The Value of Multimodal Imaging in Early Phenotyping of Cardiomyopathies: A Family Case Report
Abstract
1. Introduction
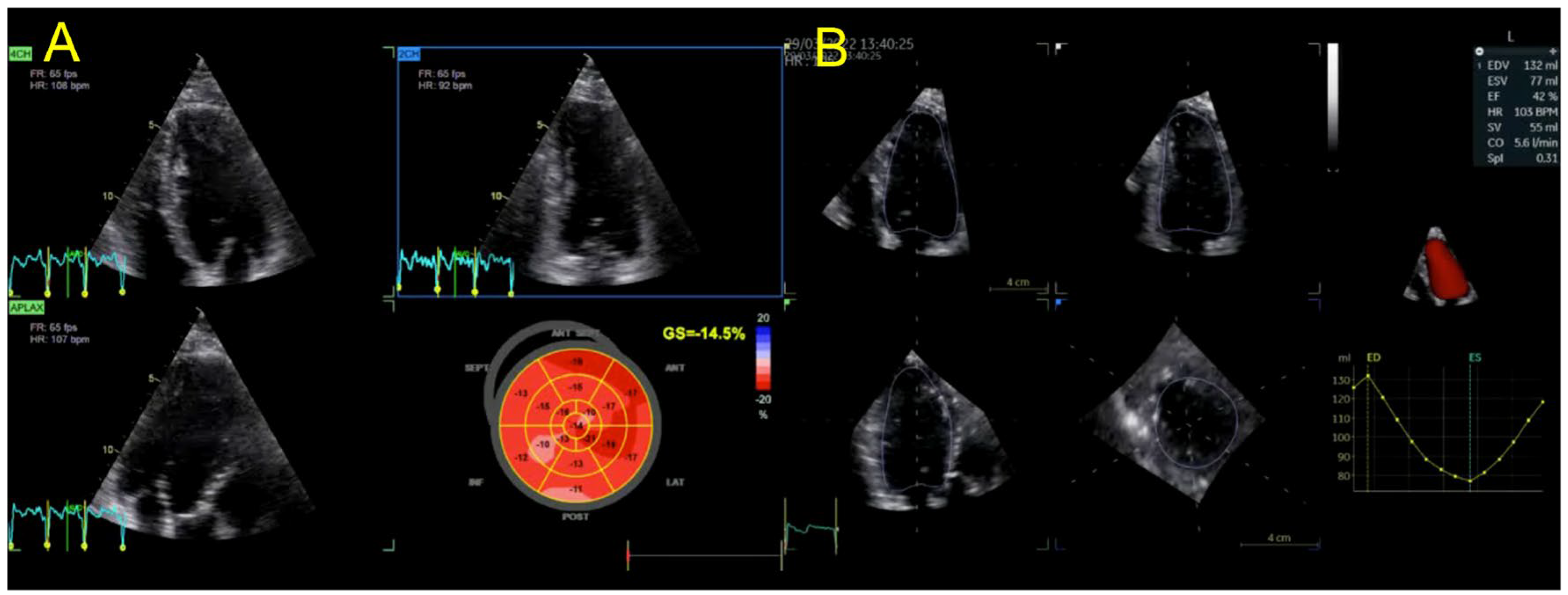
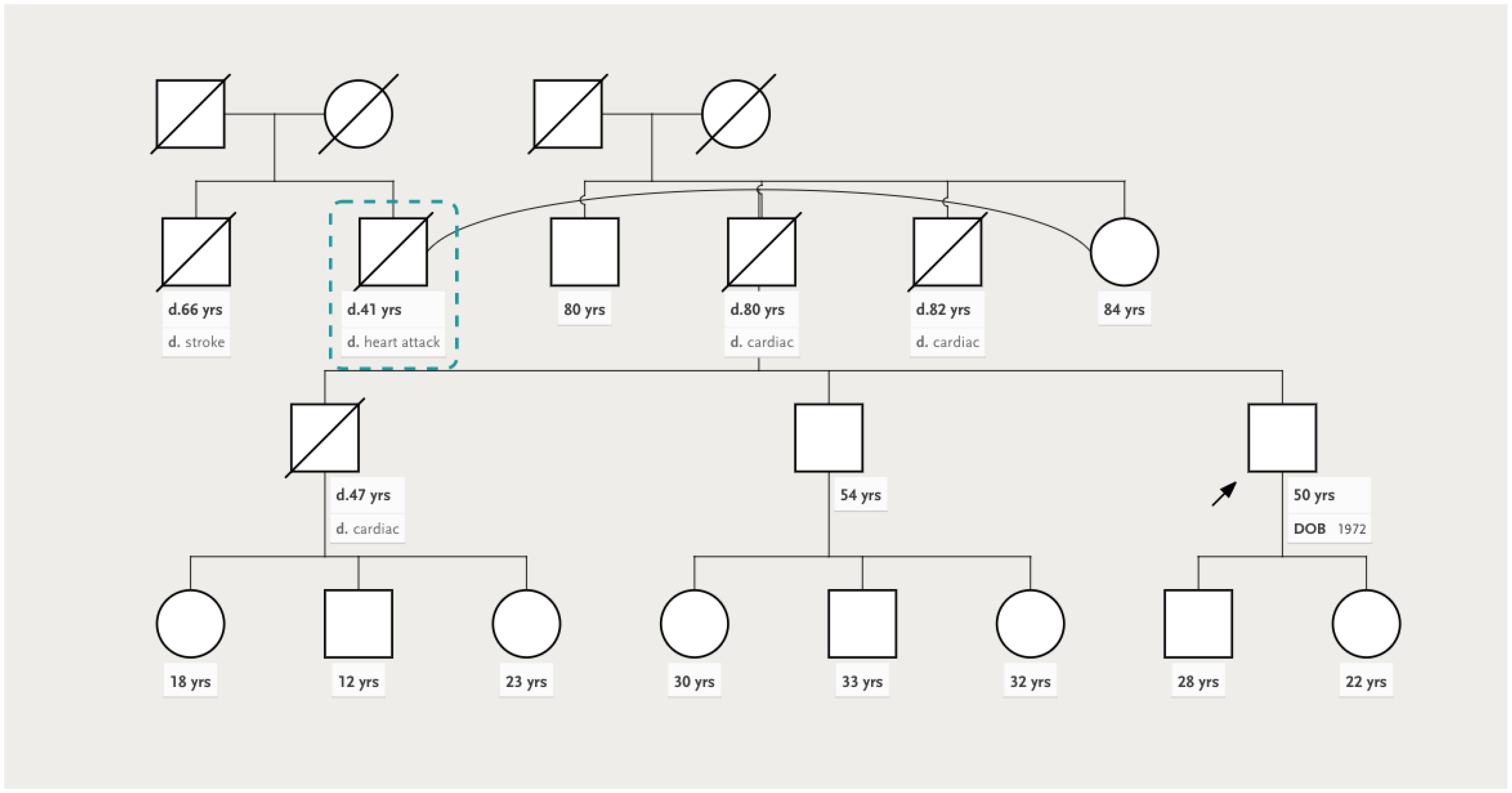
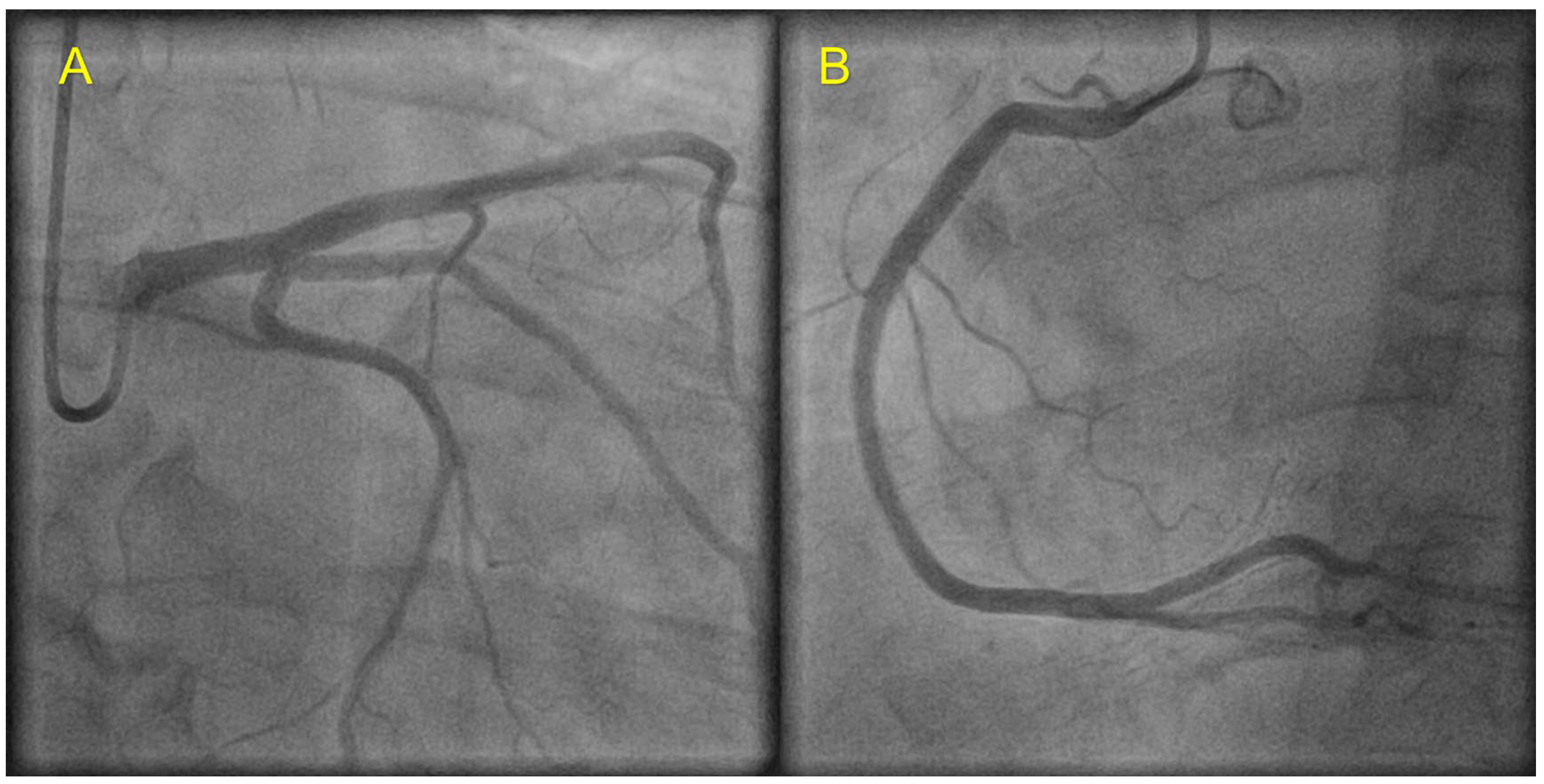
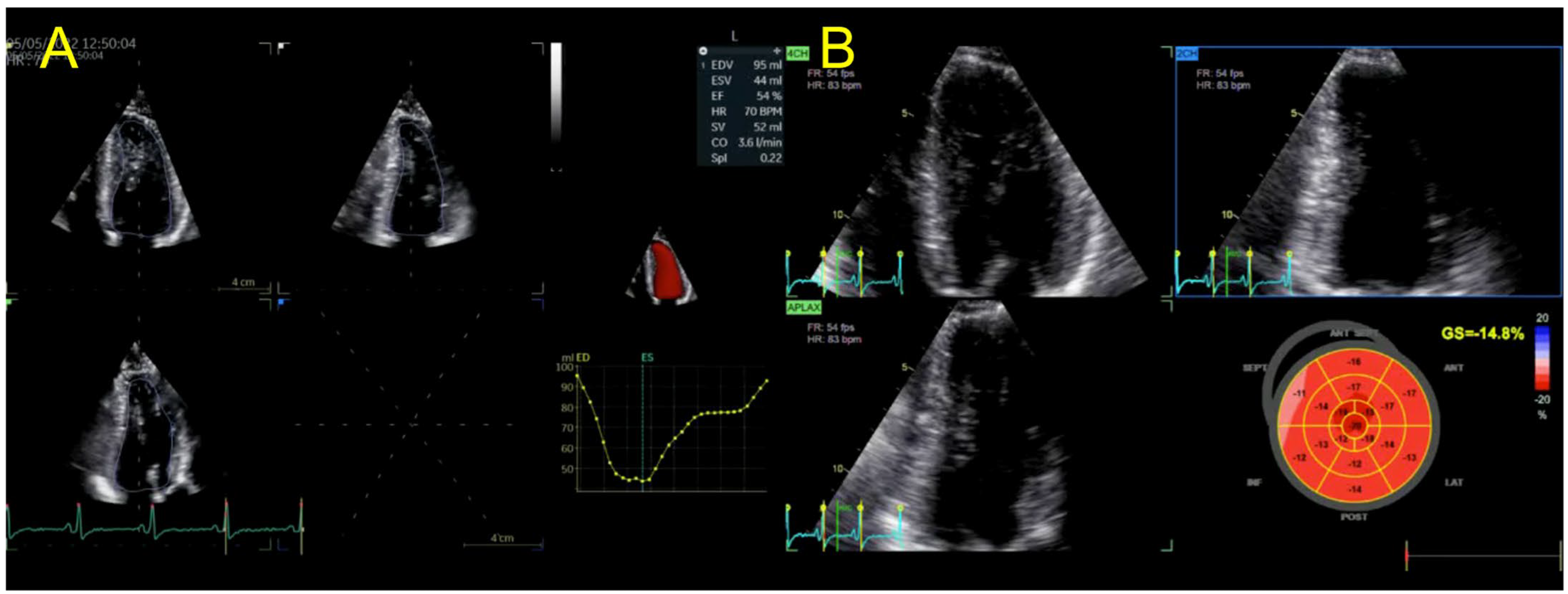
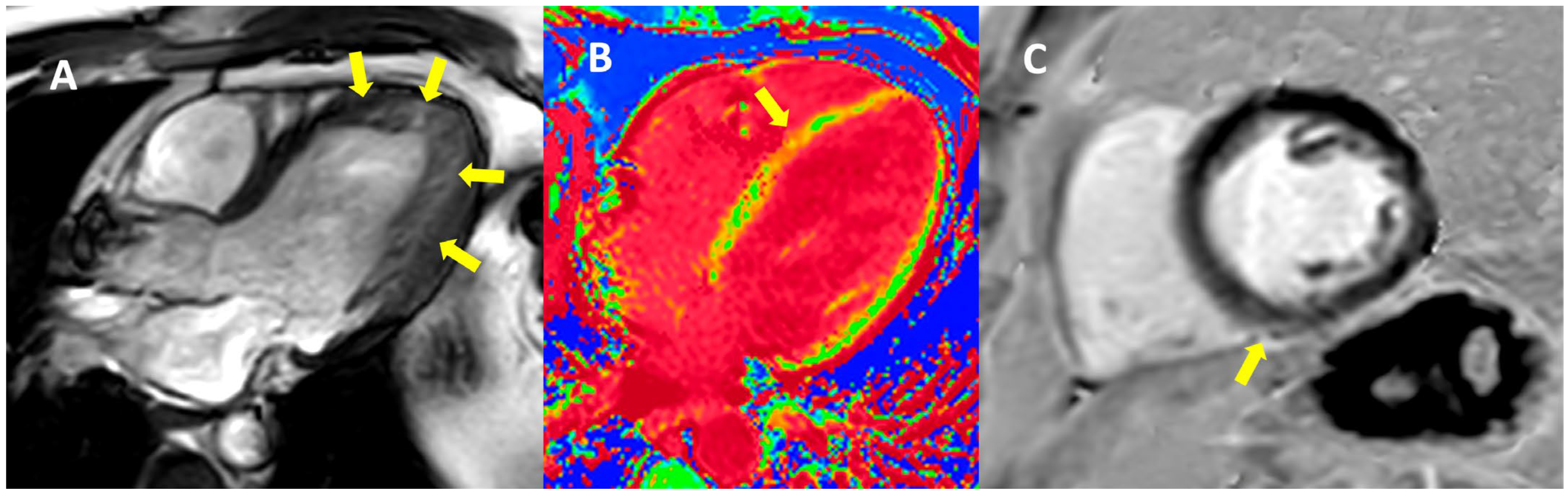
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. Proposal for a Revised Definition of Dilated Cardiomyopathy, Hypokinetic Non-Dilated Cardiomyopathy, and Its Implications for Clinical Practice: A Position Statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Cannatà, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving Concepts in Dilated Cardiomyopathy. Eur. J. Heart Fail 2018, 20, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Z.; Guo, L.; Yu, S.; Li, T.; Zheng, L.; Pan, G.; Yang, J.; Sun, Y.; Hui, R.; et al. Prevalence of Hypokinetic Non-Dilated Cardiomyopathy in a Large General Chinese Population. Int. J. Cardiol. 