Targeted Treatment of Soft-Tissue Sarcoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strings
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
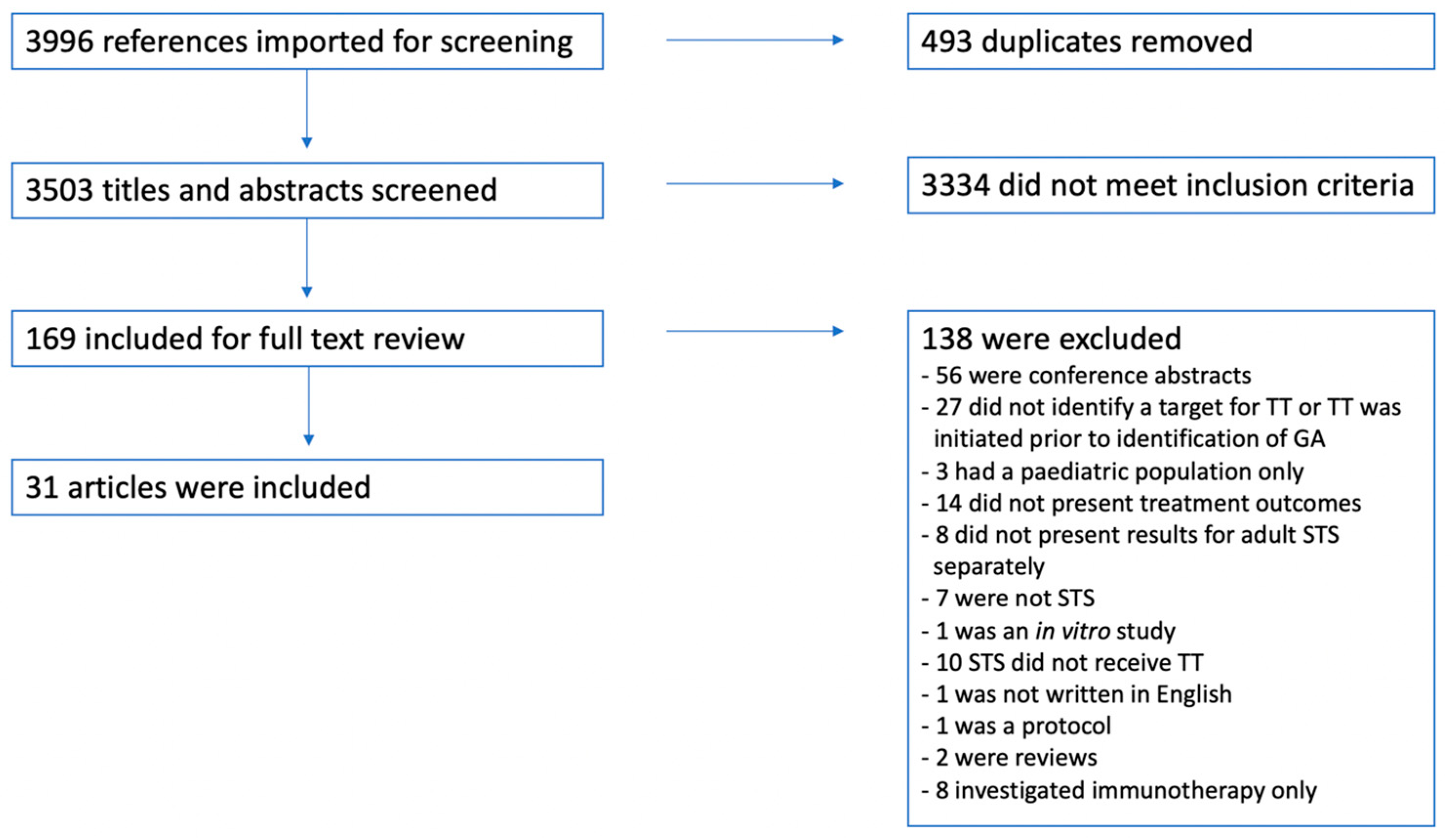
3. Results
3.1. Study Selection
3.2. Study Description and Quality Assessment
3.2.1. MDM2 Inhibitors
3.2.2. Crizotinib
3.2.3. Ceritinib
3.2.4. 90Y-OTSA
| First Author, Year | Study Design | Quality Assessment | Histological Subtype (n) | Genetic Alteration | Targeted Agent | Treatment Outcome |
|---|---|---|---|---|---|---|
| Arnaud-Coffin, 2020 [2] | Prospective cohort study | Good | Advanced STS | (PFS/OS) | ||
| LMS (1) | AKT2 amplification | Everolimus | 2.6/10.9 months | |||
| MPNST (1) | ERBB2 mutation | Lapatinib | 1.9/3.8 months | |||
| Angiosarcoma (1) | FLT4 mutation | Pazopanib | 3.1/10.7 months | |||
| UPS (1) | AKT2 deletion | Everolimus | 1.4/4.1 months | |||
| GIST (3) | CDKN2A deletion | Palbociclib | 0.8/4.9, 0.9/2.6, 3.7/22.9 months | |||
| Brian Dalton, 2017 [46] | Cohort study | Good | Advanced cancer | |||
| MPNST (1) | EGFR duplication | Afatinib | PD 2 months | |||
| RMS (1) | FGFR1 mutation | Pazopanib | PD 4 months | |||
| Cecchini, 2018 [47] | Case report | Fair | High-grade sarcoma (1) | ATM frameshift mutation | Olaparib | PD 2 months |
| Chen, 2021 [33] | Case report | Good | Mesenchymal sarcoma (1) | NTRK1–KHDRBS1 fusion | Crizotinib | CR 40+ months |
| Dembla, 2018 [13] | Retrospective cohort study | Fair | LPS (6) | MDM2 amplification | MDM2 inhibitor | 3 had PR, 2 had SD (15.7 and 4.7 months, respectively) 1 n/a |
| Elvin, 2017 [48] | Case report | Good | LMS (1) | CDKN2A deletion | Palbociclib | SD 4 months, radiological progression at 8 months |
| Forde, 2016 [31] Kinne, 2019 [32] | Case report Update | Fair | IMS (1) | ALKrearrangements | Crizotinib | CR 3 months, CR 164 weeks |
| Giraudet, 2018 [45] | Phase 1 cohort study | Good | SS (8) | FZD10 | 90Y-OTSA-101 | |
| 3 received 370 MBq of 90Y | 1 had SD, 2 had PD | |||||
| 5 received 1110 MBq of 90Y | 2 had SD, 3 had PD 1 with SD received a second injection resulting in PFS for 21.4 weeks | |||||
| Groisberg, 2017 [11] | Cohort study | Good | Advanced sarcoma | |||
| Gliosarcoma (1) | BRAF V600E | Vemurafenib | PR 16 months | |||
| DDLPS (1) | ROS1 amplification | Ceritinib | SD 5 months | |||
| DDLPS (1) | MDM2 amplification | MDM2 inhibitor | PR 3 cycles | |||
| GIST (1) | KIT +AKT Amplification | Imatinib, sunitinib, regorafenib, AKT inhibitor | PD x3 | |||
