Causes of Polyserositis: A Systematic Review
Abstract
1. Introduction
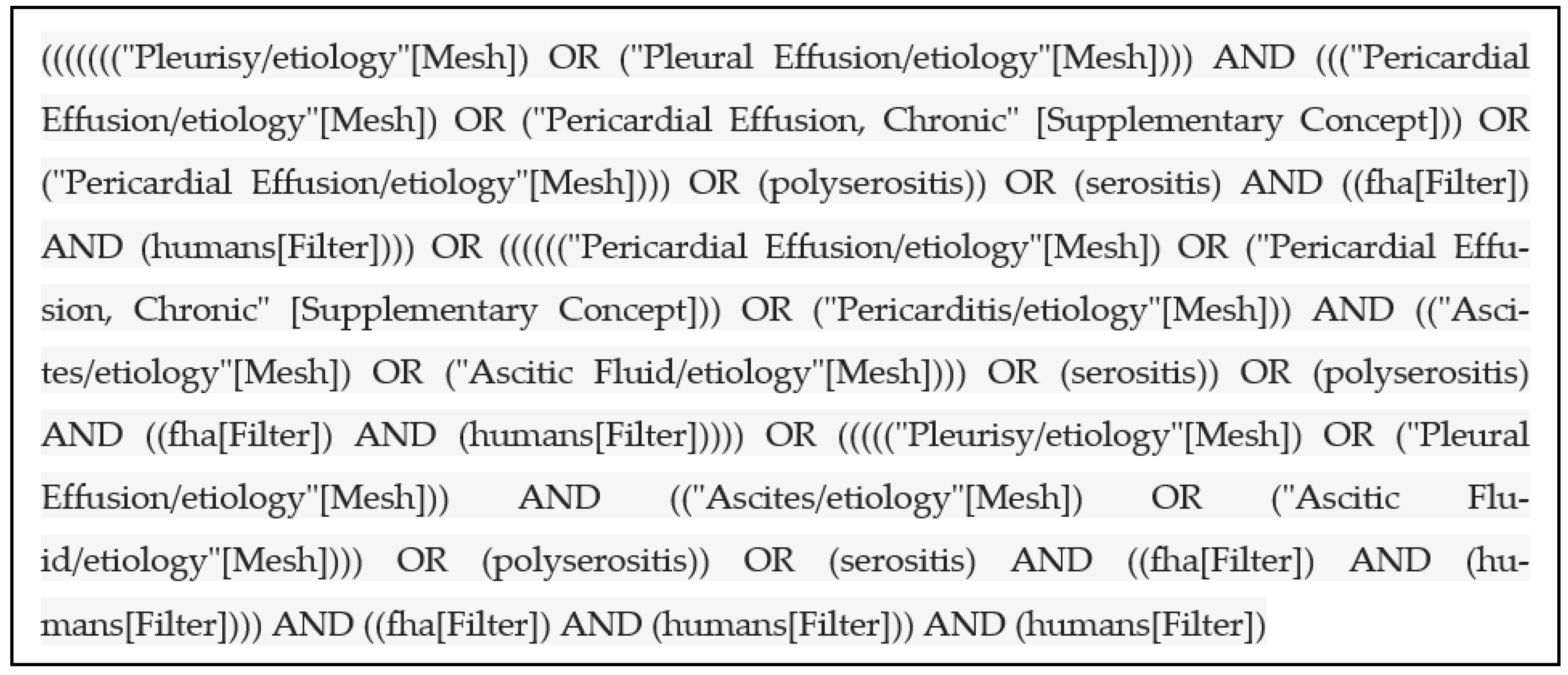
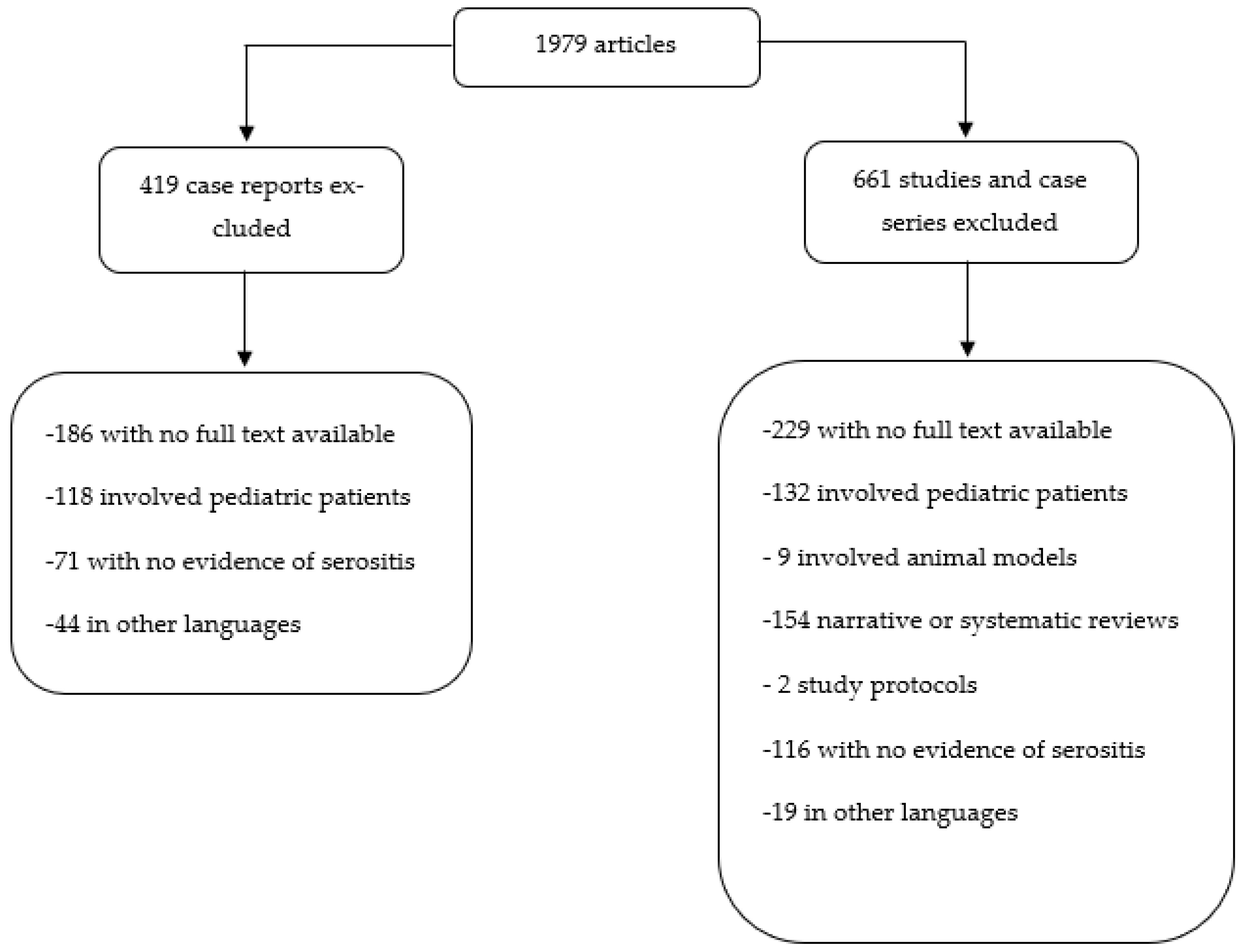
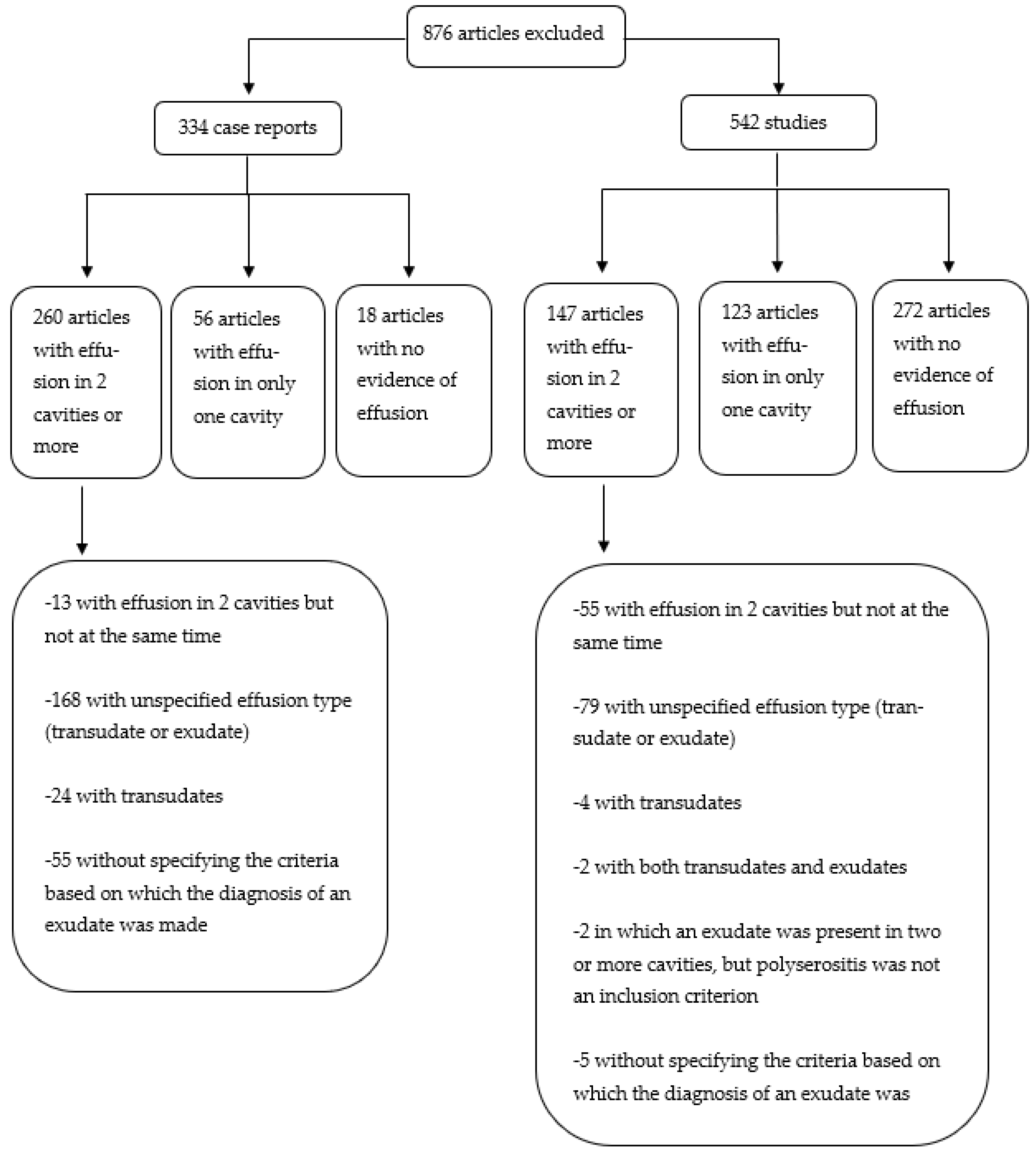
2. Materials and Methods
3. Results
| Ref | G | Diagnosis | Serosal Involvement | Means of Diagnosis | Differential Diagnosis | Treatment | Follow-Up |
|---|---|---|---|---|---|---|---|
| [24] | F | Adult Still’s disease | Pleura (bilateral)–pericardium–peritoneum | Positive Cush and Yamaguchi criteria, bone marrow biopsy, axillary lymph node biopsy, exploratory laparoscopy | Infectious diseases, autoimmune diseases, neoplastic processes | CS, Cyclophosphamide, paracentesis | Successfully treated, no recurrence for four years |
| [21] | F | SLE | Pleura (left)–peritoneum | SLICC criteria, bone marrow biopsy, fluid analysis, exploratory laparoscopy | Other autoimmune diseases, tuberculosis, neoplasia | CS, mycophenolate mofetil, granulocyte colony stimulating factor | At 6 months, residual minimal left-sided pleural effusion |
| [25] | F | Dermatomyositis | Pleura (bilateral)–peritoneum | Typical skin manifestations, elevated muscle enzymes, muscle weakness, and myogenic pattern on electromyography, lower limbs MRI | Autoimmune diseases, infectious diseases, tuberculosis | CS, Cyclophosphamide, Tacrolimus, intravenous immunoglobulins | No recurrence of pleural effusion or ascites after 70 days |
| [26] | M | Rheumatoid arthritis | Pleura (bilateral)–pericardium–peritoneum | Clinical manifestations, ultrasound, pleural biopsy, subcutaneous nodules biopsies, imagistic investigations, fluid analysis | Autoimmune diseases, infectious diseases, neoplasia | CS, methotrexate, NSAIDs | Disease remission |
| [22] | M | SLE | Pleura (bilateral)–peritoneum | Clinical manifestations, serology, fluid analysis | Other autoimmune diseases, hypothyroidism | CS | Remission |
