Abstract
The detection of ROS1 rearrangements in metastatic non-squamous non-small cell lung carcinoma (NS-NSCLC) permits administration of efficient targeted therapy. Detection is based on a testing algorithm associated with ROS1 immunohistochemistry (IHC) screening followed by ROS1 FISH and/or next generation sequencing (NGS) to confirm positivity. However, (i) ROS1 rearrangements are rare (1–2% of NS-NSCLC), (ii) the specificity of ROS1 IHC is not optimal, and (iii) ROS1 FISH is not widely available, making this algorithm challenging to interpret time-consuming. We evaluated RNA NGS, which was used as reflex testing for ROS1 rearrangements in NS-NSCLC with the aim of replacing ROS1 IHC as a screening method. ROS1 IHC and RNA NGS were prospectively performed in 810 NS-NSCLC. Positive results were analyzed by ROS1 FISH. ROS1 IHC was positive in 36/810 (4.4%) cases that showed variable staining intensity while NGS detected ROS1 rearrangements in 16/810 (1.9%) cases. ROS1 FISH was positive in 15/810 (1.8%) of ROS1 IHC positive cases and in all positive ROS1 NGS cases. Obtaining both ROS1 IHC and ROS1 FISH reports took an average of 6 days, while obtaining ROS1 IHC and RNA NGS reports took an average of 3 days. These results showed that systematic screening for the ROS1 status using IHC must be replaced by NGS reflex testing.
1. Introduction
The detection of ROS1 rearrangement in stage IIIB/IV non-small cell lung cancer (NSCLC) patients makes them eligible for targeted therapy, notably for administration of the tyrosine-kinase inhibitor crizotinib [1,2]. Therefore, it is necessary to systematically look for ROS1 rearrangements in cases of metastatic non-squamous NSCLC (NS-NSCLC) at the baseline [3,4]. Most of the algorithms for ROS1 status assessment in routine clinical practice, notably in pathology laboratories, are based on ROS1 immunohistochemistry (IHC) as a screening test, followed by a molecular biology test [ROS1 FISH, next generation sequencing (NGS), or RT-qPCR approaches] to confirm ROS1 IHC positivity [3,5,6,7,8,9,10,11,12]. It is noteworthy that ROS1 targeted therapy can be administered only based on a positive molecular biology result. Therefore, this two-step algorithm is necessary considering the variable specificity and the high sensitivity of ROS1 IHC staining, which may give false positive results and, less frequently, false negative results [5].
The progressive discovery of several therapeutic molecular targets for NS-NSCLC has increased the number of biomarkers to analyze. Thus, it is recommended, more and more often, to evaluate not only the EGFR, ALK, and ROS1 status, but also that of BRAF, RET, NTRK, MET, KRAS, and HER2 before any treatment [4]. Therefore, it is difficult in daily practice to sequentially look for the genomic alterations of each of these genes for several reasons. First, the turnaround times (TAT) to obtain most of the results can delay the initiation of targeted treatment. Second, some of these tests cannot be performed or lead to uncertain or false negative results due to small tissue biopsies and/or a low percentage of tumor cells, which give an insufficient amount of extracted nucleic acid and/or tumor cells visible on tissue sections [13,14]. Consequently, at present, the need to screen for ROS1 with IHC is under discussion, and its use is being challenged. This paves the way for DNA and RNA NGS reflex testing, which, in a single step, can look at all the necessary genomic alterations associated with the currently available targeted therapies used in routine clinical practice [4].
This study aimed to compare, prospectively, the results of ROS1 rearrangement screening by ROS1 IHC and RNA NGS for 810 NS-NSCLC patients. The results were then compared to those of the ROS1 FISH. Finally, the TAT to obtain the different results were also compared.
2. Patients and Methods
Between September 2021 and February 2023, 810 NS-NSCLC patients were tested systematically by ROS1 IHC and DNA and RNA NGS (Laboratory of Clinical and Experimental Pathology, Pasteur Hospital, University Côte d’Azur Nice, France; Figure 1).
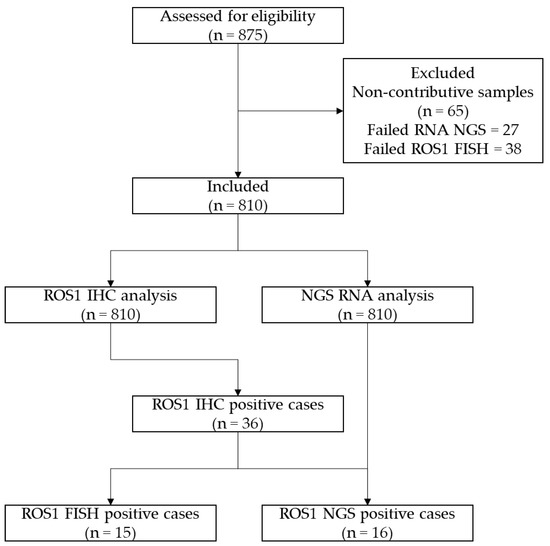
Figure 1.
