Abstract
Pregnant women are more prone to experience severe COVID-19 disease, including intensive care unit (ICU) admission, use of invasive ventilation, extracorporeal membrane oxygenation (ECMO), and mortality compared to non-pregnant individuals. Additionally, research suggests that SARS-CoV-2 infection during pregnancy is linked to adverse pregnancy outcomes, such as preterm birth, preeclampsia, and stillbirth, as well as adverse neonatal outcomes, including hospitalization and admission to the neonatal intensive care unit. This review assessed the available literature from November 2021 to 19 March 2023, concerning the safety and effectiveness of COVID-19 vaccination during pregnancy. COVID-19 vaccination administered during pregnancy is not linked to significant adverse events related to the vaccine or negative obstetric, fetal, or neonatal outcomes. Moreover, the vaccine has the same effectiveness in preventing severe COVID-19 disease in pregnant individuals as in the general population. Additionally, COVID-19 vaccination is the safest and most effective method for pregnant women to protect themselves and their newborns from severe COVID-19 disease, hospitalization, and ICU admission. Thus, vaccination should be recommended for pregnant patients. While the immunogenicity of vaccination in pregnancy appears to be similar to that in the general population, more research is needed to determine the optimal timing of vaccination during pregnancy for the benefit of the neonate.
1. Introduction
The effectiveness of COVID-19 vaccination was first reported in December 2020. Subsequently, mass vaccination of the general population took place [1]. Nonetheless, pregnant women were not included in initial COVID-19 vaccine trials due to concerns about the lack of prior experience with mRNA vaccines in this population and uncertainty about the potential risks to both the mother and fetus [1,2,3]. As a result, the available information on the long-term safety and efficacy of COVID-19 vaccines in pregnant individuals is currently limited to observational data [1,4]. This limited knowledge has contributed to vaccine hesitancy among pregnant women [1].
Compared to non-pregnant women, pregnant women are at an elevated risk of experiencing severe disease following SARS-CoV-2 infection [1,5,6,7,8]. Indeed, pregnant patients with SARS-CoV-2 infection are three times more likely to require admission to an intensive care unit (ICU), 2.9 times more likely to require invasive ventilation, 2.4 times more likely to need extracorporeal membrane oxygenation (ECMO) and 1.7 times more likely to die [3,5,6,7,8,9,10,11,12,13,14,15,16]. Additionally, pregnant patients are at a higher risk of experiencing sepsis, acute respiratory distress syndrome, thromboembolic events, acute renal failure, and adverse cardiac events compared to non-pregnant patients with COVID-19 [7,12,13,17].
Regarding pregnancy outcomes, pregnant women with SARS-CoV-2 infection are at a heightened risk of preeclampsia/eclampsia, preterm birth, stillbirth, fetal distress, premature rupture of membranes, gestational diabetes, impaired fetal growth, and cesarean section, compared to pregnant women without the infection [7,13,14,15,16,17,18,19,20,21].
Concerning infants, those born to women with laboratory-confirmed SARS-CoV-2 infection are 1.45 times more likely of neonatal adverse outcomes, 1.24 times more likely to require neonatal ICU, 1.61 times more likely to require prolonged neonatal hospitalization and associate a 2-fold higher risk of death, compared to those born without the infection [8,19,22]. The main reason for neonatal morbidity in pregnant women with SARS-CoV-2 infection is prematurity due to the increased rates of preterm deliveries in pregnant women with severe COVID-19 [18].
Given that COVID-19 is associated with maternal, fetal, and neonatal adverse outcomes, the vast majority of scientific societies, including the American College of Obstetricians and Gynecologists (ACOG), the Royal College of Obstetricians and Gynecologists (RCOG), and the World Health Organization (WHO) strongly support COVID-19 vaccination during pregnancy [4]. However, in some countries, COVID-19 vaccination in pregnant women is still not recommended. In Africa, although the vast majority of countries have not been positioned yet, from those who had, Argelia, Libia, and Egypt do not recommend vaccination in pregnant women. In Asia, China, and Kazakhstan, neither recommend the vaccine, whereas, in Europe and South America, it is limited to Lituania and Nicaragüa, respectively [23]. Given that data regarding COVID-19 vaccines in pregnancy is constantly evolving, an update regarding COVID-19 vaccination during pregnancy is presented in this literature review.
2. Methods
2.1. Search Strategy and Selection Criteria
A literature review was conducted by searching for published studies in the PubMed database written in Spanish or English from November 2021 to 19 March 2023. The search terms used were: (pregnant OR pregnancy) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination) AND (maternal outcomes OR pregnancy complications) AND (neonatal outcomes OR infant complications).
The inclusion criteria were: (1) retrospective or prospective studies that provided a detailed description of the methodological approach used, including cohort studies, case-control studies, case reports, case series, narrative reviews, literature reviews, and meta-analyses, (2) studies with full-text available, (3) studies written in Spanish or English from November 2021 to 19 March 2023, (4) studies that investigated the efficacy and safety of COVID-19 vaccination during pregnancy, (5) studies that addressed the maternal and neonatal impact of COVID-19 vaccines administered in pregnant women, (6) studies that evaluated the immunological patterns of the COVID-19 vaccine in mothers and their neonates during pregnancy. The following studies were excluded: (1) studies that did not provide a detailed description of the methodology used, as well as the number of patients or studies included, (2) editorials or conference papers, (3) studies with no full text available, (4) studies written languages other than Spanish or English, and (5) those irrelevant to the subject of the literature review after reading the title and the abstract.
