertile women in Calabar, Nigeria. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 804. [Google Scholar] [CrossRef]
- Sefrioui, O.; Madkour, A.; Kaarouch, I.; Louanjli, N. Luteal estradiol pretreatment of poor and normal responders during GnRH antagonist protocol. Gynecol. Endocrinol. 2019, 35, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Davar, R.; Hoseini, M.; Saeed, L. Vaginal compared oral administration of estradiol in women with thin endometrium: A cross-sectional study. Int. J. Reprod. BioMedicine (IJRM) 2020, 19, 591–596. [Google Scholar] [CrossRef]
- Chua, S.J.; Danhof, N.A.; Mochtar, M.H.; Van Wely, M.; McLernon, D.J.; Custers, I.; Lee, E.; Dreyer, K.; Cahill, D.J.; Gillett, W.R.; et al. Age-related natural fertility outcomes in women over 35 years: A systematic review and individual participant data meta-analysis. Hum. Reprod. 2020, 35, 1808–1820. [Google Scholar] [CrossRef]
- Koo, H.S.; Song, I.O.; Cha, S.H.; Park, C.W.; Kim, H.O. The likelihood of achieving pregnancy through timed coitus in young infertile women with decreased ovarian reserve. Clin. Exp. Reprod. Med. 2018, 45, 31. [Google Scholar] [CrossRef]
- Frank-Herrmann, P.; Jacobs, C.; Jenetzky, E.; Gnoth, C.; Pyper, C.; Baur, S.; Freundl, G.; Goeckenjan, M.; Strowitzki, T. Natural conception rates in subfertile couples following fertility awareness training. Arch. Gynecol. Obstet. 2017, 295, 1015–1024. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, I.; Cho, S.; Kim, H.I.; Baek, H.W.; Lee, J.H.; Park, Y.J.; Kim, H.; Yun, B.H.; Seo, S.K.; et al. Predictive Factors of Conception and the Cumulative Pregnancy Rate in Subfertile Couples Undergoing Timed Intercourse with Ultrasound. Front. Endocrinol. 2021, 12, 650883. [Google Scholar] [CrossRef]