CONSORT flow diagram of the study.
The main epidemiological, clinical, and pathological data are shown in Table 1.

Table 1.
Main epidemiological and pathological data.
ROS1 IHC and/or ROS1 NGS positive cases were analyzed with ROS1 FISH. The TAT was considered to be the time between obtaining the histological diagnosis and electronic validation of the reports.
2.1. ROS1 Immunohistochemistry
The formalin-fixed, paraffin-embedded (FFPE) tissue, with a thickness of 4 μm, was subjected to immunohistochemistry. A rabbit monoclonal ROS1 antibody (D4D6) provided by Cell Signaling Technology (Danvers, MA, USA) was used at a 1:75 dilution for a duration of 2 hours. The Benchmark ULTRA autostainer (Ventanaa, Tucson, AZ, USA) was used to perform staining, which was achieved through the OptiView DAB IHC detection kit (Roche) and an amplification kit (Ventana). To evaluate the staining intensity for each case, an assessment was conducted. An H-score, ranging from 0 to 300, was calculated for each case by multiplying the staining intensity (0 indicating no staining, 1 representing weak staining but more than the background staining, 2 indicating moderate staining, and 3 indicating strong staining) by the percentage of positive tumor cells. The positive controls included ROS1-rearranged lung adenocarcinoma tissue that tested FISH positive.
2.2. Next Generation Sequencing
The nucleic acid extraction was conducted using either the Maxwell RSC Instrument (Promega, Madison, WI, USA) in combination with the Maxwell RSC FFPE Plus DNA kit or the Maxwell RSC RNA FFPE kit (Promega). After the extraction of nucleic acid, the concentration was measured by employing the Qubit Fluorometric quantification assay (Thermo Fisher Scientific, Waltham, MA, USA), utilizing the Qubit RNA HS Assay Kit and the Qubit dsDNA HS Assay Kit. The Ion Torrent™ Genexus™ Integrated Sequencer (Thermo Fisher Scientific) was used for the detection of genomic alterations by Ion semiconductor sequencing (Ion Torrent™ Technology, Thermo Fisher Scientific). The Oncomine™ Precision Assay GX panel (OPA) provided by Thermo Fisher Scientific was utilized, targeting 50 key genes. Among them, 45 genes were designed for DNA mutation detection, 18 for fusion detection, and 14 for copy number variant (CNV) detection. In addition, the panel incorporated a 5′/3′ expression imbalance strategy for the detection of novel fusions. By using this panel, up to 16 samples could be sequenced simultaneously on a single run with the Genexus sequencer.
2.3. ROS1 Fluorescence In Situ Hybridization
De-paraffinization of 4-micron formalin-fixed, paraffin-embedded tissue sections was performed before conducting a pre-treatment step of heat-induced epitope retrieval (HIER) using SPoT-Light Tissue Pretreatment Solution (Thermo Fisher Scientific) at pH 7. A proteolytic digestion of tissue sections was then carried out using Protease 1 (Abbott Molecular, Des Plaines, IL, USA), followed by a rinse in saline-sodium citrate buffer (SSC). The ZytoLight SPEC ROS1 Dual Colour Break Apart Probe (ZytoVision, Bremerhaven, Germany) was used, followed by denaturation at 95 °C for 5 min and overnight hybridization at 37 °C. The slides were then dehydrated and counterstained with SlowFade Gold DAPI (Invitrogen, Waltham, MA, USA). At least 50 tumor nuclei per case were evaluated for interphase signals using an epifluorescence microscope (Zeiss, White Plains, NY, USA) and an automated fluorescence microscope (Olympus, Tokyo, Japan) equipped with cell imaging and analysis software (BioView, Rehovot, Israel). Cases were categorized as ROS1 FISH positive if they demonstrated at least 15% of cells with split signals at least one signal distance apart or an isolated centromeric 30 (green signal) pattern. This 15% cutoff was determined by in-house validation and adheres to international guidelines [15].