Additionally, the bibliography of the papers selected was screened in the search for other studies that accomplished the criteria mentioned above to be included.
2.2. Data Extraction
The main objective of this review was to examine the safety of COVID-19 vaccination during pregnancy, including the mRNA-1273 vaccine, the adenovirus vector vaccine, and the BNT162b2 vaccine. The secondary objectives of the study were, firstly, to evaluate the efficacy of COVID-19 vaccines in decreasing unfavorable maternal and neonatal outcomes linked to COVID-19, and secondly, to address whether COVID-19 vaccination during pregnancy enhances the neonatal immune system against SARS-CoV-2 infection either through cord blood or breastfeeding transmission of antibodies.
The selected studies were analyzed for the following data: (1) basic information about the studies, such as publication year, first author, and study design; (2) characteristics of the study population, including the type of participants, sample size, and location; (3) details about the type of COVID-19 vaccines used, number of doses administered, and timing of vaccination during pregnancy; and (4) results and conclusions of the studies. The basic characteristics of the included studies are summarized in Table 1.
2.3. Data Synthesis
A narrative review was conducted using the data gathered from the selected studies. ZOTERO was employed to sort out articles and remove any duplicates. The safety of COVID-19 vaccines during pregnancy was assessed by examining the potential adverse effects described in the literature.
The effectiveness of COVID-19 vaccines in pregnant women vaccinated during or before pregnancy was evaluated based on reported pregnancy outcomes. These included the incidence of SARS-CoV-2 infection, severe illness, hospitalization related to COVID-19, ICU admission, mortality, and other adverse maternal, fetal, or neonatal outcomes.
Ultimately, data reported in the literature concerning the benefits of maternal COVID-19 vaccination on the neonatal immune system was carefully reviewed.
3. Results
3.1. Included Articles
When the initial literature research was conducted up to 19 March 2023, 177 articles were identified. After reviewing the titles and abstracts, 110 articles were excluded. Then, 33 more articles were excluded during the full-text review based on the inclusion and exclusion criteria. Screening the bibliography of the already selected ones, three additional articles were selected. As a result, 37 studies were included in the literature review. The literature retrieval flow diagram is presented in Figure 1.
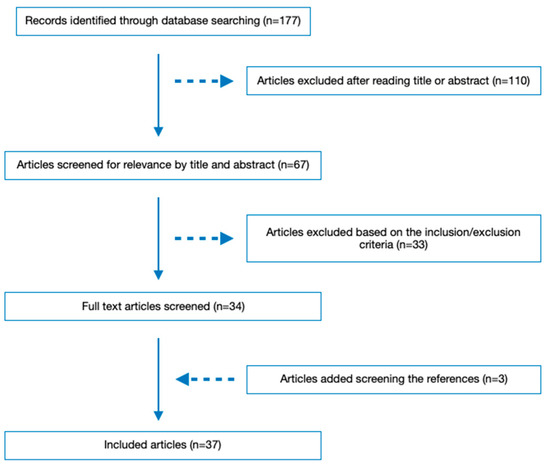
Figure 1.
Flow diagram of included articles.
3.2. Safety of COVID-19 Vaccination during Pregnancy
Observational studies and large case series investigating COVID-19 vaccines in pregnancy have not found any significant adverse events related to the vaccines, apart from the ones commonly described for the general population, such as injection site pain, fever, rash, myalgia, arthralgia, fatigue, headache, chills, lymphadenopathy or lymphadenitis, and nausea or vomiting [24,25]. Moreover, there have been no reports of obstetric, fetal, or neonatal adverse outcomes [1,3,10,24,26,27,28,29,30]. Additionally, there is no evidence of clinically relevant levels of transplacental transfer of mRNA vaccine products [31,32,33].
Hence, there is no proof of an elevated risk of adverse obstetric outcomes, such as miscarriage, preterm birth, earlier gestation at birth, small for gestational age at birth, fetal growth restriction, placental abruption, eclampsia/preeclampsia, gestational hypertension, stillbirth or chorioamnionitis, associated with COVID-19 vaccination during pregnancy [1,11,27,28,34]. Furthermore, COVID-19 vaccination does not appear to increase the risk of birth trauma, mode of delivery, postpartum hemorrhage, maternal death, pulmonary embolism, intensive care unit admission, puerperal fever, the incidence of thromboembolism or length of hospital stay [25,35,36,37,38].
3.3. Effectiveness of COVID-19 Vaccines in Reducing Adverse Maternal, Obstetric, and Neonatal Outcomes Associated with COVID-19
Regarding the effectiveness of COVID-19 vaccines in pregnant women, it has been estimated that COVID-19 vaccines are equally effective in pregnant women as in the general population [1,27,39]. In this line, several studies have demonstrated that COVID-19 vaccines prevent the risk of COVID-19 infection, severe COVID-19, hospitalization, ICU admission, and maternal death among pregnant individuals [25,38,39,40,41,42,43,44].
Ma Y et al. and Goldshtein et al. reported a 50% reduction in the risk of SARS-CoV-2 infection and COVID-19-related hospitalization with COVID-19 vaccination [10,45]. Other studies have also reported that vaccinated pregnant women have a lower frequency of infection before delivery compared to unvaccinated women [25,39,45]. Moreover, a study published by Kim et al. found that 4.1% and 25% of patients in the vaccination and non-vaccination groups, respectively, had severe symptoms, and 2.6% and 16.2% required oxygen therapy [46]. In this line, Ilter et al. reported in vaccinated pregnant people a significant reduction in the requirement for oxygen support (0.0 vs. 9.6%, p = 0.015) and the admission to an intensive care unit (0.0 vs. 3.8%) compared to the unvaccinated group [44]. Furthermore, in a recent study by Eid et al., a significant benefit was found in the vaccinated group with reduced COVID-19 severity, improved clinical results, and fewer hospital or ICU admissions [47].