2016, 223, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Yukimitsu, N.; Yokoyama, N.; Ikeda, Y.; Ishibashi, R.; Takamura, S.; Kozuma, K.; Hatsuno, M. One-year outcome in hypokinetic non-dilated cardiomyopathy detected by cardiac magnetic resonance: Idiopathic dilated cardiomyopathy comparison. J. Am. Coll. Cardiol. 2022, 79, 359. [Google Scholar] [CrossRef]
- Gigli, M.; Stolfo, D.; Merlo, M.; Barbati, G.; Ramani, F.; Brun, F.; Pinamonti, B.; Sinagra, G. Insights into Mildly Dilated Cardiomyopathy: Temporal Evolution and Long-Term Prognosis. Eur. J. Heart Fail 2017, 19, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, M.; Ditaranto, R.; Rapezzi, C.; Pasquale, F.; Lovato, L.; Leone, O.; Parisi, V.; Potena, L.; Ferrara, V.; Minnucci, M.; et al. Clinical Presentations Leading to Arrhythmogenic Left Ventricular Cardiomyopathy. Open Heart 2022, 9, e001914. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical Risk Prediction in Patients with Left Ventricular Myocardial Noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Mirea, O.; Berceanu, M.; Constantin, A.; Mănescu, M.; Târtea, G.C.; Donoiu, I.; Militaru, C.; Istrătoaie, O. Non-compaction cardiomyopathy–brief review. J. Mind Med. Sci. 2017, 4, 115–124. