Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Determination and Power Analysis
2.2. Inclusion Criteria
2.3. Population
2.4. Research Locations
2.5. Assessments
2.6. Functional Dysmetria
2.7. Pediatric Evaluation of Disability Inventory-Computer Adaptive Test (PEDI-CAT)
2.8. Neuro Postural Optimization (NPO)
2.9. Neuro Psycho Physical Optimization Treatments
2.10. Statistics
3. Results
3.1. NPO and Functional Dysmetria
3.2. PEDI-CAT
3.2.1. Activities of Daily Living
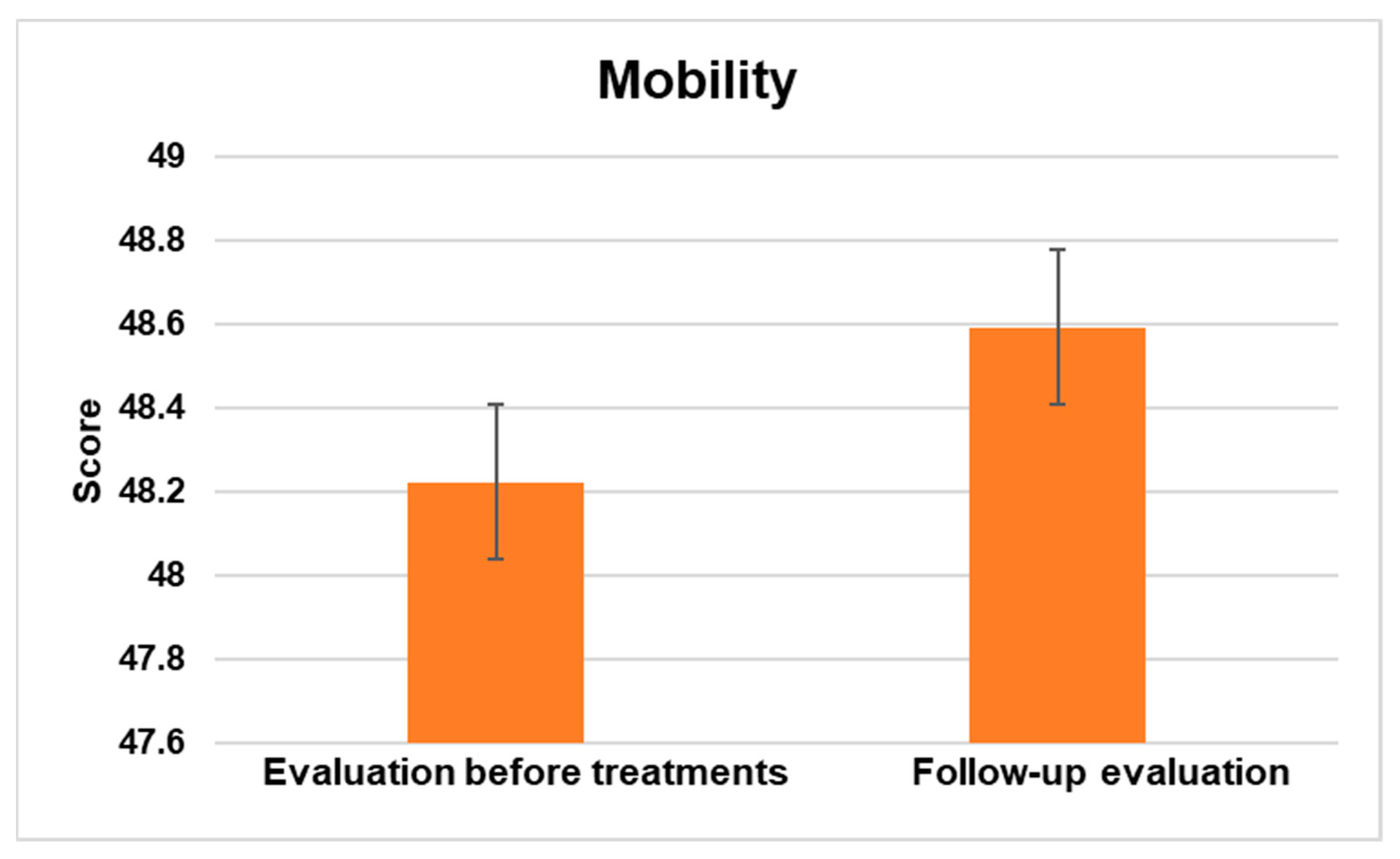
3.2.2. Mobility
3.2.3. Social Cognition
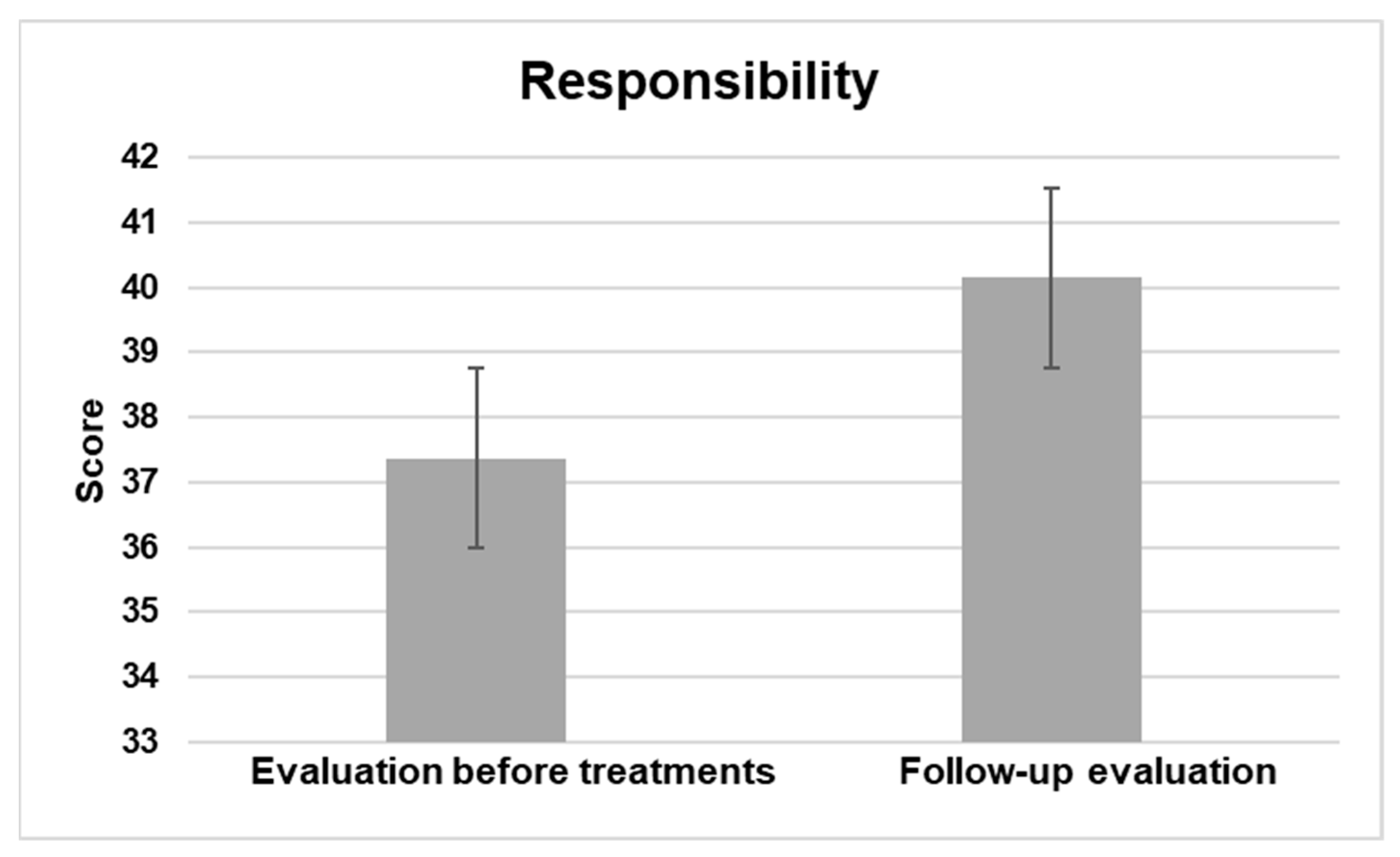
3.2.4. Responsibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Reynolds, K.; Ji, Y.; Gu, R.; Rai, S.; Zhou, C.J. Impaired neurodevelopmental pathways in autism spectrum disorder: A review of signaling mechanisms and crosstalk. J. Neurodev. Disord. 2019, 11, 10. [Google Scholar] [CrossRef]
- Kaat, A.J.; Lecavalier, L. Disruptive behavior disorders in children and adolescents with autism spectrum disorders: A review of the prevalence, presentation, and treatment. Res. Autism Spectr. Disord. 2013, 7, 1579–1594. [Google Scholar] [CrossRef]
- Schachar, R.J.; Dupuis, A.; Arnold, P.D.; Anagnostou, E.; Kelley, E.; Georgiades, S.; Nicolson, R.; Townes, P.; Burton, C.L.; Crosbie, J. Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder: Shared or Unique Neurocognitive Profiles? Res. Child Adolesc. Psychopathol. 2023, 51, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Pazuniak, M.; Pekrul, S.R. Obsessive–Compulsive Disorder in Autism Spectrum Disorder Across the Lifespan. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 419–432. [Google Scholar] [CrossRef]
- Hudson, C.C.; Hall, L.; Harkness, K.L. Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: A Meta-Analysis. J. Abnorm. Child Psychol. 2019, 47, 165–175. [Google Scholar] [CrossRef]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Canitano, R.; Pallagrosi, M. Autism Spectrum Disorders and Schizophrenia Spectrum Disorders: Excitation/Inhibition Imbalance and Developmental Trajectories. Front. Psychiatry 2017, 8, 69. [Google Scholar] [CrossRef]
- Carroll, L.S.; Owen, M.J. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 2009, 1, 102. [Google Scholar] [CrossRef]
- Vaquerizo-Serrano, J.; de Pablo, G.S.; Singh, J.; Santosh, P. Autism Spectrum Disorder and Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. J. Autism Dev. Disord. 2022, 52, 1568–1586. [Google Scholar] [CrossRef]
