The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease
Abstract
1. Introduction
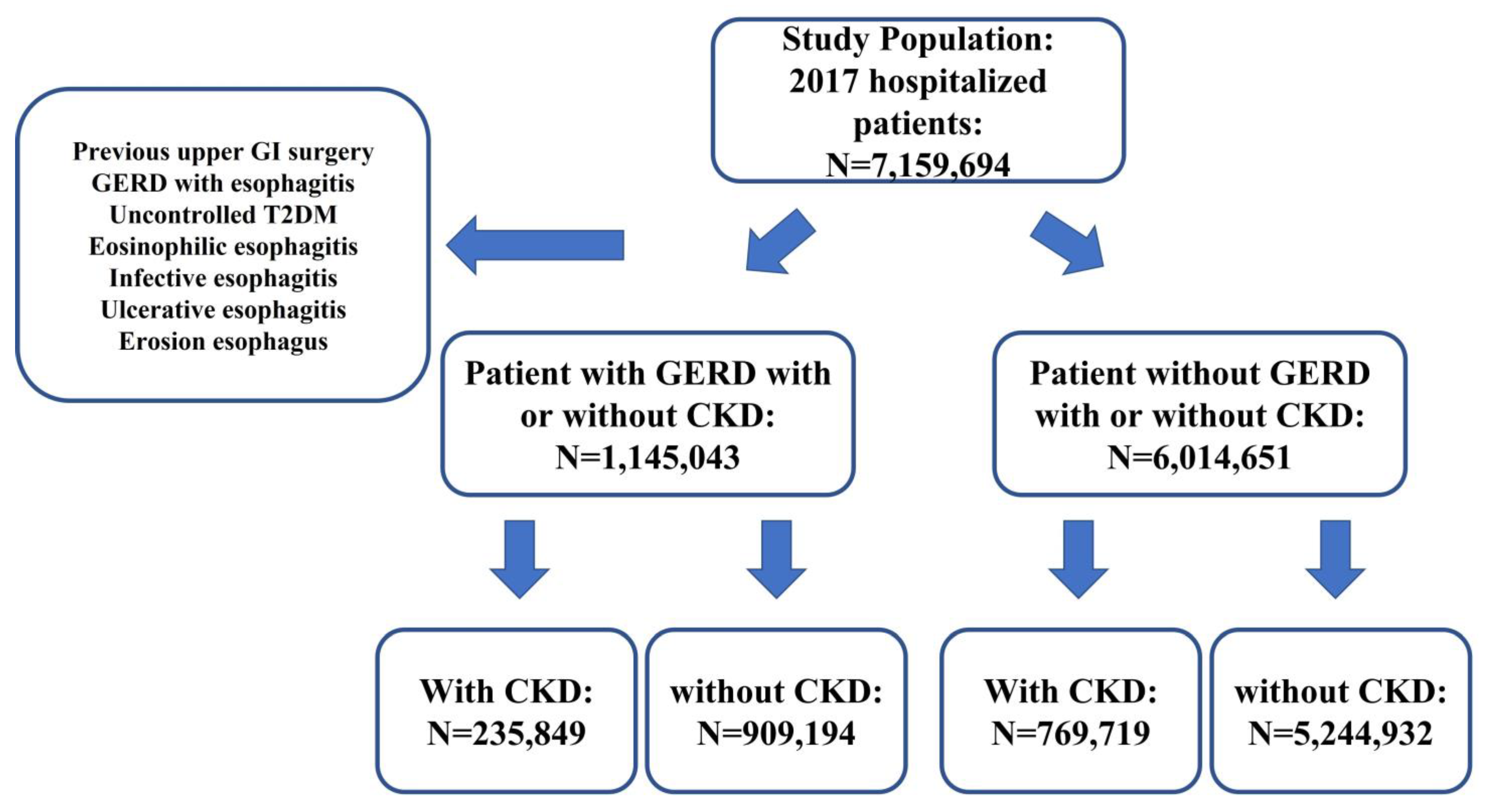
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fass, R.; Boeckxstaens, G.E.; El-Serag, H.; Rosen, R.; Sifrim, D.; Vaezi, M.F. Gastro-oesophageal reflux disease. Nat. Rev. Dis. Prim. 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Off. J. Am. Coll. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920; quiz 1943. [Google Scholar] [CrossRef] [PubMed]
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal Reflux Disease: A Review. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef]
- Giannini, E.G.; Zentilin, P.; Dulbecco, P.; Vigneri, S.; Scarlata, P.; Savarino, V. Management strategy for patients with gastroesophageal reflux disease: A comparison between empirical treatment with esomeprazole and endoscopy-oriented treatment. Am. J. Gastroenterol. 2008, 103, 267–275. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Time trends of gastroesophageal reflux disease: A systematic review. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 17–26. [Google Scholar] [CrossRef]
- Hampel, H.; Abraham, N.S.; El-Serag, H.B. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005, 143, 199–211. [Google Scholar] [CrossRef]
- Kahrilas, P.J.; Shaheen, N.J.; Vaezi, M.F. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008, 135, 1392–1413.e5. [Google Scholar] [CrossRef]
- Pandolfino, J.E.; Kahrilas, P.J. Smoking and gastro-oesophageal reflux disease. Eur. J. Gastroenterol. Hepatol. 2000, 12, 837–842. [Google Scholar] [CrossRef]
- Johansen, K.L.; Chertow, G.M.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Li, S.; Li, S.; Liu, J.; et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022, 79, A8–A12. [Google Scholar] [CrossRef]
- Milito, G.; Taccone-Gallucci, M.; Brancaleone, C.; Nardi, F.; Filingeri, V.; Cesca, D.; Casciani, C.U. Assessment of the upper gastrointestinal tract in hemodialysis patients awaiting renal transplantation. Am. J. Gastroenterol. 1983, 78, 328–331. [Google Scholar] [PubMed]
- Milito, G.; Taccone-Gallucci, M.; Brancaleone, C.; Nardi, F.; Cesca, D.; Boffo, V.; Casciani, C.U. The gastrointestinal tract in uremic patients on long-term hemodialysis. Kidney Int. Suppl. 1985, 17, S157–S160. [Google Scholar]