| [8] | M | Adult Still’s disease | Pleura (bilateral)–pericardium | Yamaguchi criteria exclusion of autoimmune diseases | Autoimmune disease | CS, intravenous immunoglobulins | Remission |
| [23] | M | SLE | Pleura (bilateral)–pericardium | Clinical manifestations, serology, imagistic investigations, fluid analysis | Heart failure, other autoimmune diseases, infectious diseases, neoplasia | CS, mycophenolate mofetil | Serositis improvement |
| [29] | F | Acute myelogenous leukemia with myeloid sarcoma | Pleura (bilateral)–pericardium | Axillary lymph node biopsy, bone marrow biopsy, flow cytometric and cytogenetic analysis of both effusions | Other types of hematological neoplasms | Antibiotics, antifungals, cytostatic regimens | Death after 69 days |
| [18] | F | Pseudo-Meigs Syndrome associated to ovary leiomyoma | Pleura (right)–peritoneum | Fluid analysis, CT scan, omental biopsy, histopathological analysis of the mass arising from the left ovary | Infectious diseases, heart failure, constrictive pericarditis, cirrhosis, hypothyroidism, neoplastic processes | Surgery (histerectomy and bilateral salpingo-oophorectomy), drainage of the pleural effusion | Complete resolution of PS |
| [27] | F | Primary effusion lymphoma | Pleura (bilateral)–pericardium | Imagistic investigations, effusion analysis (including cytology, immunohistochemistry, and FISH analysis) | Autoimmune diseases, hypothyroidism, infectious diseases | Pleural drainage, pericardial effusion resolved with the first pleural drainage | One unilateral recurrence which resolved with drainage, and without any additional recurrence |
| [28] | M | Mycobacterium tuberculosis infection after orthotopic liver transplantation | Pleura (unilateral)–peritoneum | Fluid analysis with positive Mycobacterium tuberculosis PCR, but negative cultures | Other bacterial and viral infections | Isoniazid, ethambutol, streptomycin, ciprofloxacin | No recurrence of serositis |