Regarding obstetric outcomes, Stock et al. and Hui et al. have reported a decrease in stillbirth in pregnant individuals who received the COVID-19 vaccine [39,48]. In addition, a systematic review and meta-analysis found that COVID-19 vaccination was linked to a 15% reduction in stillbirth cases [1]. Watanabe et al. also provided evidence that the administration of COVID-19 vaccines in pregnancy reduced intrauterine fetal death [38].
Concerning preterm delivery, Hui et al. reported a significant decrease in total preterm births <37 weeks (5.1% vs. 9.2%), spontaneous preterm birth (2.4% vs. 4.0%), and iatrogenic preterm birth (2.7% vs. 5.2%) with COVID-19 vaccination [48]. In addition, Carbone et al. and Schrag et al. reported a reduced likelihood of lower gestational age at delivery, non-reassuring fetal monitoring, and a decreased rate of premature delivery in pregnant women who received the COVID-19 vaccine compared to those unvaccinated [43,49].
Table 1 summarizes the main maternal and neonatal benefits and risks of COVID-19 vaccination during pregnancy from the studies included in the review.

Table 1.
Main maternal and neonatal benefits and risks of COVID-19 vaccination in pregnancy from the studies included in the review.
Table 1.
Main maternal and neonatal benefits and risks of COVID-19 vaccination in pregnancy from the studies included in the review.
| Main Benefits of COVID-19 Vaccination in Pregnancy | Main Risks of COVID-19 Vaccination in Pregnancy |
|---|---|
| Reduction in the risk of SARS-CoV-2 infection [10,25,38,39,40,41,42,43,44,45] | Injection site pain [24,25] |
| Reduction in the risk of severe SARS-CoV-2 infection [25,38,39,40,41,42,43,44,46,47] | Fever [24,25] |
| Reduction in the risk of COVID-19-related hospitalization [10,25,38,39,40,41,42,43,44,45,47] | Rash [24,25] |
| Reduction in the risk of ICU admission [25,38,39,40,41,42,43,44,47] | Fatigue [24,25] |
| Reduction in the risk of maternal mortality [25,38,39,40,41,42,43,44] | Arthralgia [24,25] |
| Decrease in stillbirth [1,38,39,48] | Myalgia [24,25] |
| Decrease in total preterm births [43,48,49] | Headache [24,25] |
| Reduction in the risk of SARS-CoV-2 infection in infants <6 months [50,51] | Nausea or vomiting [24,25] |
| Reduction in the risk of severe SARS-CoV-2 infection in infants, including MIS-C [3,52] | Chills [24,25] |
| Reduction in the risk of hospitalization for COVID-19 in infants <6 months [17,25,40,43,53,54] | Lymfadenopathy [24,25] |
| Reduction in the risk of ICU admission in infants <6 months [3] | Lymfadenithis [24,25] |
3.4. COVID-19 Vaccination and Potential Benefits for the Neonatal Immune System against SARS-CoV-2 Infection
Regarding hospitalization of newborns, a test-negative and case-control study conducted in 17 US states from July 2021 to January 2022 assessed the efficacy of mRNA vaccines during pregnancy against COVID-19-related hospitalization in infants under six months of age. The study revealed a vaccine effectiveness rate of 61%. Moreover, when administered within the first 20 weeks of gestation, two doses of the vaccine were 32% effective in preventing COVID-19-associated hospitalization in infants, whereas administering the vaccine from 21 weeks gestation through to 14 days before delivery resulted in an efficacy rate of 80% [17,25,40,43,53]. Another study found that maternal vaccination was 52% effective in preventing hospitalization for COVID-19 in infants. The effectiveness was higher at 69% when maternal vaccination was administered above 20 weeks of gestation and lower at 38% during the first 20 weeks of pregnancy [54]. Halasa et al. also pointed out that administering the SARS-CoV-2 vaccine during pregnancy could decrease the risk of infant hospitalization for COVID-19 by 30–70% up to 4–6 months of age [17]. In this line, Kugelam et al. recommend COVID-19 vaccination for pregnant individuals in the second trimester to provide protection for the mother and ensure the safety of the newborn [55].
Regarding infection rates, early neonatal SARS-CoV-2 infections were mainly observed among unvaccinated mothers. This indicates that infants born to mothers vaccinated with the mRNA vaccine type had a significantly reduced risk of infection with SARS-CoV-2 compared to infants born to unvaccinated mothers [50,51]. Fully vaccinated mothers had an effectiveness of 61.6% (95% CI, 31.9–78.4), whereas partially vaccinated mothers’ effectiveness was not significant [53].
Referring to adverse neonatal outcomes, various studies showed that the administration of the COVID-19 vaccine to pregnant individuals in the third trimester reduced the risk of neonatal adverse outcomes, including multisystem inflammatory syndrome in children (MIS-C) [3,52]. An extensive safety study conducted in Canada indicated that maternal vaccination might protect against low Apgar scores and admission to neonatal intensive care units [43]. Accordingly, Rottenstreich et al. reported that infants born to mothers vaccinated with two doses of mRNA vaccine during gestation had a 30% lower likelihood of COVID-19-associated admission to the ICU in the first six months of life [3].