- Mosner, M.G.; Kinard, J.L.; Shah, J.S.; McWeeny, S.; Greene, R.K.; Lowery, S.C.; Mazefsky, C.A.; Dichter, G.S. Rates of Co-occurring Psychiatric Disorders in Autism Spectrum Disorder Using the Mini International Neuropsychiatric Interview. J. Autism Dev. Disord. 2019, 49, 3819–3832. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Liu, G.; Kay, S.-I.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front. Cell. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef]
- Kubota, T.; Mochizuki, K. Epigenetic Effect of Environmental Factors on Autism Spectrum Disorders. Int. J. Environ. Res. Public Health 2016, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Khogeer, A.A.; AboMansour, I.S.; Mohammed, D.A. The Role of Genetics, Epigenetics, and the Environment in ASD: A Mini Review. Epigenomes 2022, 6, 15. [Google Scholar] [CrossRef]
- Frye, R.E. A Personalized Multidisciplinary Approach to Evaluating and Treating Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Maioli, M.; Martins, M.C.M.; de Castro, P.C.F.; Silva, N.A.P.d.O.; de Mattos, J.A.V.; Fontani, V.; Rinaldi, S. REAC Non-invasive Neurobiological Stimulation for Mitigating the Impact of Internalizing Disorders in Autism Spectrum Disorder. Adv. Neurodev. Disord. 2021, 5, 446–456. [Google Scholar] [CrossRef]
- Rinaldi, A.; Martins, M.C.M.; Maioli, M.; Rinaldi, S.; Fontani, V. REAC Noninvasive Neurobiological Stimulation in Autism Spectrum Disorder for Alleviating Stress Impact. Adv. Neurodev. Disord. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Rinaldi, S.; Mura, M.; Castagna, A.; Fontani, V. Long-lasting changes in brain activation induced by a single REAC technology pulse in Wi-Fi bands. Randomized double-blind fMRI qualitative study. Sci. Rep. 2014, 4, srep05668. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Castagna, A.; Lotti, M. Noninvasive radioelectric asymmetric conveyor brain stimulation treatment improves balance in individuals over 65 suffering from neurological diseases: Pilot study. Ther. Clin. Risk Manag. 2012, 8, 73–78. [Google Scholar] [CrossRef]
- Barcessat, A.R.P.; Bittencourt, M.N.; Ferreira, L.D.; Neri, E.D.S.; Pereira, J.A.C.; Bechelli, F.; Rinaldi, A. REAC Cervicobrachial Neuromodulation Treatment of Depression, Anxiety, and Stress During the COVID-19 Pandemic. Psychol. Res. Behav. Manag. 2020, 13, 929–937. [Google Scholar] [CrossRef]
- Rinaldi, A.; Rinaldi, C.; Pereira, J.A.C.; Margotti, M.L.; Bittencourt, M.N.; Barcessat, A.R.P.; Fontani, V.; Rinaldi, S. Radio electric asymmetric conveyer neuromodulation in depression, anxiety, and stress. Neuropsychiatr. Dis. Treat. 2019, 15, 469–480. [Google Scholar] [CrossRef]