| [17] | F | Meigs syndrome | Pleura (right)–peritoneum | Fluid analysis with elevated CA-125, exploratory laparotomy, histopathological analysis of the mass arising from the left ovary | nd | Pleural abrasion with talc pleurodesis; histerectomy and bilateral salpingo-oophorectomy | No recurrence of pleural effusion 12 weeks after surgery |
| [12] | F | Meigs syndrome | Pleura (bilateral)–pericardium–peritoneum | CT scan, fluid analysis, histopathological analysis of the mass arising from one ovary | Neoplastic processes, infectious diseases | Right pleural drainage, histerectomy and bilateral salpingo-oophorectomy | No relapse 8 months after surgery |
| [9] | M | Drug-induced lupus to dasatinib | Pleura (bilateral)–pericardium | Presence of autoantibodies (ANA, anti-RNP/Sm) which became undetectable after switching to Nilotinib, natural evolution (remission of effusion with dasatinib discontinuation, and reoccurrence with dasatinib resumption | Malignancy, other autoimmune diseases, tuberculosis | CS, mycophenolate mofetil, diuretics, pleural drainage | Remission |
| [19] | F | Hypothyroidism | Pleura (left)–pericardium | Low levels of fT4 and pathologically elevated TSH during continued high-dose methimazole, exclusion of other causes | Neoplastic, infectious, and autoimmune diseases, heart failure | Discontinuation of methimasole dose, short course of levothyroxine, diuretics | Nearly remission of PS at 3 months |
| [20] | F | Drug-induced lupus secondary to trimethoprim/sulfamethoxazole | Pleura (bilateral)–pericardium | Recent administration of trimethoprim/sulfamethoxazole, fluid analysis, serology (anti-histone antibody positivity) | Autoimmune and infectious diseases | Short course of CS; pericardiocentesis (Cardiac tamponade) | PS remission 6 months after discharge |
| [13] | F | Ergotamine-induced serositis | Pleura (bilateral)–pericardium | Prolonged use of ergotamine and caffeine, exclusion of other causes, fluid analysis, pleural biopsy | Malignancy, autoimmune diseases, infectious diseases (including tuberculosis) | NSAIDs, colchicine, stopping ergotamine | PS remission4 months after discharge |