- Furukawa, N.; Iwakiri, R.; Koyama, T.; Okamoto, K.; Yoshida, T.; Kashiwagi, Y.; Ohyama, T.; Noda, T.; Sakata, H.; Fujimoto, K. Proportion of reflux esophagitis in 6010 Japanese adults: Prospective evaluation by endoscopy. J. Gastroenterol. 1999, 34, 441–444. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Mine, T.; Kawana, I.; Yasuzaki, H.; Kokuho, T.; Toya, Y.; Ohnishi, T.; Umemura, S. Gastroesophageal reflux disease in chronic renal failure patients: Evaluation by endoscopic examination. Tokai J. Exp. Clin. Med. 2009, 34, 80–83. [Google Scholar]
- Karahan, D.; Şahin, İ. Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrol. 2022, 23, 261. [Google Scholar] [CrossRef]
- Abdulrahman, I.S.; Al-Quorain, A.A. Prevalence of gastroesophageal reflux disease and its association with Helicobacter pylori infection in chronic renal failure patients and in renal transplant recipients. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2008, 14, 183–186. [Google Scholar] [CrossRef]
- Kuwahara, C.M.; Rosa, E.S.L.; Mocelin, A.J.; Zebian, M.; Pontes, R.M.; Dantas, R.O. Esophageal Dysmotility in Chronic Hemodialysis Patients After Ingestion of Liquids With Different Viscosities. Gastroenterol. Res. 2011, 4, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Kamiya, T.; Hirako, M.; Misu, N.; Kobayashi, Y.; Shikano, M.; Matsuhisa, E.; Kataoka, H.; Sasaki, M.; Ohara, H.; et al. Improvement of gastric motility by hemodialysis in patients with chronic renal failure. J. Smooth Muscle Res. Nihon Heikatsukin Gakkai Kikanshi 2007, 43, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kamionkowski, S.; Shibli, F.; Ganocy, S.; Fass, R. The relationship between gastroesophageal reflux disease and autism spectrum disorder in adult patients in the United States. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2022, 34, e14295. [Google Scholar] [CrossRef]