Table 2 summarizes the results from the studies included in the analysis.

Table 2.
Essential characteristics, results, and conclusions of the studies included in the review.
4. Discussion
The present narrative review has evaluated the impact of SARS-CoV-2 vaccination on maternal, obstetric, and neonatal outcomes. The findings indicate that the uptake of the COVID-19 vaccine during pregnancy does not result in significant adverse events related to the vaccine or poorer outcomes for mothers, obstetric/fetal health, or neonates [1,3,7,10,12,18,24,26,27,28,29,30,57]. In this line, data from the CDC v-safe registry, one of the largest international registries on COVID-19 vaccines during pregnancy, suggest that there are no apparent safety concerns related to COVID-19 vaccination in pregnancy in terms of obstetric or neonatal outcomes [58]. Additionally, the timing of COVID-19 vaccination during pregnancy does not have a significant impact on maternal–fetal outcomes [45]. The available evidence affirms that COVID-19 vaccination during pregnancy is safe and effective, regardless of the gestational age at which it is administered [58]. The main adverse effects of COVID-19 vaccination during pregnancy are the same as in the general population. Therefore, they usually appear within the first seven days, with local complications. Pain at the injection site is the most common one. Regarding systemic complications, fever would be the most frequent and potentially harmful to the fetus. Nonetheless, the use of antipyretics could help to reduce this risk [24,25,59,60]. It is important to note that the COVID-19 vaccine has not been specifically studied in pregnant women who have had complications associated with COVID-19. However, they may benefit from vaccination against COVID-19, as the vaccine can help to reduce the risk of severe illness and adverse outcomes [61].
Moreover, the vaccine’s effectiveness appears to be comparable to that observed in non-pregnant individuals [1,5,27,39]. Studies examining vaccine effectiveness have reported varying rates ranging from 41% to 96%, depending on variables including the predominant COVID-19 variant at the time of the study, the vaccine dosage administered, and the outcome variable assessed (severe COVID-19, symptomatic COVID-19, or COVID-19 infection), among other variables [1,27,39]. In general, it is recommended that pregnant women follow the same dosage and administration interval recommendations for COVID-19 vaccination as those applied to the general population. Most COVID-19 vaccines approved so far require two doses, administered at a specific time interval depending on the vaccine type, to achieve maximum efficacy [58,62]. On the other hand, the FDA states that the results from SARS-CoV-2 antibody tests currently authorized should not be used to determine a person’s level of immunity or protection against COVID-19 at any time, including gestation. These tests have not been validated to assess the level of protection, and if results are misinterpreted, there is a risk that individuals may take fewer precautions against SARS-CoV-2 exposure or may or may even choose not to get vaccinated when it is recommended [63].
Regarding maternal benefits of vaccination, the available evidence suggests that pregnant women who are COVID-19 vaccinated experience a lower incidence of infection, severe illness, hospitalization, ICU admission, and need for oxygen therapy prior to delivery when compared to unvaccinated pregnant women [4,5,14,15,25,38,39,40,41,42,43,44,57,64,65]. Additionally, a reduction in preterm birth and stillbirth in vaccinated pregnant women has been proven [1,20,38,48]. It has been reported that stillbirth in pregnant women with COVID-19 is associated with placental insufficiency and infection caused by high levels of viremia [65,66]. Thus, COVID-19 vaccines reduce viremia and may be highly effective in decreasing the risk of stillbirth [67].
Referring to neonates born from vaccinated mothers, studies show a lower rate of SARS-CoV-2 infection, hospitalization, ICU admission, and adverse neonatal outcomes such as MIS-C [3,5,15,17,25,40,43,50,51,52,53,64,65,68]. This benefit to neonates is due to the fetal transfer across the placenta of anti-Spike IgG maternal antibodies induced by the vaccine, which offers stronger protection against SARS-CoV-2 infection during the first few months, with longer disease-free intervals correlated with higher antibodies levels at birth [69]. However, the protection afforded by infant antibodies against SARS-CoV-2 significantly diminishes after six months, highlighting the need for vaccination at this point to maximize defense against COVID-19 [69]. Since COVID-19 vaccines are not authorized for infants under six months, these findings support the notion that newborns benefit from maternal vaccination in terms of protection [25].
Noticeably, maternal vaccination induces a higher antibody response than natural infection, not only through cord blood antibody transmission but also in relation to the transfer of antibodies during breastfeeding [56,70,71]. In fact, breast milk from vaccinated mothers provides immediate protection through antibodies and long-term protection through cellular immunity [72]. Similarly, when referring to transplacental transfer, evidence suggests a direct association between the antibody levels found in maternal serum and cord blood, commonly defined as the placental transfer ratio [25,70,71]. The extent of maternal protection through the transfer of antibodies across the placenta relies on the concentration of antibodies in the maternal bloodstream, which is influenced by the moment of vaccination and delivery, suggesting that the interval between vaccination and birth may be a crucial factor in determining the degree of protection that the mother offers to her newborn [25,56].