| [16] | F | Ovarian hyperstimulation syndrome | Pleura (right)–peritoneum | History (recent ovarian hyper stimulation), abdominal ultrasound (enlarged ovaries with multiple follicular cysts), pleural effusion analysis | nd | Pleural drainage | Remission |
| [10] | M | Graft versus host disease after allogenic hematopoietic stem cell transplantation | Pleura (bilateral)–peritoneum | Exclusion of other causes, associated bronchiolitis obliterans | Malignancy, infectious diseases | Inhaled CS, antibiotics | Remission of PS |
| [14] | F | Polyserositis induced by the 13-valent pneumococcal conjugated vaccine | Pleura (left)–pericardial | Exclusion of other causes, case history (temporal association with recent administration of vaccine), presence of a persistent local reaction to the 13-valent pneumococcal conjugated vaccine | Autoimmune diseases, infectious diseases, malignancy, autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) | CS, pericardial and pleural drainage | No recurrence for one and a half year since discharge |
| [15] | M | Idiopathic (labeled by the authors as “inflammatory ascites”) | Pleura (not mentioned if unilateral or bilateral)–pericardium–ascites | Exclusion of all other causes | Neoplasia, hypothyroidism, infectious diseases, autoimmune diseases | CS, paracentesis | Complete resolution after one month |
| [11] | M | Disseminated cryptococcosis | Pleura (right)–peritoneum | Blood cultures, serum, pleural, peritoneal and cerebrospinal fluid positive for cryptococcal antigen; transbronchial lung biopsy from pulmonary lesions | nd | Antifungals, antibiotics (for later superimposed bacterial infection) | Death |
| Serosal Involvement | Etiology | Number |
|---|---|---|
| Pleura–pericardium (n = 84) | Autoimmune | 13 |
| SLE | 8 | |
| Adult Still’s disease | 2 | |
| Sjogren’s syndrome | 1 | |
| Ankylosing spondylitis | 1 | |
| IgG4 related disease | 1 | |