3. Results
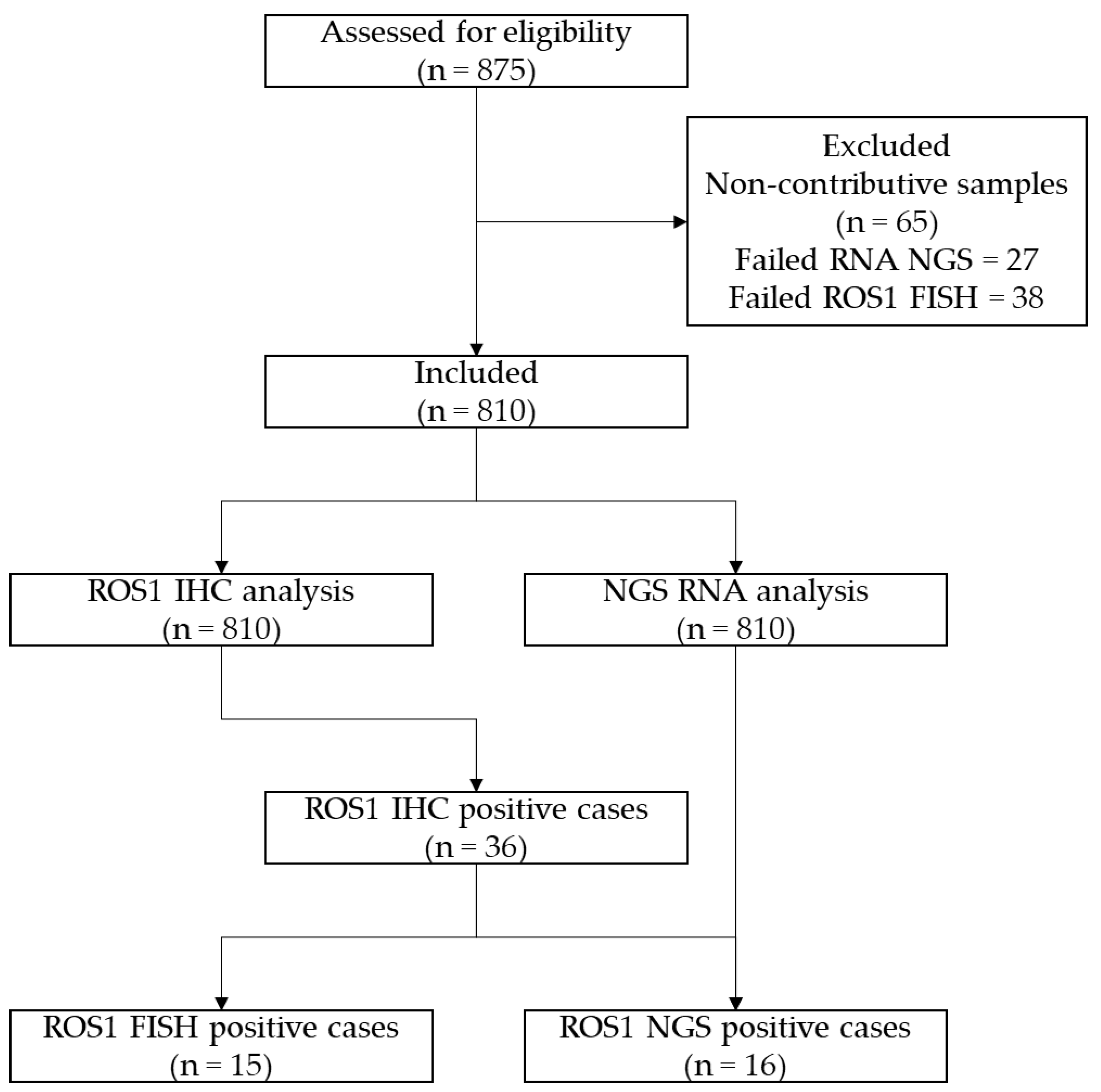
ROS1 IHC was scored in 810 cases of NS-NSCLC. Detectable staining was seen in 36/810 (4.4%) of cases showing a variable intensity and giving an H-score (Figure 2; Table 2).
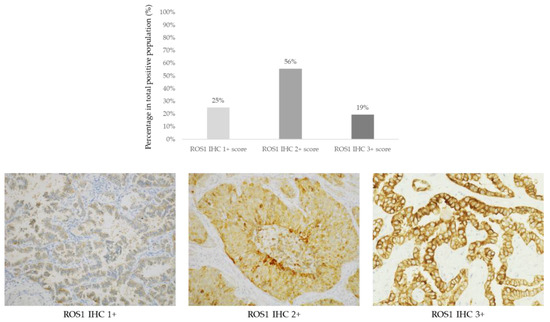
Figure 2.
Upper panel: Prevalence of ROS1 positive cases (n = 36) according to IHC scores 1+ (9/36, 25%), 2+ (20/36, 56%), and 3+ (7/36, 19%). Lower panel: Representative images of non-squamous non-small cell lung cancer cases according to ROS1 IHC scores (D4D6 clone, immunoperoxidase, original magnification ×200).

Table 2.
Number of ROS1 positive cases according to the testing methods.