Nonetheless, the ideal timing of COVID-19 vaccination in pregnancy to optimize neonatal benefit is still unclear [25,68]. Although the levels of maternal IgG and the transfer ratio of IgG across the placenta tend to rise as pregnancy progresses, the transfer of antibodies through the placenta usually begins during the second trimester, with the highest efficiency observed in the third trimester [25]. Administering the vaccine as soon as possible during gestation is essential to maximize the duration of maternal defense against infection. However, infant protection would depend on the efficiency and concentration of transplacentally acquired antibodies, which most studies suggest is higher during the third trimester [68]. Some studies have reported that all neonatal benefits are higher when mothers are vaccinated after 20 weeks of pregnancy [5,54]. Based on this hypothesis, some experts have recommended administering the booster vaccine in the early third trimester [25]. However, other studies support COVID-19 vaccination before conception or as early as possible during pregnancy to decrease the rate of severe COVID-19 disease during gestation and COVID-19 obstetric complications [40,73]. Nevertheless, at present, given that the majority of pregnant women are immunized against COVID-19, it should be noted that the absolute reduction in the risk after administering a booster dose in this population is likely to be small. Therefore, strategies for the complete vaccination of unvaccinated pregnant women would be more effective in reducing COVID-19 complications than offering booster doses to all those who are already immunized [74,75,76].
This study has several strengths, including its thorough search for evidence, rigorous selection criteria for the relevant literature, meticulous data collection, and objective analysis. Nonetheless, there are also some limitations to mention. Firstly, the retrospective nature of the study design introduces the potential probability of biases that are intrinsic to this type of study. Additionally, unknown factors could influence the findings, such as the impact of different COVID-19 variants and the gestational week at the moment of vaccination, both of which could affect maternal and neonatal outcomes. These factors should be further evaluated in future studies.
5. Conclusions
The safest and most effective method for pregnant women to safeguard themselves and their newborns from severe COVID-19 disease, hospitalization, and ICU admission is through COVID-19 vaccination. According to available data, there is no evidence of an increased risk of adverse outcomes for pregnant women, obstetric complications, or neonatal health problems following COVID-19 vaccination. Therefore, vaccination is recommended. The immunogenicity of vaccination in pregnant women appears to be comparable to that of the general population. However, the best timing for vaccination during pregnancy to benefit the newborn is still unclear, underscoring the need for further research.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, and writing have been performed by A.M.-V. and C.J.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this review study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prasad, S.; Kalafat, E.; Blakeway, H.; Townsend, R.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Ladhani, S.; et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022, 13, 2414. [Google Scholar] [CrossRef] [PubMed]
- Pregnant Women: Scientific and Ethical Considerations for Inclusion in Clinical Trials Guidance for Industry. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 25 April 2021).
- Rottenstreich, M.; Sela, H.Y.; Rotem, R.; Kadish, E.; Wiener-Well, Y.; Grisaru-Granovsky, S. COVID-19 vaccination during the third trimester of pregnancy: Rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: Overview of current recommendations and pre-and post-marketing evidence for vaccine efficacy and safety. Drug Saf. 2021, 44, 1247–1269. [Google Scholar] [CrossRef] [PubMed]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J. COVID-19 and Pregnancy. Infect. Dis. Clin. N. Am. 2022, 36, 423–433. [Google Scholar] [CrossRef]
- Delara, M.; Sadarangani, M. Immunization in pregnancy to protect pregnant people and their newborns against COVID-19. Expert Rev. Vaccines 2022, 21, 593–595. [Google Scholar] [CrossRef]
- Munoz, F.M.; Beigi, R.H.; Posavad, C.M.; Richardson, B.A.; Chu, H.Y.; Bok, K.; Campbell, J.; Cardemil, C.; DeFranco, E.; Frenck, R.W.; et al. Multi-site observational maternal and infant COVID-19 vaccine study (MOMI-vax): A study protocol. BMC Pregnancy Childbirth 2022, 22, 402. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., III; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1641. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Effectiveness and Safety of COVID-19 Vaccine among Pregnant Women in Real-World Studies: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 246. [Google Scholar] [CrossRef]