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.G.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of Titin Causing Dilated Cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, A.C.; Seidman, J.G.; Seidman, C.E. Genetic Pathogenesis of Hypertrophic and Dilated Cardiomyopathy. Heart Fail Clin. 2018, 14, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Linschoten, M.; Teske, A.J.; Baas, A.F.; Vink, A.; Dooijes, D.; Baars, H.F.; Asselbergs, F.W. Truncating Titin (TTN) Variants in Chemotherapy-Induced Cardiomyopathy. J. Card Fail 2017, 23, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, E.J.; Takayama, H.; Ferrari, G. Stretch Your Heart—But Not Too Far: The Role of Titin Mutations in Dilated Cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2018, 156, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Heart J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Paterick, T.E.; Tajik, A.J. Left Ventricular Noncompaction—A Diagnostically Challenging Cardiomyopathy. Circ. J. 2012, 76, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Tayal, U.; Newsome, S.; Buchan, R.; Whiffin, N.; Walsh, R.; Barton, P.J.; Ware, J.S.; Cook, S.A.; Prasad, S.K. Truncating Variants in Titin Independently Predict Early Arrhythmias in Patients with Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 69, 2466–2468. [Google Scholar] [CrossRef] [PubMed]
- Corden, B.; Jarman, J.; Whiffin, N.; Tayal, U.; Buchan, R.; Sehmi, J.; Harper, A.; Midwinter, W.; Lascelles, K.; Markides, V.; et al. Association of Titin-Truncating Genetic Variants with Life-threatening Cardiac Arrhythmias in Patients with Dilated Cardiomyopathy and Implanted Defibrillators. JAMA Netw. Open 2019, 6, e196520. [Google Scholar] [CrossRef] [PubMed]




| Proband | Brother | Nephew | |
|---|---|---|---|
| Clinical | Arterial hypertension Dyslipidemia | NYHA II HFrEF Persistent atrial fibrillation Arterial hypertension | Anabolic steroid use Resistance training Arterial hypertension |