- Conroy, S.; Evans, T.; Butler-Moburg, D.; Beuttler, R.; Robinson, J.; Huebert, M.; Mahony, E.O.; Grant-Beuttler, M. Clinical application and feasibility of utilizing the PEDI-CAT to assess activity and participation among children receiving physical therapy incorporating hippotherapy. Physiother. Theory Pract. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cordeiro, L.; Villagomez, A.; Swain, D.; Deklotz, S.; Tartaglia, N. Adaptive Skills in FXS: A Review of the Literature and Evaluation of the PEDI-Computer Adaptive Test (PEDI-CAT) to Measure Adaptive Skills. Brain Sci. 2020, 10, 351. [Google Scholar] [CrossRef]
- Kramer, J.M.; Coster, W.J.; Kao, Y.-C.; Snow, A.; Orsmond, G.I. A New Approach to the Measurement of Adaptive Behavior: Development of the PEDI-CAT for Children and Youth with Autism Spectrum Disorders. Phys. Occup. Ther. Pediatr. 2012, 32, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Mura, M.; Castagna, A.; Fontani, V. Preliminary pilot fMRI study of neuropostural optimization with a noninvasive asymmetric radioelectric brain stimulation protocol in functional dysmetria. Neuropsychiatr. Dis. Treat. 2012, 8, 149–154. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, A.; Rinaldi, C.; Araldi, L.; Azzarà, A.; Carta, A.M.; Casale, N.; Castagna, A.; Del Medico, M.; Di Stasio, M.; et al. Long-Lasting Efficacy of Radio Electric Asymmetric Conveyer Neuromodulation Treatment on Functional Dysmetria, an Adaptive Motor Behavior. Cureus 2022, 14, e25768. [Google Scholar] [CrossRef]
- Cruz, A.V.G.d.O.; Gonçalves, R.G.; Nunes, L.; de Oliveira, J.D.Q.; Monteiro, E.S.L.; Eneias, I.S.; Lima, T.C.G.; Ferreira, L.D.; Neri, E.S.; Pena, J.L.d.C.; et al. Neuro Postural Optimization Neuromodulation Treatment of Radio Electric Asymmetric Conveyer Technology on Stress and Quality of Life in Institutionalized Children in a Capital City of the Brazilian Amazon. Cureus 2022, 14, e26550. [Google Scholar] [CrossRef]
- Olazarán, J.; González, B.; López-Álvarez, J.; Castagna, A.; Osa-Ruiz, E.; Herrero-Cano, V.; Agüera-Ortiz, L.; Rinaldi, S.; Martínez-Martín, P. Motor Effects of REAC in Advanced Alzheimer’s Disease: Results from a Pilot Trial. J. Alzheimer’s Dis. 2013, 36, 297–302. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Aravagli, L.; Lotti, M.; Castagna, A.; Mannu, P. Neuropsychophysical optimization by REAC technology in the treatment of: Sense of stress and confusion. Psychometric evaluation in a randomized, single blind, sham-controlled naturalistic study. Patient Prefer. Adherence 2012, 6, 195–199. [Google Scholar] [CrossRef]