- Lipkind, H.S.; Vazquez-Benitez, G.; DeSilva, M.; Vesco, K.K.; Ackerman-Banks, C.; Zhu, J.; Boyce, T.G.; Daley, M.F.; Fuller, C.C.; Getahun, D.; et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—Eight Integrated Health Care Organizations, United States, December 15, 2020–July 22, 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 26. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Zovi, A.; Trama, U.; Boccellino, M. Pregnancy and COVID-19, focus on vaccine and pharmacological treatment. J. Reprod. Immunol. 2022, 151, 103630. [Google Scholar] [CrossRef]
- Twanow, J.-D.E.; McCabe, C.; Ream, M. The COVID-19 Pandemic and Pregnancy: Impact on Mothers and Newborns. Semin. Pediatr. Neurol. 2022, 42, 100977. [Google Scholar] [CrossRef]
- Grünebaum, A.; Dudenhausen, J.; Chervenak, F.A. Covid and pregnancy in the United States—An update as of August 2022. J. Perinat. Med. 2022, 51, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Safadi, M.A.; Spinardi, J.; Swerdlow, D.; Srivastava, A. COVID-19 disease and vaccination in pregnant and lactating women. Am. J. Reprod. Immunol. 2022, 88, e13550. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2021, 226, 177–186. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 Months—17 States, July 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 264. [Google Scholar]
- Şahin, D.; Tanaçan, A.; Webster, S.N.; Tekin, Ö.M. Pregnancy and COVID-19: Prevention, vaccination, therapy, and beyond. Turk. J. Med. Sci. 2021, 51, 3312–3326. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Chemaitelly, H.; Al Khal, A.; Coyle, P.V.; Saleh, H.; Kaleeckal, A.H.; Latif, A.N.; Bertollini, R.; Abou-Samra, A.B.; Abu-Raddad, L.J. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J. Clin. Investig. 2021, 131, e153662. [Google Scholar] [CrossRef]
- Piekos, S.N.; Roper, R.T.; Hwang, Y.M.; Sorensen, T.; Price, N.D.; Hood, L.; Hadlock, J.J. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: A retrospective multicentre cohort study. Lancet Digit. Health 2022, 4, e95–e104. [Google Scholar] [CrossRef]
- Radan, A.P.; Fluri, M.M.; Nirgianakis, K.; Mosimann, B.; Schlatter, B.; Raio, L.; Surbek, D. Gestational diabetes is associated with SARS-CoV-2 infection during pregnancy: A case-control study. Diabetes Metab. 2022, 48, 101351. [Google Scholar] [CrossRef]
- Facciolà, A.; Micali, C.; Visalli, G.; Venanzi Rullo, E.; Russotto, Y.; Laganà, P.; Laganà, A.; Nunnari, G.; Di Pietro, A. COVID-19 and pregnancy: Clinical outcomes and scientific evidence about vaccination. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2610–2626. [Google Scholar] [PubMed]
- Zavala, E.; Krubiner, C.B.; Jaffe, E.F.; Nicklin, A.; Gur-Arie, R.; Wonodi, C.; Faden, R.R.; Karron, R.A. Global disparities in public health guidance for the use of COVID-19 vaccines in pregnancy. BMJ Glob. Health 2022, 7, e007730. [Google Scholar] [CrossRef]
- Favre, G.; Maisonneuve, E.; Pomar, L.; Winterfeld, U.; Daire, C.; Martinez de Tejada, B.; Delecraz, D.; Campelo, S.; Moser, M.; Todesco-Bernasconi, M.; et al. COVID-19 mRNA vaccine in pregnancy: Results of the Swiss COVI-PREG registry, an observational prospective cohort study. Lancet Reg. Health-Eur. 2022, 18, 100410. [Google Scholar] [CrossRef]
- Badell, M.L.; Dude, C.M.; Rasmussen, S.A.; Jamieson, D.J. COVID-19 vaccination in pregnancy. BMJ 2022, 378, e069741. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Dhinsa, T.; Alton, G.D.; Török, E.; Dimanlig-Cruz, S.; Regan, A.K.; Sprague, A.E.; Buchan, S.A.; Kwong, J.C.; Wilson, S.E.; et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA 2022, 327, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Örtqvist, A.K.; Dahlqwist, E.; Ljung, R.; Skår, F.; Oakley, L.; Macsali, F.; Pasternak, B.; Gjessing, H.K.; Håberg, S.E.; et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022, 327, 1469–1477. [Google Scholar] [CrossRef]
- Peretz-Machluf, R.; Hirsh-Yechezkel, G.; Zaslavsky-Paltiel, I.; Farhi, A.; Avisar, N.; Lerner-Geva, L.; Meyer, R.; Tsur, A.; Yinon, Y. Obstetric and Neonatal Outcomes following COVID-19 Vaccination in Pregnancy. J. Clin. Med. 2022, 11, 2540. [Google Scholar] [CrossRef]
- Mascolo, A.; di Mauro, G.; Fraenza, F.; Gaio, M.; Zinzi, A.; Pentella, C.; Rossi, F.; Capuano, A.; Sportiello, L. Maternal, Fetal, and Neonatal Outcomes Among Pregnant Women Receiving COVID-19 Vaccination: The Preg-Co-Vax Study. Front. Immunol. 2022, 13, 965171. [Google Scholar] [CrossRef]
- Citu, I.M.; Citu, C.; Gorun, F.; Sas, I.; Tomescu, L.; Neamtu, R.; Motoc, A.; Gorun, O.M.; Burlea, B.; Bratosin, F.; et al. Immunogenicity following administration of BNT162b2 and Ad26. COV2. S COVID-19 vaccines in the pregnant population during the third trimester. Viruses 2022, 14, 307. [Google Scholar] [CrossRef]
- Prahl, M.; Golan, Y.; Cassidy, A.G.; Matsui, Y.; Li, L.; Alvarenga, B.; Chen, H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy. Nat. Commun. 2022, 13, 4422. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Wachman, E.M.; Boateng, J.; Clarke, K.; Snyder-Cappione, J.; Taglauer, E.S. The impact of maternal SARS-CoV-2 vaccination and first trimester infection on feto-maternal immune responses. Am. J. Reprod. Immunol. 2022, 88, e13625. [Google Scholar] [CrossRef]