| Echocardiography | Hypokinetic non-dilated cardiomyopathy LVEF = 42% Mild mitral regurgitation IAS aneurysm | Hypokinetic non-dilated cardiomyopathy LVEF = 34% Mild mitral regurgitation IAS aneurysm | Left ventricular concentric hypertrophy LVEF = 54% |
| Cardiac magnetic resonance imaging | Hypokinetic non-dilated cardiomyopathy Left ventricular non-compaction LVEF = 50% | Hypokinetic non-dilated cardiomyopathy Regional sub-epicardial fibrosis LVEF = 41% | Not carried out |
| Coronary angiography | Normal | Normal | Not carried out |
| Genetic testing | Heterozygous TTN variant—deletion in exon 3 (exon 326, c.70482_70483del p.Tyr23494*) | Heterozygous TTN variant—deletion in exon 3 (exon 326, c.70482_70483del p.Tyr23494*) | No pathogenic/likely pathogenic mutations identified |
| Management | CV risk factor control HTN treatment optimization Clinical and echo follow up | CV risk factor control HFrEF treatment optimization Clinical and echo follow up after 3 months of OMT | CV risk factor control Cessation of steroid use Clinical and echo follow up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovănescu, M.L.; Hădăreanu, D.R.; Militaru, S.; Florescu, C.; Militaru, C.; Donoiu, I. The