- Pereira, J.A.C.; Rinaldi, A.; Fontani, V.; Rinaldi, S. REAC neuromodulation treatments in subjects with severe socioeconomic and cultural hardship in the Brazilian state of Pará: A family observational pilot study. Neuropsychiatr. Dis. Treat. 2018, 14, 1047–1054. [Google Scholar] [CrossRef]
- Alanzi, T.; Alhashem, A.; Dagriri, K.; Alzahrani, F.; Alkuraya, F.S. A de novo splicing variant supports the candidacy of TLL1 in ASD pathogenesis. Eur. J. Hum. Genet. 2020, 28, 525–528. [Google Scholar] [CrossRef]
- Beopoulos, A.; Géa, M.; Fasano, A.; Iris, F. RNA epitranscriptomics dysregulation: A major determinant for significantly increased risk of ASD pathogenesis. Front. Neurosci. 2023, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Castora, F.J. Mitochondrial function and abnormalities implicated in the pathogenesis of ASD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 83–108. [Google Scholar] [CrossRef]
- Cervera, J.; Meseguer, S.; Mafe, S. The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci. Rep. 2016, 6, 35201. [Google Scholar] [CrossRef]
- Pietak, A.; Levin, M. Bioelectric gene and reaction networks: Computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation. J. R. Soc. Interface 2017, 14, 20170425. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef]
- Roohi-Azizi, M.; Azimi, L.; Heysieattalab, S.; Aamidfar, M. Changes of the brain’s bioelectrical activity in cognition, consciousness, and some mental disorders. Med. J. Islam. Repub. Iran 2017, 31, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, E.A.; Lushchekin, V.S.; Strelets, V.B. Bioelectric Brain Activity in Children with Autistic Spectrum Disorders: Population Heterogeneity. Hum. Physiol. 2018, 44, 386–393. [Google Scholar] [CrossRef]
- Mathews, J.; Levin, M. The body electric 2.0: Recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotechnol. 2018, 52, 134–144. [Google Scholar] [CrossRef]
- Fröhlich, F.; McCormick, D.A. Endogenous Electric Fields May Guide Neocortical Network Activity. Neuron 2010, 67, 129–143. [Google Scholar] [CrossRef]
- Qiu, C.; Shivacharan, R.S.; Zhang, M.; Durand, D.M. Can Neural Activity Propagate by Endogenous Electrical Field? J. Neurosci. 2015, 35, 15800–15811. [Google Scholar] [CrossRef]