| Neoplasm | 19 | |
| Lung adenocarcinoma | 9 | |
| Lymphoma | 4 | |
| Leukemia | 1 | |
| Other lung neoplasm | 2 | |
| Other | 3 | |
| Infectious | 14 | |
| Coxsackie virus | 6 | |
| Cytomegalovirus | 1 | |
| Epstein-Barr virus | 1 | |
| Coxiella burnetti | 1 | |
| Mycobacterium tuberculosis | 1 | |
| Other | 4 | |
| Idiopathic | 32 | |
| Other | 6 | |
| Pleura–ascites (n = 22) | Neoplasm | 9 |
| Ovarian adenocarcinoma | 2 | |
| Gastric adenocarcinoma | 2 | |
| Lymphoma | 1 | |
| Other | 4 | |
| Autoimmune | 4 | |
| SLE | 3 | |
| Dermatomyositis | 1 | |
| Infectious | 2 | |
| Mycobacterium tuberculosis | 1 | |
| Cryptococcus neoformans | 1 | |
| Other | 7 | |
| Pericardium-ascites (n = 1) | Neoplasm | 1 |
| Pancreatic adenocarcinoma | 1 | |
| Pleura–pericardium–ascites (n = 7) | Autoimmune | 2 |
| Adult Still’ s disease | 1 | |
| Rheumathoid arthritis | 1 | |
| Neoplasm | 1 | |
| Unknown origin adenocarcinoma | 1 | |
| Other | 1 | |
| Idiophatic | 3 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Losada, I.; González-Moreno, J.; Roda, N.; Ventayol, L.; Borjas, Y.; Domínguez, F.J.; Fernández-Baca, V.; García-Gasalla, M.; Payeras, A. Polyserositis: A diagnostic challenge. Intern. Med. J. 2018, 48, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Munguti, J.; Mutua, V.; Cheruiyot, I.; Csefalvay, C.; Opare-Addo, P.; Kiko, N.; Wanjiru, R. Tuberculous polyserositis in endemic areas with an emphasis on empiric therapy: A case report. Med. Case Rep. Study Protoc. 2022, 3, e0228. [Google Scholar] [CrossRef]
- Hernández-Perera, J.C.; Piñeiro-Pérez, D.; Martínez-Muñiz, J.O.; Correa-Padilla, J.M.; de Armas-Fernández, M.C.; Jordán-González, J.A.; Dávila-Gómez, C.A.; Domínguez-Romero, A.; Contino-López, R. Polyserositis as a Post-COVID-19 Complication. MEDICC Rev. 2022, 24, 57–60. [Google Scholar] [CrossRef]
- Merriam-Webster. Available online: https://www.merriam-webster.com/medical/polyserositis (accessed on 15 March 2023).