- Saleh, S.; Trujillo, S.; Ghoneim, S.; Thomas, C.; Fass, R. Effect of Hormonal Replacement Therapy on Gastroesophageal Reflux Disease and its Complications in Postmenopausal Women. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 549–551.e3. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Mine, T.; Kawana, I.; Yasuzaki, H.; Kokuho, T.; Toya, Y.; Ohnishi, T.; Umemura, S. Gastroesophageal reflux disease in hemodialysis patients. Tokai J. Exp. Clin. Med. 2009, 34, 48–52. [Google Scholar] [PubMed]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Reiher, A.E.; Mazeh, H.; Schaefer, S.; Gould, J.; Chen, H.; Sippel, R.S. Symptoms of gastroesophageal reflux disease improve after parathyroidectomy. Surgery 2012, 152, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Politz, D.; Lopez, J.; Boone, D.; Stojadinovic, A. Surgical cure of primary hyperparathyroidism ameliorates gastroesophageal reflux symptoms. World J. Surg. 2015, 39, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Mowschenson, P.M.; Rosenberg, S.; Pallotta, J.; Silen, W. Effect of hyperparathyroidism and hypercalcemia on lower esophageal sphincter pressure. Am. J. Surg. 1982, 143, 36–39. [Google Scholar] [CrossRef]
- Björkman, E.; Edebo, A.; Casselbrant, A.; Helander, H.F.; Bratlie, S.O.; Vieth, M.; Fändriks, L. The renin-angiotensin system in the esophageal mucosa of healthy subjects and patients with reflux disease. Scand. J. Gastroenterol. 2013, 48, 147–159. [Google Scholar] [CrossRef]
- Souza, R.F.; Bayeh, L.; Spechler, S.J.; Tambar, U.K.; Bruick, R.K. A new paradigm for GERD pathogenesis. Not acid injury, but cytokine-mediated inflammation driven by HIF-2α: A potential role for targeting HIF-2α to prevent and treat reflux esophagitis. Curr. Opin. Pharmacol. 2017, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N. Inflammation and oxidative stress in gastroesophageal reflux disease. J. Clin. Biochem. Nutr. 2007, 40, 13–23. [Google Scholar] [CrossRef]
- Yoshida, N.; Uchiyama, K.; Kuroda, M.; Sakuma, K.; Kokura, S.; Ichikawa, H.; Naito, Y.; Takemura, T.; Yoshikawa, T.; Okanoue, T. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand. J. Gastroenterol. 2004, 39, 816–822. [Google Scholar] [CrossRef]
- Yoshida, N.; Yoshikawa, T. Defense mechanism of the esophageal mucosa and esophageal inflammation. J. Gastroenterol. 2003, 38 (Suppl. 15), 31–34. [Google Scholar] [PubMed]