- Fetih, A.; Habib, D.; Abdelaal, I.; Hussein, M.; Fetih, G.; Othman, E. Adding sildenafil vaginal gel to clomiphene citrate in infertile women with prior clomiphene citrate failure due to thin endometrium: A prospective self-controlled clinical trial. Facts Views Vis. ObGyn 2017, 9, 21. [Google Scholar]
- Berman, J.; Berman, L.; Toler, S.; Gill, J.; Haughie, S. Sildenafil Study Group Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: A double-blind, placebo controlled study. J. Urol. 2003, 170 Pt 1, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Basson, R.; McInnes, R.; Smith, M.D.; Hodgson, G.; Koppiker, N. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J. Women’s Health Gend.-Based Med. 2002, 11, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Barnhart, H.X.; Schlaff, W.D.; Carr, B.R.; Diamond, M.P.; Carson, S.A.; Steinkampf, M.P.; Coutifaris, C.; McGovern, P.G.; Cataldo, N.A.; et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2007, 356, 551–566. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Group 1 (n = 48) | Group 2 (n = 50) | Control Group (n = 50) | p-Value |
|---|---|---|---|---|
| Age | ||||
| Age in years (mean ± SD) | 27.6 ± 3.84 | 26 ± 3.72 | 31.4 ± 4.79 | 0.230 |
| Age range in years | 20–35 | 19–34 | 22–39 | |
| Age Categories | ||||
| Age from 18 to 24 number (%) | 12 (25) | 14 (28) | 7 (14) | 0.003 * |
| Age From 25 to 29 number (%) | 19 (40) | 25 (50) | 14 (28) | |
| Age From 30 to 34 number (%) | 15 (31) | 11 (22) | 17 (34) | |
| Age above 35 number (%) | 2 (4) | 0 (0) | 12 (24) | |
| Body Mass Index (BMI) | ||||
| BMI in Kg/m2 (mean ± SD) | 23.5 ± 3.38 | 24.7 ± 2.87 | 28.6 ± 1.3 | 0.184 |
| BMI range in Kg/m2 | 18–35 | 19–34 | 27–31 | |
| Type of infertility | ||||
| Primary infertility number (%) | 24 (50) | 22 (44) | 19 (38) | 0.489 |
| Secondary infertility number (%) | 24 (50) | 28 (56) | 31 (62) | |
| Duration of infertility | ||||