Value of Multimodal Imaging in Early Phenotyping of Cardiomyopathies: A Family Case Report. J. Pers. Med. 2023, 13, 742. https://doi.org/10.3390/jpm13050742
Iovănescu ML, Hădăreanu DR, Militaru S, Florescu C, Militaru C, Donoiu I. The Value of Multimodal Imaging in Early Phenotyping of Cardiomyopathies: A Family Case Report. Journal of Personalized Medicine. 2023; 13(5):742. https://doi.org/10.3390/jpm13050742
Chicago/Turabian StyleIovănescu, Maria Livia, Diana Ruxandra Hădăreanu, Sebastian Militaru, Cristina Florescu, Constantin Militaru, and Ionuț Donoiu. 2023. "The Value of Multimodal Imaging in Early Phenotyping of Cardiomyopathies: A Family Case Report" Journal of Personalized Medicine 13, no. 5: 742. https://doi.org/10.3390/jpm13050742
APA StyleIovănescu, M. L., Hădăreanu, D. R., Militaru, S., Florescu, C., Militaru, C., & Donoiu, I. (2023). The Value of Multimodal Imaging in Early Phenotyping of Cardiomyopathies: A Family Case Report. Journal of Personalized Medicine, 13(5), 742. https://doi.org/10.3390/jpm13050742