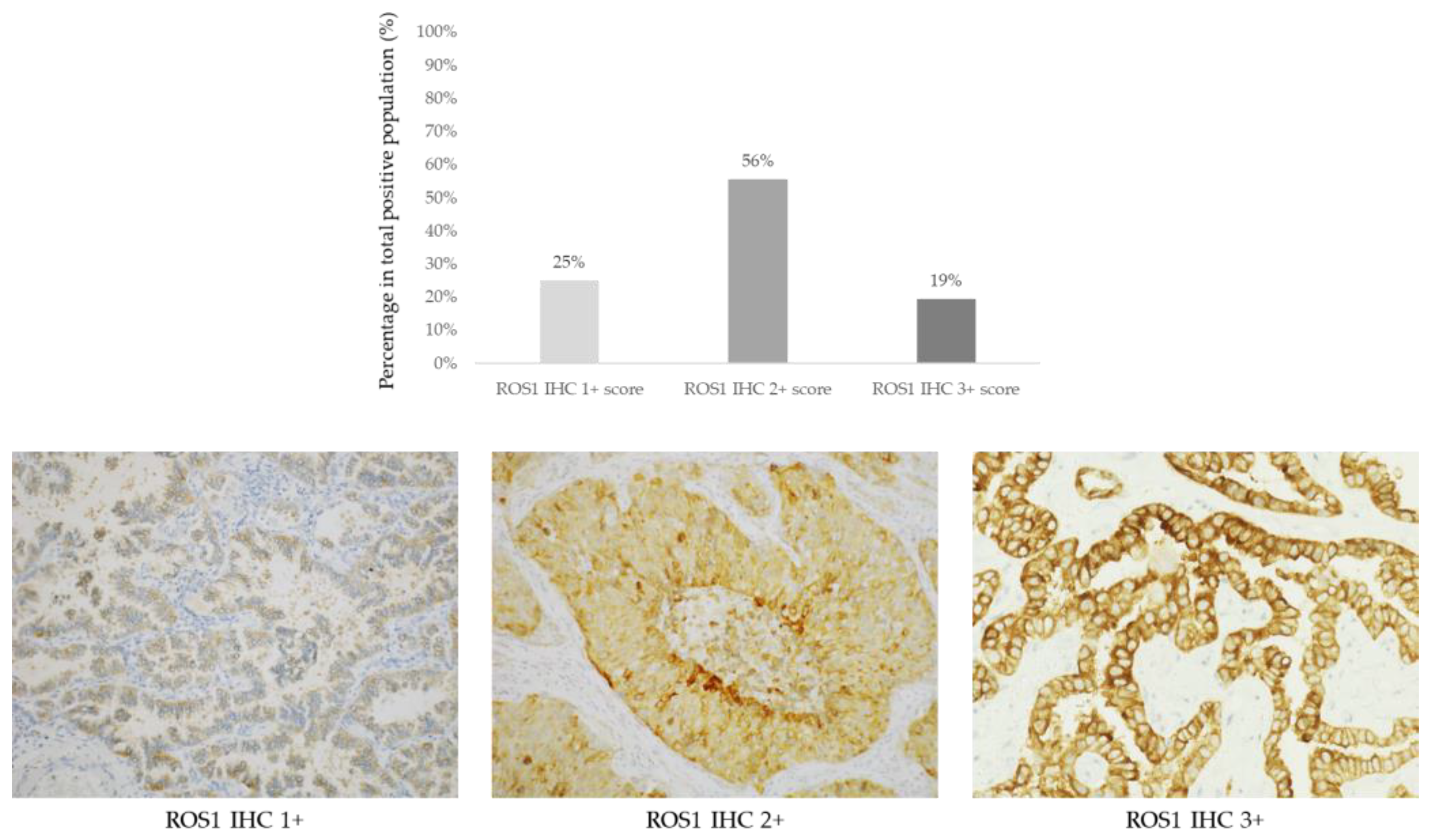
ROS1 FISH was positive in 15 of the 36 cases (42%, Figure 3).
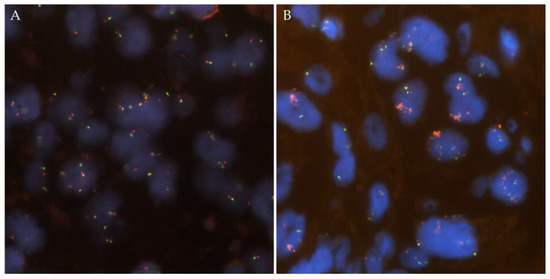
Figure 3.
Representative images from ROS1 copy number alterations detected by FISH. (A) ROS1 wild-type case with two green/orange fusion signals per tumor cell. (B) ROS1 amplified case with ROS1 cluster amplification in tumor nuclei with two green probes.
RNA NGS detected ROS1 rearrangement in 16 of the 36 (44%) ROS1 IHC positive cases and included one negative case by ROS1 FISH (Table 2). Two cases were excluded as false positive as an EML4-ALK fusion was detected by RNA sequencing, and no ROS1 structural variants were seen despite good coverage of all ROS1 introns and exons. These cases were positive for ALK IHC and ALK FISH and had an H-score of 100 for ROS1. None of the negative ROS1 IHC was found to be positive for ROS1 rearrangement when using RNA NGS.