| Duration of infertility in years (mean ± SD) | 2.3 ± 1.18 | 2.4 ± 1.17 | 2.3 ± 1.19 | 0.164 |
| Infertility duration > 2 years as n (%) | 17 (35) | 19 (38) | 12 (24) | 0.283 |
| Characteristic | Group 1 (n = 48) | Group 2 (n = 50) | Control Group (n = 50) | p-Value |
|---|---|---|---|---|
| Endometrial Thickness and Number of Follicles | ||||
| Number of follicles as n (%) | 0.038 * | |||
| One | 33 (69) | 38 (76) | 45 (90) | |
| Two | 13 (27) | 11 (22) | 3 (6) | |
| Three | 2 (4) | 1 (2) | 2 (4) | |
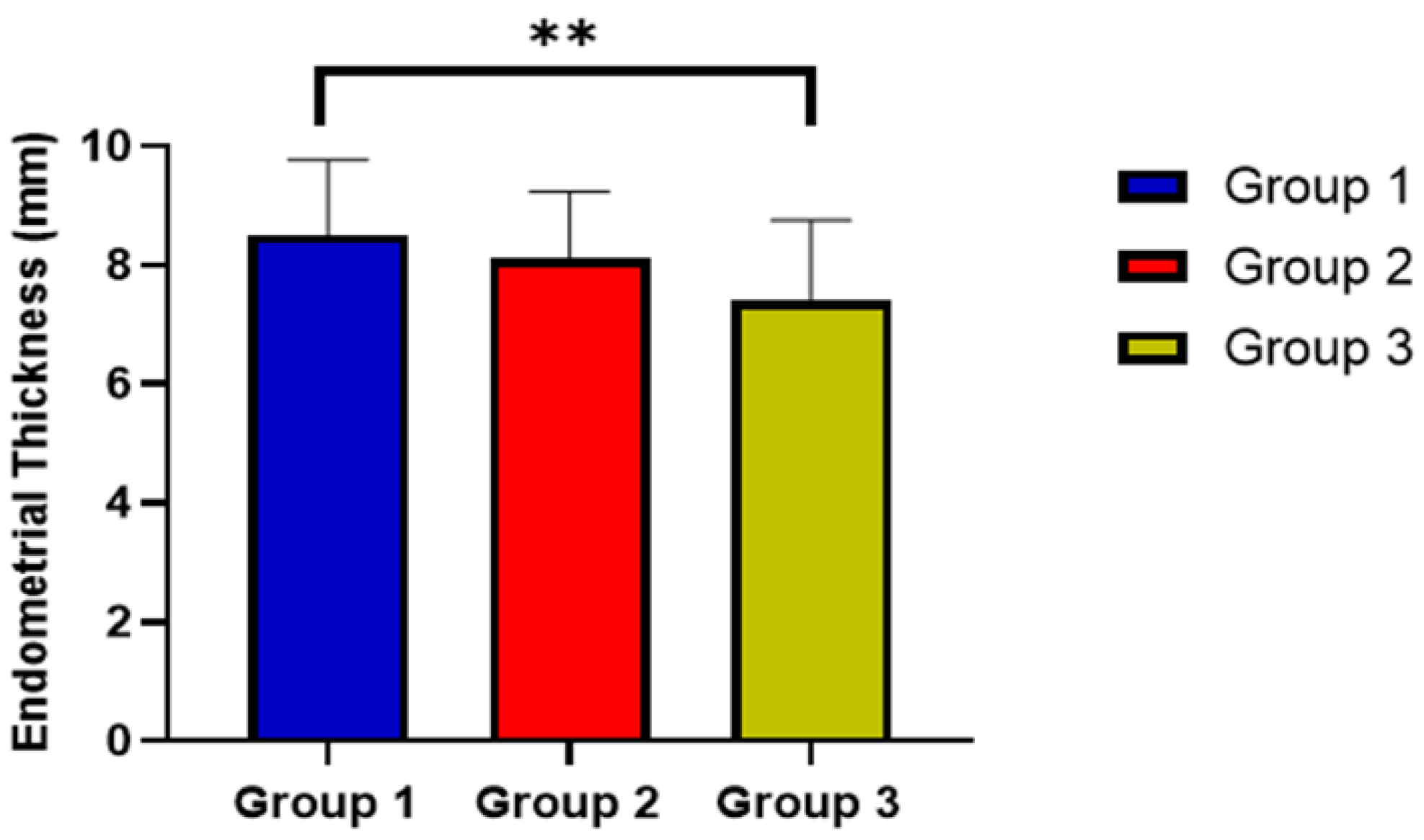
| Endometrial thickness, mean ± SD | 8.5 ± 1.27 | 8.1 ± 1.14 | 7.4 ± 1.35 | 0.0004 * |
| Ovulation | ||||
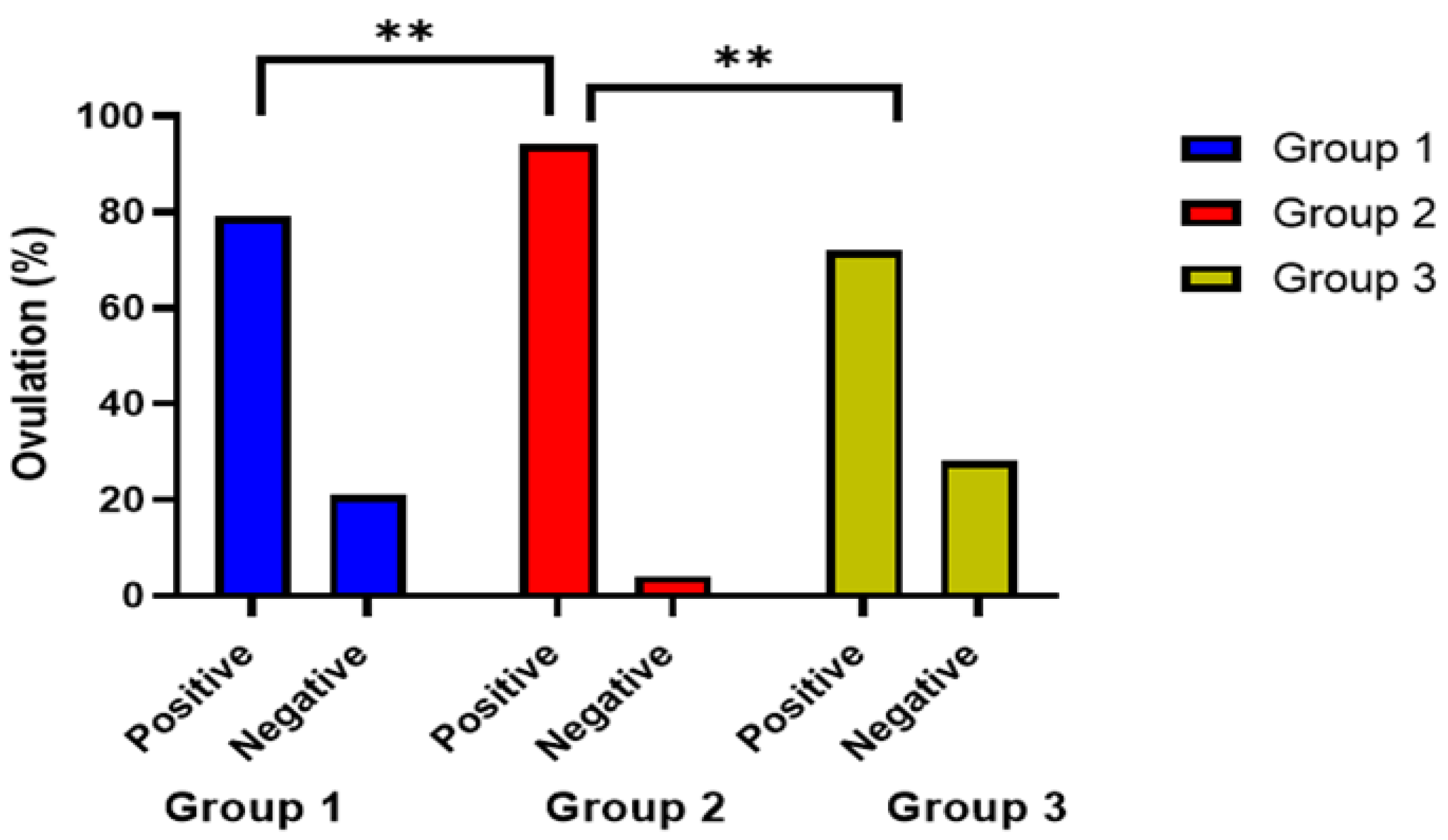
| Positive no. (%) | 38 (79) | 48 (96) | 36 (72) | 0.007 * |
| Negative no. (%) | 10 (21) | 2 (4) | 14 (28) | |
| Pregnancy | ||||
| Positive no. (%) | 28 (58) | 23 (46) | 13 (26) | 0.005 * |
| Negative no. (%) | 20 (42) | 27 (54) | 37 (74) | |
| Treatment Group | Subgroup | Endometrial Thickness in mm Mean ± SD | p-Value |
|---|---|---|---|
| Group 1 | Infertility ≤ 2 years | 8.6 ± 1.11 | 0.003 * |
| Infertility > 2 years | 8.2 ± 1.54 | ||
| Group 2 | Infertility ≤ 2 years | 7.5 ± 1.49 | |
| Infertility > 2 years | 7.2 ± 1.61 | ||
| Control group | Infertility ≤ 2 years | 8.0 ± 0.98 | |
| Infertility > 2 years | 8.5 ± 1.04 |
| Subgroup | Subpopulation | Adjusted Significance |
|---|---|---|
| Group 1; infertility ≤ 2 years | Group 1; infertility > 2 years | 0.405 |
| Group 2; infertility ≤ 2 years | 0.001 * | |
| Group 2; infertility > 2 years | 0.001 * | |
| Group 3; infertility > 2 years | 0.061 | |
| Group 3; infertility ≤ 2 years | 0.820 | |
| Group 1; infertility > 2 years | Group 2; infertility ≤ 2 years | 0.065 |
| Group 2; infertility > 2 years | 0.031 * | |
| Group 3; infertility > 2 years | 0.509 | |
| Group 3; infertility ≤ 2 years | 0.641 | |