- Cheng, L.; Harnett, K.M.; Cao, W.; Liu, F.; Behar, J.; Fiocchi, C.; Biancani, P. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagitis. Gastroenterology 2005, 129, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
| GERD w/ CKD | GERD w/o CKD | p Value | No GERD w/CKD | No GERD w/o CKD | p Value | |
|---|---|---|---|---|---|---|
| Age | 71.6 ± 0.1 | 62.5 ± 0.1 | <0.01 | 69.9 ± 0.1 | 43.4 ± 0.1 | <0.01 |
| Sex | ||||||
| Female | 124,690 (52.9%) | 546,663 (60.1%) | <0.01 | 344,777 (44.8%) | 3,021,122 (57.6%) | <0.01 |
| Male | 111,150 (47.1%) | 362,502 (39.9%) | <0.01 | 424,924 (55.2%) | 2,222,916 (42.4%) | <0.01 |
| Race | ||||||
| White | 161,472 (68.5%) | 679,222 (74.7%) | <0.01 | 461,824 (60.0%) | 3,142,505 (59.9%) | >0.05 |
| Black | 41,320 (17.5%) | 98,546 (10.8%) | <0.01 | 161,606 (21.0%) | 748,476 (14.3%) | <0.01 |
| Hispanic | 16,298 (6.9%) | 62,590 (6.9%) | <0.01 | 78,580 (10.2%) | 698,702 (13.3%) | <0.01 |
| Asian | 4377 (1.9%) | 13,420 (1.5%) | <0.01 | 22,083 (2.9%) | 173,819 (3.3%) | <0.05 |
| Complications | ||||||
| T2DM | 108,257 (45.9%) | 55,930 (6.2%) | <0.01 | 362,579 (47.1%) | 151,975 (2.9%) | <0.01 |
| HTN | 123,433 (52.3%) | 494,247 (54.4%) | <0.01 | 336,194 (43.7%) | 1,509,476 (28.8%) | <0.01 |
| HLD | 129,974 (55.1%) | 362,456 (39.9%) | <0.01 | 338,254 (50.4%) | 918,667 (17.5%) | <0.01 |
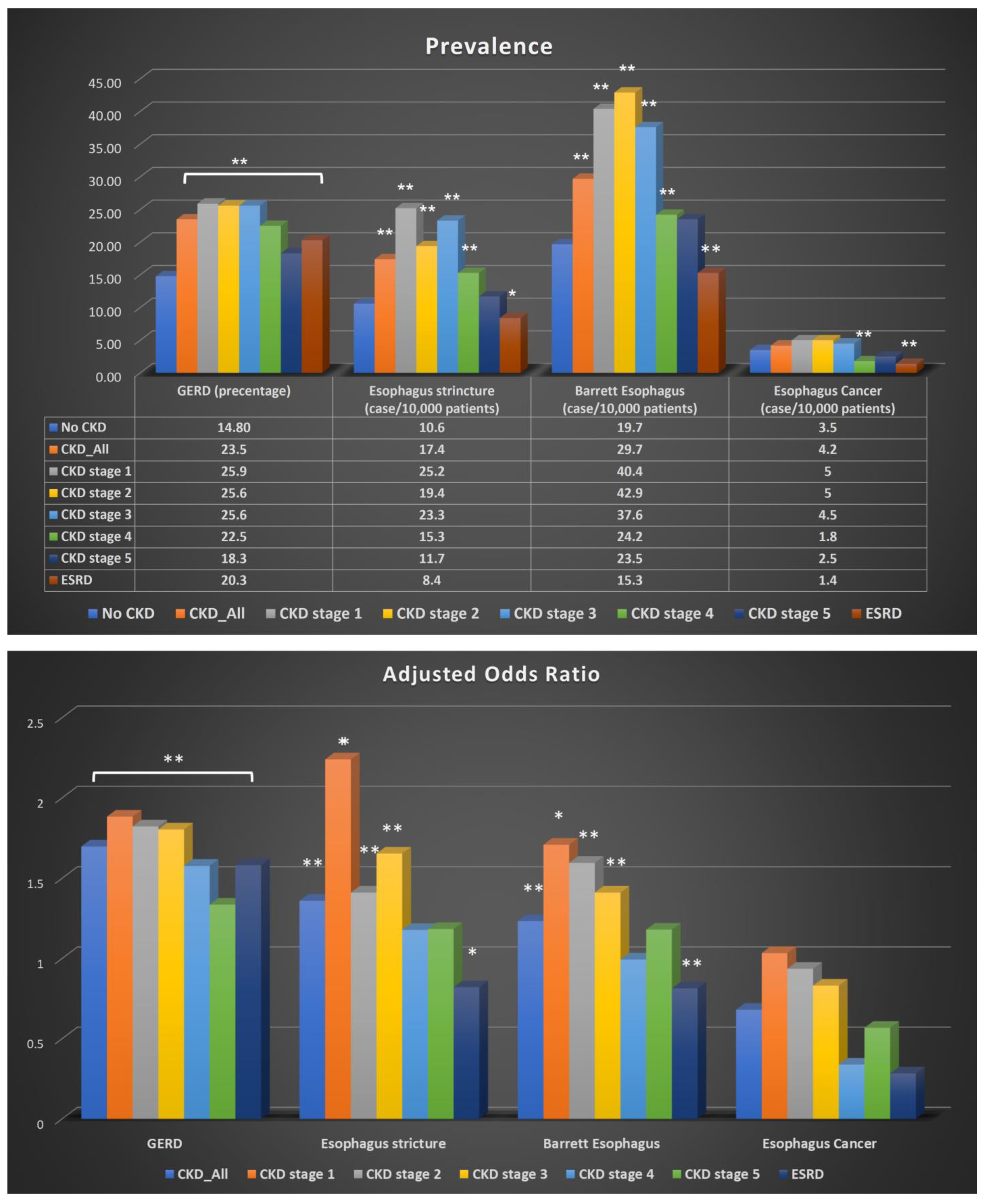
| GERD | ||||||
| GERD Number | GERD Percentage | Odds Ratio | p Value | Adjusted Odds Ratio | p Value | |
| CKD_ALL | 1.768 | <0.01 | 1.697 | <0.01 | ||
| YES | 235,849 | 23.5% | ||||
| No | 909,149 | 14.8% | ||||
| CKD stage 1 | 1384 | 25.9% | 2.013 | <0.01 | 1.883 | <0.01 |
| CKD stage 2 | 12,444 | 25.6% | 1.986 | <0.01 | 1.822 | <0.01 |
| CKD stage 3 | 102,156 | 25.6% | 1.968 | <0.01 | 1.803 | <0.01 |
| CKD stage 4 | 24,689 | 22.5% | 1.675 | <0.01 | 1.577 | <0.01 |
| CKD stage 5 | 2675 | 18.3% | 1.293 | <0.01 | 1.336 | <0.01 |
| ESRD | 41,502 | 20.3% | 1.469 | <0.01 | 1.581 | <0.01 |
| Esophageal Stricture | ||||||
| Cases | Cases per 100,000 Patients | OR | p Value | Adjusted OR | p Value | |
| CKD_ALL | 1.642 | <0.01 | 1.36 | <0.01 | ||
| YES | 1335 | 17.4 | ||||
| No | 5547 | 10.6 | ||||
| CKD stage 1 | 10 | 25.2 | 2.392 | <0.01 | 2.24 | 0.012 |
| CKD stage 2 | 70 | 19.4 | 1.834 | <0.01 | 1.411 | <0.01 |