The TAT was 4 days (range 3–8 days) to obtain DNA and RNA NGS reports. The TAT was, in total, 5 days (range 4–9 days) to obtain both the ROS1 IHC (2 days; range 2–4 days) and the ROS1 FISH (3 days; range 2–5 days) reports. All driver mutations were mutually exclusive.
4. Discussion
This study showed that an ultrafast DNA and RNA NGS setting at the baseline can be greatly involved in reflex testing for ROS1 rearrangements in NS-NSCLC [16,17]. Here, the NGS results were obtained in an average of four working days and a positive result was confirmed in 15/16 cases by FISH. Therefore, one false negative ROS1 FISH was observed due to the presence of short deletion, as previously reported [18]. In comparison, the ROS1 IHC results were obtained in an average of two working days, but the positivity was only confirmed after an additional three working days by ROS1 FISH in 42% of the cases, highlighting the relatively weak specificity of ROS1 IHC. Moreover, as already reported, false positive ROS1 IHC cases due to the presence of an ALK rearrangement were observed in our series [19].
Taken together, this led us to abandon the ROS1 IHC screening approach and the sequential ROS1 IHC/ROS1 FISH algorithm in our laboratory. Thus, a new algorithm was set up to evaluate the ROS1 status by first systematically performing ROS1 RNA NGS and then confirming the positive results with ROS1 FISH. Globally, the percentage of positive cases of ROS1 rearrangement was a little bit lower than that described in the literature since our cohort of NS-NSCLC patients contains more than 30% of patients with early-stage disease, which is associated with a lower number of rearranged ROS1 tumors [20].
The shift in routine clinical practice away from ROS1 IHC screening of patients’ NS-NSCLC was warranted for different reasons, notably based on the results obtained in the present work. ROS1 status evaluation using IHC has a couple of limitations. It has been shown to give a number of false positive results, and the low specificity has been confirmed by discrepancies with the ROS1 FISH results [5]. Moreover, it is important to consider which ROS1 antibody is being used since the specificity and sensitivity of these antibodies can be variable [21,22,23,24]. The determination of an H-score from ROS1 IHC can have added value to set up a cutoff for whether to perform ROS1 FISH to confirm the results [25]. This was not confirmed in the present study. One reason for this could be the low number of positive ROS1 cases detected in our series. Therefore, in contrast to ALK IHC, which can be used as a companion diagnostic test, ROS1 IHC always has to be confirmed by at least one molecular biology test [26,27]. The relatively low frequency of ROS1 rearrangements in NS-NSCLC, present in approximatively 0.9–2.6% according to the series, which leads to a ROS1 IHC for a low number of positive results, needs to be taken into consideration [28]. Moreover, more rarely, some false negative results have been noted with ROS1 IHC [7,8,29]. Finally, for many cases, both ROS1 IHC and ROS1 FISH are unnecessarily performed, resulting in associated costs, an increase in the work load in the pathology laboratory, and an increase in the TAT to obtain reports; this can also lead to exhaustion of the tumor tissue, notably in cases of small biopsies and/or low percentages of tumor cells [13,14].
Our results demonstrated perfect concordance between the ROS1 FISH results and those obtained with RNA NGS. RNA NGS was combined simultaneously with DNA NGS, which allowed assessment of the status of the current necessary genes at the baseline for NS-NSCLC [4]. One previous limitation to the use of only NGS for ROS1 rearrangement evaluation was the delay in obtaining the results, which would not be suitable for the administration of a targeted therapy according to the organization, workflow of the samples, and sequencing approaches. However, the development of a new ultrafast NGS system has allowed us to obtain, in another study, the results in an average of four days for positive cases, which is even faster than the TAT when sequentially using ROS1 IHC and then ROS1 FISH [16,17]. It is also noteworthy that RNA NGS can lead to the identification of all the fusion partners of ROS1 and allow the detection of short deletions sometimes not visible with ROS1 FISH [30,31]. Although DNA-based sequencing can detect rearrangements of fusion genes in intron regions, those genes often differ from messenger RNA (mRNA) fusions, emphasizing that RNA NGS is certainly the ideal approach for the evaluation of the ROS1 status [30,32,33]. Performing DNA/RNA NGS at the same time allows for the investigation of the different genomic alterations that are currently necessary to evaluate at the baseline in NS-NSCLC [4]. In this context, it is noteworthy that concurrent classic driver oncogene mutations with ROS1 rearrangement may predict a superior clinical outcome in NSCLC patients [34]. However, a number of restrictions can limit the possibility of developing, in daily practice, the new algorithm described above. First, in contrast to immunohistochemical platforms that are largely available in the majority of pathology laboratories, NGS approaches are not equally distributed in all countries or even in organizations and institutions in a single country [35,36]. Second, even if available, access to some NGS approaches could be limited, due to the cost and absence of reimbursement of costs, and can also be associated with a long TAT to obtain the results, which is not compatible with international guidelines [15]. Due to the lower cost and the shorter TAT, IHC and/or rapid RT-PCR can be easily performed as an alternative for ROS1 status assessment [16,36,37,38]. More importantly, IHC and FISH methods can sometimes be the only approaches that detect some therapeutic targets in very small tissue biopsies and/or, if only a low percentage of tumor cells are present, knowing that NGS can lead to some false negative results due to the low quantity and/or quality of the extracted nucleic acid [39,40]. As mentioned previously, in contrast to RNA NGS, ROS1 FISH can also lead to false negative results, notably in the presence of small deletions [41]. Moreover, ROS1 FISH has a relatively high price tag and technical difficulties (a sufficient number of tumor cells are needed) and can be time-consuming for the operator.