- Concato, L.M. Sulla poliorromennite scrofolosa o tisi delle sierose. G. Int. Sci. Med 1881, 3, 1037. [Google Scholar]
- Hoffman, F.G. Idiopathic polyserositis. Arch. Intern. Med. 1961, 108, 872–883. [Google Scholar] [CrossRef]
- Light, R.W.; Macgregor, M.I.; Luchsinger, P.C.; Ball, W.C., Jr. Pleural effusions: The diagnostic separation of transudates and exudates. Ann. Intern. Med. 1972, 77, 507–513. [Google Scholar] [CrossRef]
- Neto, N.S.; Waldrich, L.; de Carvalho, J.F.; Pereira, R.M. Adult-onset Still’s disease with pulmonary and cardiac involvement and response to intravenous immunoglobulin. Acta Reumatol. Port. 2009, 34, 628–632. [Google Scholar]
- Maral, S.; Bakanay, S.M.; Kucuksahin, O.; Dilek, I. Lupus-like symptoms with anti-RNP/Sm and anti-nuclear antibodies positivity: An extremely rare adverse event of dasatinib. J. Oncol. Pharm. Pract. 2020, 26, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Sakai, S.; Ueda, H.; Fujikura, K.; Imai, Y.; Ishikawa, T. Encapsulating peritoneal sclerosis in a patient after allogeneic hematopoietic stem cell transplantation: A case report. BMC Gastroenterol. 2019, 19, 12. [Google Scholar] [CrossRef]
- Kamiya, H.; Ishikawa, R.; Moriya, A.; Arai, A.; Morimoto, K.; Ando, T.; Ikushima, S.; Oritsu, M.; Takemura, T. Disseminated cryptococcosis complicated with bilateral pleural effusion and ascites during corticosteroid therapy for organizing pneumonia with myelodysplastic syndrome. Intern. Med. 2008, 47, 1981–1986. [Google Scholar] [CrossRef]
- Okuda, K.; Noguchi, S.; Narumoto, O.; Ikemura, M.; Yamauchi, Y.; Tanaka, G.; Takai, D.; Fukayama, M.; Nagase, T. A case of Meigs’ syndrome with preceding pericardial effusion in advance of pleural effusion. BMC Pulm. Med. 2016, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Helsen, V.; Decoutere, L.; Spriet, I.; Fagard, K.; Boonen, S.; Tournoy, J. Ergotamine-induced pleural and pericardial effusion successfully treated with colchicine. Acta Clin. Belg. 2013, 68, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, P.; Gertner, E.; McEvoy, C.E. Severe polyserositis induced by the 13-valent pneumococcal conjugate vaccine: A case report. J. Med. Case Rep. 2017, 11, 142. [Google Scholar] [CrossRef]
- Guidetti, E.; Galassi, M.; Croci, L.; Stagni, B.; Crespi, C.; Tovoli, F.; Bolondi, L. A case of inflammatory ascites. Acta Clin. Belg. 2014, 69, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Barile, D.; Bataille, Y.; Duysinx, B.C.; Lambermont, B.; Louis, R. Unilateral pleural effusion in ovarian hyperstimulation syndrome. Rev. Med. Liege 2008, 63, 474–479. [Google Scholar] [PubMed]
- Riker, D.; Goba, D. Ovarian mass, pleural effusion, and ascites: Revisiting Meigs syndrome. J. Bronchol. Interv. Pulmonol. 2013, 20, 48–51. [Google Scholar] [CrossRef]
- Pauls, M.; MacKenzie, H.; Ramjeesingh, R. Hydropic leiomyoma presenting as a rare condition of pseudo-Meigs syndrome: Literature review and a case of a pseudo-Meigs syndrome mimicking ovarian carcinoma with elevated CA125. BMJ Case Rep. CP 2019, 12, e226454. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.; Park, M.J.; Jo, Y.S. Massive pleural and pericardial effusion due to hypothyroidism in a patient with a surgically treated thyroid-stimulating hormone-producing pituitary adenoma. Acta Clin. Belg. 2018, 73, 398–401. [Google Scholar] [CrossRef]
- Jose, A.; Cramer, A.K.; Davar, K.; Gutierrez, G. A case of drug-induced lupus erythematosus secondary to trimethoprim/sulfamethoxazole presenting with pleural effusions and pericardial tamponade. Lupus 2017, 26, 316–319. [Google Scholar] [CrossRef]
- Kakar, A.; Pipaliya, K.; Gogia, A. A rare combination: Chylous polyserositis and autoimmune myelofibrosis as a presentation of systemic lupus erythematosus. Int. J. Rheum. Dis. 2019, 22, 516–520. [Google Scholar] [CrossRef]