- Otero, S.; Miller, E.S.; Sunderraj, A.; Shanes, E.D.; Sakowicz, A.; Goldstein, J.A.; Mithal, L.B. Maternal Antibody Response and Transplacental Transfer Following SARS-CoV-2 Infection or Vaccination in Pregnancy. Clin. Infect. Dis. 2022, 76, 220–228. [Google Scholar] [CrossRef]
- Pratama, N.R.; Wafa, I.A.; Budi, D.S.; Putra, M.; Wardhana, M.P.; Wungu, C.-D.K. mRNA COVID-19 vaccines in pregnancy: A systematic review. PLoS ONE 2022, 17, e0261350. [Google Scholar] [CrossRef]
- Hagrass, A.I.; Almadhoon, H.W.; Al-Kafarna, M.; Almaghary, B.K.; Nourelden, A.Z.; Fathallah, A.H.; Hasan, M.T.; Mohammed, Y.A.; Al-Nabahin, A.O.; Wafi, D.S.; et al. Maternal and neonatal safety outcomes after SAR-CoV-2 vaccination during pregnancy: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2022, 22, 581. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Dimanlig-Cruz, S.; Regan, A.K.; Håberg, S.E.; Gravel, C.A.; Oakley, L.; Alton, G.D.; Török, E.; Dhinsa, T.; Shah, P.S.; et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after COVID-19 vaccination during pregnancy: Population based retrospective cohort study. BMJ 2022, 378, e071416. [Google Scholar] [CrossRef]
- Popescu, D.E.; Cîtu, C.; Jura, A.M.C.; Lungu, N.; Navolan, D.; Craina, M.; Semenescu, A.; Gorun, F.; Jura, M.A.; Belengeanu, V.; et al. The Benefits of Vaccination against SARS-CoV-2 during Pregnancy in Favor of the Mother/Newborn Dyad. Vaccines 2022, 10, 848. [Google Scholar] [CrossRef]
- Watanabe, A.; Yasuhara, J.; Iwagami, M.; Miyamoto, Y.; Yamada, Y.; Suzuki, Y.; Takagi, H.; Kuno, T. Peripartum outcomes associated with COVID-19 vaccination during pregnancy: A systematic review and meta-analysis. JAMA Pediatr. 2022, 176, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.E.M.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022, 28, 504–512. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- De Rose, D.U.; Salvatori, G.; Dotta, A.; Auriti, C. SARS-CoV-2 vaccines during pregnancy and breastfeeding: A systematic review of maternal and neonatal outcomes. Viruses 2022, 14, 539. [Google Scholar] [CrossRef]
- Morgan, J.A.; Biggio, J.R., Jr.; Martin, J.K.; Mussarat, N.; Chawla, H.K.; Puri, P.; Williams, F.B. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet. Gynecol. 2022, 139, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Verani, J.R.; Dixon, B.E.; Page, J.M.; Butterfield, K.A.; Gaglani, M.; Vazquez-Benitez, G.; Zerbo, O.; Natarajan, K.; Ong, T.C.; et al. Estimation of COVID-19 mRNA Vaccine Effectiveness Against Medically Attended COVID-19 in Pregnancy During Periods of Delta and Omicron Variant Predominance in the United States. JAMA Netw. Open 2022, 5, e2233273. [Google Scholar] [CrossRef] [PubMed]
- Ilter, P.B.; Prasad, S.; Berkkan, M.; Mutlu, M.A.; Tekin, A.B.; Celik, E.; Ata, B.; Turgal, M.; Yildiz, S.; Turkgeldi, E.; et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet. Gynecol. 2022, 59, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Goldshtein, I.; Nevo, D.; Steinberg, D.M.; Rotem, R.S.; Gorfine, M.; Chodick, G.; Segal, Y. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 2021, 326, 728–735. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S.; Kim, H.M.; Kim, M.J.; Kwon, K.T.; Cha, H.H.; Seong, W.J. Impact of vaccination and the omicron variant on COVID-19 severity in pregnant women. Am. J. Infect. Control 2023, 51, 351–353. [Google Scholar] [CrossRef]
- Eid, J.; Abdelwahab, M.; Williams, H.; Caplan, M.; Hajmurad, S.; Venkatesh, K.K.; Costantine, M.M.; Rood, K.M. Decreased severity of COVID-19 in vaccinated pregnant individuals during predominance of different SARS-CoV-2 variants. Am. J. Reprod. Immunol. 2022, 88, e13596. [Google Scholar] [CrossRef]
- Hui, L.; Marzan, M.B.; Rolnik, D.L.; Potenza, S.; Pritchard, N.; Said, J.M.; Palmer, K.R.; Whitehead, C.L.; Sheehan, P.M.; Ford, J.; et al. Reductions in stillbirths and preterm birth in COVID-19 vaccinated women: A multi-center cohort study of vaccination uptake and perinatal outcomes. Am. J. Obstet. Gynecol. 2022. online ahead of print. [Google Scholar]
- Carbone, L.; Trinchillo, M.G.; Di Girolamo, R.; Raffone, A.; Saccone, G.; Iorio, G.G.; Gabrielli, O.; Maruotti, G.M. COVID-19 vaccine and pregnancy outcomes: A systematic review and meta-analysis. Int. J. Gynecol. Obstet. 2022, 159, 651–661. [Google Scholar] [CrossRef]
- Adhikari, E.H.; MacDonald, L.; SoRelle, J.A.; Morse, J.; Pruszynski, J.; Spong, C.Y. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with delta (B. 1.617. 2) and omicron (B. 1.1. 529) variant predominance. JAMA 2022, 327, 1500–1502. [Google Scholar] [CrossRef]
- Carlsen, E.Ø.; Magnus, M.C.; Oakley, L.; Fell, D.B.; Greve-Isdahl, M.; Kinge, J.M.; Håberg, S.E. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern. Med. 2022, 182, 825–831. [Google Scholar] [CrossRef]
- Mangat, C.; Yarrarapu SN, S.; Singh, G.; Bansal, P. Maternal COVID-19 Vaccine May Reduce the Risk of MIS-C in Infants: A Narrative Review. Vaccines 2022, 10, 1454. [Google Scholar] [CrossRef]