Thus, it seems important to integrate different approaches for ROS1 status evaluation into a pathology laboratory. ROS1 FISH is useful, notably as an orthogonal tool, in addition to ROS1 IHC to validate some uncertain NGS and/or RT-PCR results, notably in young and non-smoker patients. In addition, new in situ technologies, such as multiplex IHC, could be associated with different antibodies, including a ROS1 antibody. This approach can not only save tissue material, but it can also reduce the TAT to obtain, at the same time, results associated with different targetable molecules [42].
5. Conclusions
In conclusion, the present study demonstrated that performing IHC to evaluate the ROS1 rearrangement status in advanced NS-NSCLC should be abandoned nowadays in favor of ultrafast RNA NGS reflex testing [16,17,43]. ROS1 FISH is still useful for validation of the diagnosis in the case of uncertain NGS results and/or of very small tissue biopsies with a few tumor cells. Finally, ROS1 FISH can also be performed if RNA NGS is negative under certain circumstances (young and/or non-smoker patients, or specific requests by physicians).
Author Contributions
Original draft writing, resources, reviewing, and graphical design, V.H.; reviewing and editing, S.G., C.B., E.L.-M., S.L. and M.I.; supervision, administration, resources, reviewing, and editing, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Centre Hospitalier Universitaire de Nice.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
P.H. received research grants from Thermo-Fisher Scientific, Biocartis, and BMS, and participated on advisory boards form AstraZeneca, Janssen, Abbvie, Roche, BMS, Thermo-Fisher Scientific, Biocartis, Pfizer, AMGEN, Qiagen, and Bayer. The remaining authors declare no conflict of interest.
References
- D’Angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in non-small cell lung cancer: A review with recommendations. Virchows Arch. 2016, 469, 489–503. [Google Scholar] [CrossRef]
- Cheung, C.C.; Smith, A.C.; Albadine, R.; Bigras, G.; Bojarski, A.; Couture, C.; Cutz, J.C.; Huang, W.Y.; Ionescu, D.; Itani, D.; et al. Canadian ROS proto-oncogene 1 study (CROS) for multi-institutional implementation of ROS1 testing in non-small cell lung cancer. Lung Cancer 2021, 160, 127–135. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Smith, D.; Le, C.H.; Liu, W.W.; Ordinario, E.; Manohar, C.; Lee, M.; Rajamani, J.; Truong, H.; Li, J.; et al. Correlation of ROS1 Immunohistochemistry With ROS1 Fusion Status Determined by Fluorescence In Situ Hybridization. Arch. Pathol. Lab. Med. 2020, 144, 735–741. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Gottberg-Williams, A.; Vang, P.; Yang, S.; Britt, N.; Kaur, J.; Haberberger, J.; Danziger, N.; Owens, C.; Beckloff, S.E.; et al. Correlating ROS1 Protein Expression With ROS1 Fusions, Amplifications, and Mutations. JTO Clin. Res. Rep. 2020, 2, 100100. [Google Scholar] [CrossRef]
- Makarem, M.; Ezeife, D.A.; Smith, A.C.; Li, J.J.N.; Law, J.H.; Tsao, M.S.; Leighl, N.B. Reflex ROS1 IHC Screening with FISH Confirmation for Advanced Non-Small Cell Lung Cancer-A Cost-Efficient Strategy in a Public Healthcare System. Curr. Oncol. 2021, 28, 3268–3279. [Google Scholar] [CrossRef]
- Prall, O.W.J.; Browning, J.; Nastevski, V.; Caporarello, S.; Bates, B.; Hewitt, C.A.; Arenas, A.; Lamb, G.; Howlett, K.; Arnolda, R.; et al. ROS1 rearrangements in non-small cell lung cancer: Screening by immunohistochemistry using proportion of cells staining without intensity and excluding cases with MAPK pathway drivers improves test performance. Pathology 2022, 54, 279–285. [Google Scholar] [CrossRef]