- Awazawa, R.; Yamamoto, Y.; Mine, Y.; Nakamura, I.; Kishimoto, K.; Kinjyo, F.; Hagiwara, K.; Fujita, J.; Uezato, H.; Takahashi, K. Systemic lupus erythematosus complicated with protein-losing enteropathy: A case report and review of the published works. J. Dermatol. 2012, 39, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Jatwani, S.; Handa, R.; Jatwani, K.; Chugh, K. Bronchiolitis obliterans organising pneumonia as an initial manifestation in a patient with systemic lupus erythematosus: A rare presentation. BMJ Case Rep. 2018, e2017224094. [Google Scholar] [CrossRef] [PubMed]
- Falkenbach, A.; Lembcke, B.; Schneider, M.; Wigand, R.; Mulert-Ernst, R.; Caspary, W. Polyserositis in adult Still’s disease with onset during pregnancy. Clin. Rheumatol. 1994, 13, 513–517. [Google Scholar] [CrossRef]
- Matsuoka, N.; Asano, T.; Sato, S.; Sasajima, T.; Fujita, Y.; Temmoku, J.; Yashiro Furuya, M.; Matsumoto, H.; Suzuki, E.; Kobayashi, H.; et al. A case of dermatomyositis complicated with pleural effusion and massive ascites. Fukushima J. Med. Sci. 2020, 65, 140–145. [Google Scholar] [CrossRef]
- Szeto, M.C.; Disney, B.; Perkins, P.; Wood, G. Ascites and other incidental findings revealing undiagnosed systemic rheumatoid arthritis. BMJ Case Rep. 2015, 2015, bcr2014207142. [Google Scholar] [CrossRef] [PubMed]
- Klepfish, A.; Zuckermann, B.; Schattner, A. Primary effusion lymphoma in the absence of HIV infection-clinical presentation and management. QJM 2015, 108, 481–488. [Google Scholar] [CrossRef]
- Grasso, A.; De Leo, P.; Malfatti, F.; Toscanini, F.; Anselmo, M.; Menardo, G. Late occurrence of pleural and peritoneal effusion due to Mycobacterium tuberculosis infection (TB) in a patient with posttransplantation recurrent HCV chronic hepatitis: Safety of peginterferon and ribavirin treatment after recovery of TB:—A case report. Transplant. Proc. 2008, 40, 1783–1785. [Google Scholar] [CrossRef]
- Pi, Y.; Wang, B.; Wang, L.; Ren, H. Polyserositis as a primary clinical manifestation of CD7+ acute myelogenous leukemia with myeloid sarcoma: A case report. Medicine 2020, 99, e23615. [Google Scholar] [CrossRef]
- Pisacreta, A.M.; Mascolo, R.; Nivuori, M.; Dominioni, C.C.; Gabiati, C.; Trotta, L.; Pancrazi, M.; Marco, G.D.; Carollo, C.; Pedroli, A.; et al. Acute pericarditis with pleuropulmonary involvement, fever and elevated C-reactive protein: A systemic autoinflammatory disease? A cohort study. Eur. J. Intern. Med. 2023, S0953-6205, 00112–00117. [Google Scholar] [CrossRef]
- Wu, M.A.; Costedoat-Chalumeau, N.; Maestroni, S.; Brucato, A. Acute pericarditis or a systemic disease with pleuropulmonary involvement? Intern. Emerg. Med. 2019, 14, 731–733. [Google Scholar] [CrossRef]
- Dorland, W.A. Dorland’s Illustrated Medical Dictionary, 32nd ed.; Saunders: Philadelphia, PA, USA, 2011; ISBN 978-1-4160-6257-8. [Google Scholar]
- Nakamura, A.; Yazaki, M.; Tokuda, T.; Hattori, T.; Ikeda, S. A Japanese patient with familial Mediterranean fever associated with compound heterozygosity for pyrin variant E148Q/M694I. Intern. Med. 2005, 44, 261–265. [Google Scholar] [CrossRef]
- Liang, Y.; Leng, R.X.; Pan, H.F.; Ye, D.Q. The prevalence and risk factors for serositis in patients with systemic lupus erythematosus: A cross-sectional study. Rheumatol. Int. 2017, 37, 305–311. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Bai, Y.; Yang, D.; Xiong, Y.; Zeng, X. Clinical characteristics and follow-up analysis of adult-onset Still’s disease complicated by hemophagocytic lymphohistiocytosis. Clin. Rheumatol. 2016, 35, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumatol. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- UpToDate. Available online: https://www.uptodate.com/contents/pulmonary-manifestations-of-systemic-lupus-erythematosus-in-adults?search=lupus%20serositis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H2058057 (accessed on 27 March 2023).