| Group 2; infertility ≤ 2 years | Group 2; infertility > 2 years | 0.545 |
| Group 3; infertility > 2 years | 0.123 | |
| Group 3; infertility ≤ 2 years | 0.029 * | |
| Group 2; infertility > 2 years | Group 3; infertility > 2 years | 0.056 |
| Group 3; infertility ≤ 2 years | 0.014 * | |
| Group 3; infertility > 2 years | Group 3; infertility ≤ 2 years | 0.258 |
| Treatment Group | Subgroup | Clinical Pregnancy n (%) | p-Value |
|---|---|---|---|
| Group 1 | Infertility ≤ 2 years | 20 (41.6) | 0.003 * |
| Infertility > 2 years | 8 (16.6) | ||
| Group 2 | Infertility ≤ 2 years | 16 (32) | |
| Infertility > 2 years | 7 (14) | ||
| Control group | Infertility ≤ 2 years | 9 (18) | |
| Infertility > 2 years | 4 (8) |
| Side Effects No. (%) | Group 1 | Group 2 | Group 3 | p-Value |
|---|---|---|---|---|
| Headache no. (%) | 5 (10.4%) | 3 (6%) | 1 (2%) | 0.231 |
| Flushing no. (%) | 3 (6.3%) | 2 (4%) | 2 (4%) | |
| Blurring vision no. (%) | 0 (0%) | 6 (12%) | 2 (4%) | |
| GIT upset no. (%) | 4 (8.3%) | 7 (14%) | 1 (2%) |
| Variable | Treatment Groups | |
|---|---|---|
| Lambda Value (λ) | p-Value | |
| Type of infertility (1ry or 2ry) | 0.031 | 0.723 |
| Ovulation (positive or negative) | 0.058 | 0.114 |
| Clinical pregnancy (yes or no) | 0.142 | 0.117 |
| Overall side effects (yes or no) | 0.09 | 0.166 |
| Endometrial Thickness | Number of Follicles | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Control Group | Group 1 | Group 2 | Control Group | |||||||
| ρ | p-Value | ρ | p-Value | Ρ | p-Value | Ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age in years | −0.375 | 0.009 * | 0.012 | 0.935 | 0.14 | 0.925 | −0.111 | 0.451 | −0.014 | 0.922 | −0.021 | 0.883 |
| BMI in Kg/m2 | −0.03 | 0.983 | 0.009 | 0.951 | 0.096 | 0.506 | 0.196 | 0.182 | −0.441 | 0.001 * | 0.018 | 0.903 |
| Duration of infertility in tears | 0.05 | 0.734 | −0.182 | 0.206 | 0.121 | 0.404 | −0.071 | 0.632 | −0.296 | 0.037 * | −0.129 | 0.371 |