- Selinger, C.I.; Li, B.T.; Pavlakis, N.; Links, M.; Gill, A.J.; Lee, A.; Clarke, S.; Tran, T.N.; Lum, T.; Yip, P.Y.; et al. Screening for ROS1 gene rearrangements in non-small-cell lung cancers using immunohistochemistry with FISH confirmation is an effective method to identify this rare target. Histopathology 2017, 70, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Tsuta, K.; Wakai, S.; Arai, Y.; Asamura, H.; Shibata, T.; Furuta, K.; Kohno, T.; Kushima, R. Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod. Pathol. 2014, 27, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. The challenges of evaluating predictive biomarkers using small biopsy tissue samples and liquid biopsies from non-small cell lung cancer patients. J. Thorac. Dis. 2019, 11 (Suppl. S1), S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Kerr, K.M.; Garrido, P.; Thunnissen, E.; Dequeker, E.; Normanno, N.; Patton, S.J.; Fairley, J.; Kapp, J.; de Ridder, D.; et al. Expert opinion on NSCLC small specimen biomarker testing—Part 2: Analysis, reporting, and quality assessment. Virchows Arch. 2022, 481, 351–366. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Hofman, V.; Heeke, S.; Bontoux, C.; Chalabreysse, L.; Barritault, M.; Bringuier, P.P.; Fenouil, T.; Benzerdjeb, N.; Begueret, H.; Merlio, J.P.; et al. Ultra-fast gene fusion assessment for in non-squamous non-small cell lung cancer. JTO Clin. Res. Rep. 2022, 4, 100457. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef]
- Capizzi, E.; Dall’Olio, F.G.; Gruppioni, E.; Sperandi, F.; Altimari, A.; Giunchi, F.; Fiorentino, M.; Ardizzoni, A. Clinical significance of ROS1 5’ deletions in non-small cell lung cancer. Lung Cancer 2019, 135, 88–91. [Google Scholar] [CrossRef]
- Warth, A.; Muley, T.; Dienemann, H.; Goeppert, B.; Stenzinger, A.; Schnabel, P.A.; Schirmacher, P.; Penzel, R.; Weichert, W. ROS1 expression and translocations in non-small-cell lung cancer: Clinicopathological analysis of 1478 cases. Histopathology 2014, 65, 187–194. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Conde, E.; Hernandez, S.; Martinez, R.; Angulo, B.; De Castro, J.; Collazo-Lorduy, A.; Jimenez, B.; Muriel, A.; Mate, J.L.; Moran, T.; et al. Assessment of a New ROS1 Immunohistochemistry Clone (SP384) for the Identification of ROS1 Rearrangements in Patients with Non-Small Cell Lung Carcinoma: The ROSING Study. J. Thorac. Oncol. 2019, 14, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Hernandez, S.; Benito, A.; Caminoa, A.; Garrido, P.; Lopez-Rios, F. Screening for ROS1 fusions in patients with advanced non-small cell lung carcinomas using the VENTANA ROS1 (SP384) Rabbit Monoclonal Primary Antibody. Expert Rev. Mol. Diagn. 2021, 21, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Rouquette, I.; Long-Mira, E.; Piton, N.; Chamorey, E.; Heeke, S.; Vignaud, J.M.; Yguel, C.; Mazières, J.; Lepage, A.L.; et al. Multicenter Evaluation of a Novel ROS1 Immunohistochemistry Assay (SP384) for Detection of ROS1 Rearrangements in a Large Cohort of Lung Adenocarcinoma Patients. J. Thorac. Oncol. 2019, 14, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, G.; Zhang, G.; Song, Z. Evaluation of a new diagnostic immunohistochemistry approach for ROS1 rearrangement in non-small cell lung cancer. Lung Cancer 2020, 146, 224–229. [Google Scholar] [CrossRef]
- Fielder, T.; Butler, J.; Tierney, G.; Holmes, M.; Lam, K.Y.; Satgunaseelan, L.; Colebatch, A.J.; Mahar, A.; Gupta, R.; O’Toole, S.; et al. ROS1 rearrangements in lung adenocarcinomas are defined by diffuse strong immunohistochemical expression of ROS1. Pathology 2022, 54, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Woo, J.; Kim, S. A Systematic Review of Companion Diagnostic Tests by Immunohistochemistry for the Screening of Alectinib-Treated Patients in ALK-Positive Non-Small Cell Lung Cancer. Diagnostics 2022, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Keppens, C.; von der Thüsen, J.; Pauwels, P.; Ryska, A.; ‘t Hart, N.; Schuuring, E.; Miller, K.; Thunnissen, E.; Zwaenepoel, K.; Dequeker, E.M.C. Staining Performance of ALK and ROS1 Immunohistochemistry and Influence on Interpretation in Non-Small-Cell Lung Cancer. J. Mol. Diagn. 2020, 22, 1438–1452. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhan, P.; Zhang, X.; Lv, T.; Song, Y. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2015, 4, 300–309. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Schmidt, R.L.; Furtado, L.V.; Matynia, A.P.; Deftereos, G.; Sirohi, D. Histologic and Molecular Characterization of Non-Small Cell Lung Carcinoma With Discordant ROS1 Immunohistochemistry and Fluorescence In Situ Hybridization. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 19–26. [Google Scholar] [CrossRef]