| CKD stage 3 | 691 | 23.3 | 2.202 | <0.01 | 1.653 | <0.01 |
| CKD stage 4 | 130 | 15.3 | 1.448 | <0.01 | 1.178 | 0.06 |
| CKD stage 5 | 14 | 11.7 | 1.109 | <0.01 | 1.184 | 0.531 |
| ESRD | 136 | 8.4 | 0.789 | <0.01 | 0.821 | 0.025 |
| Barrett’s Esophagus | ||||||
| Cases | Case per 100,000 Patients | OR | p Value | Adjusted OR | p Value | |
| CKD_ALL | 1.514 | <0.01 | 1.233 | <0.01 | ||
| YES | 2288 | 29.7 | ||||
| No | 10,313 | 19.7 | ||||
| CKD stage 1 | 16 | 40.4 | 2.057 | <0.01 | 1.708 | 0.034 |
| CKD stage 2 | 18 | 42.9 | 2.185 | <0.01 | 1.595 | <0.01 |
| CKD stage 3 | 1117 | 37.6 | 1.915 | <0.01 | 1.411 | <0.01 |
| CKD stage 4 | 206 | 24.2 | 1.234 | <0.01 | 0.994 | 0.935 |
| CKD stage 5 | 28 | 23.5 | 1.194 | 0.35 | 1.18 | 0.392 |
| ESRD | 250 | 15.3 | 0.78 | <0.01 | 0.814 | <0.01 |
| Esophageal Cancer | ||||||
| Cases | Cases per 100,000 Patients | OR | p Value | Adjusted OR | p Value | |
| CKD_ALL | 0.848 | 0.01 | 0.681 | <0.01 | ||
| YES | 272 | 3.5 | ||||
| No | 2185 | 4.2 | ||||
| CKD stage 1 | 2 | 5 | 1.21 | 0.788 | 1.034 | 0.962 |
| CKD stage 2 | 18 | 5 | 1.196 | 0.451 | 0.936 | 0.781 |
| CKD stage 3 | 135 | 4.5 | 1.09 | 0.33 | 0.831 | 0.04 |
| CKD stage 4 | 15 | 1.8 | 0.423 | <0.01 | 0.339 | <0.01 |
| CKD stage 5 | 3 | 2.5 | 0.603 | 0.381 | 0.568 | 0.327 |
| ESRD | 22 | 1.4 | 0.324 | <0.01 | 0.284 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wright, Z.; Patton-Tackett, E.D.; Song, G. The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease. J. Pers. Med. 2023, 13, 827. https://doi.org/10.3390/jpm13050827
Wang X, Wright Z, Patton-Tackett ED, Song G. The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease. Journal of Personalized Medicine. 2023; 13(5):827. https://doi.org/10.3390/jpm13050827
Chicago/Turabian StyleWang, Xiaoliang, Zachary Wright, Eva D. Patton-Tackett, and Gengqing Song. 2023. "The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease" Journal of Personalized Medicine 13, no. 5: 827. https://doi.org/10.3390/jpm13050827
APA StyleWang, X., Wright, Z., Patton-Tackett, E. D., & Song, G. (2023). The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease. Journal of Personalized Medicine, 13(5), 827. https://doi.org/10.3390/jpm13050827