| Predictors | Model 1 Endometrial Thickness | Model 2 Number of Follicles | Model 3 Clinical Pregnancy | |||
|---|---|---|---|---|---|---|
| ꞵ (S.E) | p-Value | ꞵ (S.E) | p-Value | ꞵ (S.E) | p-Value | |
| Age | 0.35 (0.57) | 0.0001 * | −0.031 (0.01) | 0.71 | −0.21 (0.08) | 0.003 * |
| Body mass index (BMI) | −0.16 (0.55) | 0.002 * | −0.27 (0.13) | 0.041 * | −0.174 (0.01) | 0.013 * |
| Infertility duration | −0.15 (0.53) | 0.0003 * | −0.15 (0.03) | 0.079 | 0.06 (0.01) | 0.37 |
| Endometrial thickness | ------ | ------ | 0.05 (0.03) | 0.070 | 0.54 (0.02) | 0.0001 * |
| Intercept | 1.252 | 0.005 * | 1.739 | 0.005 * | 0.09 | 0.79 |
| R2 | 0.552 | 0.15 | 0.39 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altyar, A.E.; Boshra, M.S.; Abou Warda, A.E.; Shawkey, S.M.; Abdallah Mohamed Salem, S.; Sarhan, R.M.; Sarhan, N. Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women. J. Pers. Med. 2023, 13, 842. https://doi.org/10.3390/jpm13050842
Altyar AE, Boshra MS, Abou Warda AE, Shawkey SM, Abdallah Mohamed Salem S, Sarhan RM, Sarhan N. Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women. Journal of Personalized Medicine. 2023; 13(5):842. https://doi.org/10.3390/jpm13050842
Chicago/Turabian StyleAltyar, Ahmed E., Marian S. Boshra, Ahmed Essam Abou Warda, Sherwet M. Shawkey, Sara Abdallah Mohamed Salem, Rania M. Sarhan, and Neven Sarhan. 2023. "Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women" Journal of Personalized Medicine 13, no. 5: 842. https://doi.org/10.3390/jpm13050842
APA StyleAltyar, A. E., Boshra, M. S., Abou Warda, A. E., Shawkey, S. M., Abdallah Mohamed Salem, S., Sarhan, R. M., & Sarhan, N. (2023). Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women. Journal of Personalized Medicine, 13(5), 842. https://doi.org/10.3390/jpm13050842