- Yao, X.; Abd Hamid, M.; Sundaralingam, A.; Evans, A.; Karthikappallil, R.; Dong, T.; Rahman, N.M.; Kanellakis, N.I. Clinical perspective and practices on pleural effusions in chronic systemic inflammatory diseases. Breathe 2020, 16, 200203. [Google Scholar] [CrossRef]
- Choi, B.Y.; Yoon, M.J.; Shin, K.; Lee, Y.J.; Song, Y.W. Characteristics of pleural effusions in systemic lupus erythematosus: Differential diagnosis of lupus pleuritis. Lupus 2015, 24, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Tannor, E.K.; Yeboah-Mensah, K. Biopsy proven lupus nephritis in a black male patient in West Africa with systemic lupus erythematosus: Case report. Pan. Afr. Med. J. 2018, 31, 198. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Pan, W.; Wang, A.; Yu, T.; Song, A. Secondary angle-closure glaucoma as an ocular presentation of systemic lupus erythematosus: A case report. J. Int. Med. Res. 2020, 48, e300060520959492. [Google Scholar] [CrossRef]
- Sučić, M.; Ovčariček, S.; Hrkać, A.; Mažuran, B.; Budinčević, H. Polyserositis and severe sepsis after open suprapubic radical prostactectomy: A case report. Acta Clin. Croat. 2018, 57, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liwen, Z.; Bingfang, C.; Yanbo, D.; Jianping, C. Eosinophilic gastroenteritis with multiple serous membrane effusion as the first sign: A case report and literature review. J. Int. Med. Res. 2020, 48, e300060520917274. [Google Scholar] [CrossRef]
- Kattula, S.R.S.T.; Avula, A.; Baradhi, K.M. Anasarca; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Buoro, S.; Tombetti, E.; Ceriotti, F.; Simon, C.; Cugola, D.; Seghezzi, M.; Innocente, F.; Maestroni, S.; Del Carmen Baigorria Vaca, M.; Moioli, V.; et al. What is the normal composition of pericardial fluid? Heart 2021, 107, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Biondo, A.; Ricci, D.; Boffini, M.; Pivetta, E.; Brucato, A.; Giustetto, C.; De Ferrari, G.M.; Rinaldi, M. Contemporary biochemical analysis of normal pericardial fluid. Heart 2020, 106, 541–544. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoichitoiu, L.E.; Ionescu, G.D.; Neatu, I.; Baicus, C. Causes of Polyserositis: A Systematic Review. J. Pers. Med. 2023, 13, 834. https://doi.org/10.3390/jpm13050834
Stoichitoiu LE, Ionescu GD, Neatu I, Baicus C. Causes of Polyserositis: A Systematic Review. Journal of Personalized Medicine. 2023; 13(5):834. https://doi.org/10.3390/jpm13050834
Chicago/Turabian StyleStoichitoiu, Laura Elena, Georgeta Daniela Ionescu, Ingrid Neatu, and Cristian Baicus. 2023. "Causes of Polyserositis: A Systematic Review" Journal of Personalized Medicine 13, no. 5: 834. https://doi.org/10.3390/jpm13050834
APA StyleStoichitoiu, L. E., Ionescu, G. D., Neatu, I., & Baicus, C. (2023). Causes of Polyserositis: A Systematic Review. Journal of Personalized Medicine, 13(5), 834. https://doi.org/10.3390/jpm13050834