- Danino, D.; Ashkenazi-Hoffnung, L.; Diaz, A.; Erps, A.D.; Eliakim-Raz, N.; Avni, Y.S.; Greenberg, D.; Givon-Lavi, N.; Youngster, I. Effectiveness of BNT162b2 Vaccination During Pregnancy in Preventing Hospitalization for SARS-CoV-2 in Infants. J. Pediatr. 2022, 254, 48–53.e1. [Google Scholar] [CrossRef] [PubMed]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Pannaraj, P.S.; Boom, J.A.; Sahni, L.C.; Chiotos, K.; Cameron, M.A.; et al. Maternal Vaccination and Risk of Hospitalization for COVID-19 among Infants. N. Engl. J. Med. 2022, 387, 109–119. [Google Scholar] [CrossRef]
- Kugelman, N.; Nahshon, C.; Shaked-Mishan, P.; Cohen, N.; Sher, M.L.; Gruber, M.; Marom, I.; Zolotarevsky, A.; Lavie, O.; Damti, A.; et al. Maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at delivery after receipt of the BNT162b2 messenger RNA COVID-19 vaccine during the second trimester of pregnancy. JAMA Pediatr. 2022, 176, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Briker, S.M.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; et al. Comparison of Maternal and Neonatal Antibody Levels After COVID-19 Vaccination vs SARS-CoV-2 Infection. JAMA Netw. Open 2022, 5, e2240993. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, S.; Mysorekar, I.U. COVID-19 and pregnancy: Clinical outcomes; mechanisms, and vaccine efficacy. Transl. Res. 2022, 251, 84–95. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021, 385, 1536. [Google Scholar]
- Rasmussen, S.A.; Kelley, C.F.; Horton, J.P.; Jamieson, D.J. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: What obstetricians need to know. Obstet. Gynecol. 2021, 137, 408. [Google Scholar] [CrossRef]
- DeSilva, M.; Haapala, J.; Vazquez-Benitez, G.; Vesco, K.K.; Daley, M.F.; Getahun, D.; Zerbo, O.; Naleway, A.; Nelson, J.C.; Williams, J.T.; et al. Evaluation of Acute Adverse Events after COVID-19 Vaccination during Pregnancy. N. Engl. J. Med. 2022, 387, 187–189. [Google Scholar] [CrossRef]
- ACOG. ACOG Practice Advisory: Vaccinating Pregnant and Lactating Patients Against COVID-19. 2021. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccination (accessed on 20 March 2023).
- ACOG. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. Obstet. Gynecol. 2021, 137, e30–e35. [Google Scholar]
- Food and Drug Administration. Antibody Testing is Not Currently Recommended to Assess Immunity after COVID-19 Vaccination: FDA Safety Communication. 2021. Available online: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety (accessed on 20 March 2023).
- Liu, S.; Zhong, J.; Zhang, D. Transplacental Transfer of Maternal Antibody against SARS-CoV-2 and Its Influencing Factors: A Review. Vaccines 2022, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Mulkey, S.B.; Roberts, D.J. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: Clinical-pathologic correlations. Am. J. Obstet. Gynecol. 2023, 228, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Radan, A.P.; Baud, D.; Favre, G.; Papadia, A.; Surbek, D.; Baumann, M.; Raio, L. Low placental weight and altered metabolic scaling after severe acute respiratory syndrome coronavirus type 2 infection during pregnancy: A prospective multicentric study. Clin. Microbiol. Infect. 2022, 28, 718–722. [Google Scholar] [CrossRef]
- Schwartz, D.A. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses 2022, 14, 458. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Madhi, S.A. COVID-19 vaccines in pregnancy. Trends Mol. Med. 2022, 28, 662–680. [Google Scholar] [CrossRef]
- Burns, M.D.; Muir, C.; Atyeo, C.; Davis, J.P.; Demidkin, S.; Akinwunmi, B.; Fasano, A.; Gray, K.J.; Alter, G.; Shook, L.L.; et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 1696. [Google Scholar] [CrossRef]
- Fu, W.; Sivajohan, B.; McClymont, E.; Albert, A.; Elwood, C.; Ogilvie, G.; Money, D. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynecol. Obstet. 2022, 156, 406–417. [Google Scholar] [CrossRef]
- Joubert, E.; Kekeh, A.C.; Amin, C.N. COVID-19 and novel mRNA vaccines in pregnancy: An updated literature review. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 21–28. [Google Scholar] [CrossRef]
- Novillo, B.; Martínez-Varea, A. COVID-19 Vaccines during Pregnancy and Breastfeeding: A Systematic Review. J. Pers. Med. 2022, 13, 40. [Google Scholar] [CrossRef]
- Martínez-Varea, A.; Satorres, E.; Florez, S.; Domenech, J.; Desco-Blay, J.; Monfort-Pitarch, S.; Hueso, M.; Perales-Marín, A.; Diago-Almela, V. Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19. J. Pers. Med. 2022, 12, 2008. [Google Scholar] [CrossRef]
- Kalafat, E.; Magee, L.A.; von Dadelszen, P.; Heath, P.; Khalil, A. COVID-19 booster doses in pregnancy and global vaccine equity. Lancet 2022, 399, 907. [Google Scholar] [CrossRef] [PubMed]
- Kalafat, E.; Heath, P.; Prasad, S.; Pat, O.; Khalil, A. COVID-19 vaccination in pregnancy. Am. J. Obstet. Gynecol. 2022, 227, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Hunagund, S.; Golan, Y.; Asiodu, I.V.; Prahl, M.; Gaw, S.L. Effects of Vaccination Against Influenza, Pertussis, and COVID-19 on Human Milk Antibodies: Current Evidence and Implications for Health Equity. Front. Immunol. 2022, 13, 910383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).