- Kazdal, D.; Hofman, V.; Christopoulos, P.; Ilié, M.; Stenzinger, A.; Hofman, P. Fusion-positive non-small cell lung carcinoma: Biological principles, clinical practice, and diagnostic implications. Genes Chromosomes Cancer 2022, 61, 244–260. [Google Scholar] [CrossRef]
- Suehara, Y.; Arcila, M.; Wang, L.; Hasanovic, A.; Ang, D.; Ito, T.; Kimura, Y.; Drilon, A.; Guha, U.; Rusch, V.; et al. Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clin. Cancer Res. 2012, 18, 6599–6608. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Haberberger, J.; Sokol, E.; Schrock, A.B.; Danziger, N.; Madison, R.; Trabucco, S.; Jin, D.; Pavlick, D.; Ramanan, V.; et al. Clinicopathologic, genomic and protein expression characterization of 356 ROS1 fusion driven solid tumors cases. Int. J. Cancer 2021, 148, 1778–1788. [Google Scholar] [CrossRef]
- Li, D.; Jiang, H.; Jin, F.; Pan, L.; Xie, Y.; Zhang, L.; Li, C. Concurrent classic driver oncogenes mutation with ROS1 rearrangement predicts superior clinical outcome in NSCLC patients. Genes Genom. 2023, 45, 93–102. [Google Scholar] [CrossRef]
- Horgan, D.; Curigliano, G.; Rieß, O.; Hofman, P.; Büttner, R.; Conte, P.; Cufer, T.; Gallagher, W.M.; Georges, N.; Kerr, K.; et al. Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe. J. Pers. Med. 2022, 12, 72. [Google Scholar] [CrossRef]
- Rojo, F.; Conde, E.; Torres, H.; Cabezón-Gutiérrez, L.; Bautista, D.; Ramos, I.; Carcedo, D.; Arrabal, N.; García, J.F.; Galán, R.; et al. Clinical and economic impact of ‘ROS1-testing’ strategy compared to a ‘no-ROS1-testing’ strategy in advanced NSCLC in Spain. BMC Cancer 2022, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Buglioni, A.; Caffes, P.L.; Hessler, M.G.; Mansfield, A.S.; Lo, Y.C. Clinical Utility Validation of an Automated Ultrarapid Gene Fusion Assay for NSCLC. JTO Clin. Res. Rep. 2022, 3, 100434. [Google Scholar] [CrossRef] [PubMed]
- Depoilly, T.; Garinet, S.; van Kempen, L.C.; Schuuring, E.; Clavé, S.; Bellosillo, B.; Ercolani, C.; Buglioni, S.; Siemanowski, J.; Merkelbach-Bruse, S.; et al. Multicenter Evaluation of the Idylla GeneFusion in Non-Small-Cell Lung Cancer. J. Mol. Diagn. 2022, 24, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Rojo, F.; Gómez, J.; Enguita, A.B.; Abdulkader, I.; González, A.; Lozano, D.; Mancheño, N.; Salas, C.; Salido, M.; et al. Molecular diagnosis in non-small-cell lung cancer: Expert opinion on ALK and ROS1 testing. J. Clin. Pathol. 2022, 75, 145–153. [Google Scholar] [CrossRef]
- Hofman, V.; Lassalle, S.; Bence, C.; Long-Mira, E.; Nahon-Estève, S.; Heeke, S.; Lespinet-Fabre, V.; Butori, C.; Ilié, M.; Hofman, P. Any Place for Immunohistochemistry within the Predictive Biomarkers of Treatment in Lung Cancer Patients? Cancers 2018, 10, 70. [Google Scholar] [CrossRef]
- Davies, K.D.; Le, A.T.; Sheren, J.; Nijmeh, H.; Gowan, K.; Jones, K.L.; Varella-Garcia, M.; Aisner, D.L.; Doebele, R.C. Comparison of Molecular Testing Modalities for Detection of ROS1 Rearrangements in a Cohort of Positive Patient Samples. J. Thorac. Oncol. 2018, 13, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Beaulande, M.; Long-Mira, E.; Bontoux, C.; Zahaf, K.; Lalvée, S.; Hamila, M.; Benzaquen, J.; Cohen, C.; Berthet, J.P.; et al. Analytical validation of automated multiplex chromogenic immunohistochemistry for diagnostic and predictive purpose in non-small cell lung cancer. Lung Cancer 2022, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Heeke, S.; Bontoux, C.; Boutros, J.; Ilié, M.; Hofman, V.; Marquette, C.H.; Hofman, P.; Benzaquen, J. Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing. J. Pers. Med. 2022, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).