- Tseng, A.-S.; Levin, M. Transducing Bioelectric Signals into Epigenetic Pathways During Tadpole Tail Regeneration. Anat. Rec. 2012, 295, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J. Physiol. 2014, 592, 2295–2305. [Google Scholar] [CrossRef]
- Funk, R.H.W. Endogenous electric fields as guiding cue for cell migration. Front. Physiol. 2015, 6, 143. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef]
- Bonsi, P.; De Jaco, A.; Fasano, L.; Gubellini, P. Postsynaptic autism spectrum disorder genes and synaptic dysfunction. Neurobiol. Dis. 2021, 162, 105564. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Braeutigam, S.; Dawes, J.M.; Krsnik, Z.; Kostovic, I.; Coutinho, E.; Dewing, J.M.; Horton, C.A.; Gomez-Nicola, D.; Menassa, D.A. Autism Spectrum Disorders: Multiple Routes to, and Multiple Consequences of, Abnormal Synaptic Function and Connectivity. Neurosci. 2020, 27, 10–29. [Google Scholar] [CrossRef]
- Rebollo, B.; Telenczuk, B.; Navarro-Guzman, A.; Destexhe, A.; Sanchez-Vives, M.V. Modulation of intercolumnar synchronization by endogenous electric fields in cerebral cortex. Sci. Adv. 2021, 7, eabc7772. [Google Scholar] [CrossRef]
- Shivacharan, R.S.; Chiang, C.-C.; Zhang, M.; Gonzalez-Reyes, L.E.; Durand, D.M. Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields. Exp. Neurol. 2019, 317, 119–128. [Google Scholar] [CrossRef]
- Ferreira, F.; Moreira, S.; Barriga, E.H. Stretch-induced endogenous electric fields drive neural crest directed collective cell migration in vivo. bioRxiv 2021. bioRxiv:2021.10.11.463916. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, A.; Marins Martins, M.C.; De Almeida Martins Oliveira, A.C.; Rinaldi, S.; Fontani, V. Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study. J. Pers. Med. 2023, 13, 792. https://doi.org/10.3390/jpm13050792
Rinaldi A, Marins Martins MC, De Almeida Martins Oliveira AC, Rinaldi S, Fontani V. Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study. Journal of Personalized Medicine. 2023; 13(5):792. https://doi.org/10.3390/jpm13050792
Chicago/Turabian StyleRinaldi, Arianna, Márcia C. Marins Martins, Ana C. De Almeida Martins Oliveira, Salvatore Rinaldi, and Vania Fontani. 2023. "Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study" Journal of Personalized Medicine 13, no. 5: 792. https://doi.org/10.3390/jpm13050792
APA StyleRinaldi, A., Marins Martins, M. C., De Almeida Martins Oliveira, A. C., Rinaldi, S., & Fontani, V. (2023). Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study. Journal of Personalized Medicine, 13(5), 792. https://doi.org/10.3390/jpm13050792