| LMS (2) | ROS1 mutation | Pazopanib +crizotinib | PR 22 cycles, SD 6 months, PD | |||
| LMS (1) | PTEN deletion | PI3K inhibitor | PD | |||
| Pleomorphic sarcoma (1) | MEMO1–ALK fusion | Ceritinib | PD after 4 cycles | |||
| Myxoid LPS (1) | AKT1 mutation | AKT inhibitor | SD 1 cycle | |||
| Spindle cell sarcoma (1) | KIAA1549–BRAF fusion | Sorafenib+ bevacizumab + temsirolimus | SD 11 cycles | |||
| WDLPS (4) | MDM2 amplification | MDM2 inhibitor | SD 8 cycles, CR, SD 2 cycles, SD 23 months | |||
| Groisberg, 2020 [49] | Cohort study | Fair | Alveolar soft part sarcoma (1) | HGF amplification | Pazopanib + vorinostat | SD 28 months |
| Harttrampf, 2017 [50] | Prospective cohort study | Good | Paediatric advanced tumours Epithelioid sarcoma (1) | SMARCB1 deletion | Tazemetostat | PD 2 months |
| Ji, 2016 [51] | Case report | Good | Angiosarcoma (1) | VEGFR2 | Apatinib | PFS 12 months |
| Jin, 2021 [52] | Cohort study | Good | STS | |||
| Inflammatory myofibroblastoma (1) | MAP2K1 | Trametinib | RFS: 2 months | |||
| LPS (1) | CDK4 | Palbociclib | RFS: 4 months | |||
| Fibrosarcoma (1) | COL1A1–PDGFB fusion | Imatinib | RFS: 10 months | |||
| Clear cell sarcoma (1) | BRAF V600E | Vemurafenib | RFS: 21 months | |||
| Kato, 2018 [43] | Cohort study | Good | Advanced cancer | |||
| Desmoid tumour (1) | CTNNB1 mutation | Sorafenib + sulindac | SD, PFS 9.1+ months | |||
| ESS (1) | CDKN2A mutations and FRS2 amplification | Palbociclib + lenvatinib + anastrozole + doxorubicin | SD, PFS 3.6+ months | |||
| Myxofibrosarcoma (1) | IGFR1 amplification | Ceritinib | PD, PFS 1.8+ months | |||
| Kerr, 2021 [36] | Cohort study | Good | IMT (2) | ALK+ SLC12A1–ROS1 fusion | Crizotinib Crizotinib+ surgery | NED: 1.8 years NED: 2 years |
| Kyi, 2021 [44] | Case report | Good | IMT (1) | FN1–ALK fusion | Crizotinib→ ceritinib | SD 4 months→ SD 6 months |
| IMT (1) | TNS1–ALK fusion | Crizotinib→ Alectinib→ Ceritinib → Lorlatinib | SD 3 months → SD 12 months → SD 2 months → PD 1 month | |||
| Myofibroblastic sarcoma (1) | LBH–ALK fusion | Crizotinib→ Ceritinib | SD 30 months → SD 6 months | |||
| IMT (1) | IGFBP5–ALK fusion | Ceritinib | PR 24+ months | |||
| Li, 2020 [42] | Case report | Fair | MPNST (1) | TJP1–ROS1 fusion | Crizotinib | SD 2 months, PD after 4 months |
| Mansfield, 2016 [41] | Case report | Good | IMS (1) | ALK+ | Crizotinib → Ceritinib + surgery | PR 8 months→ progression → PR 18 months |
| Rabban, 2020 [35] | Case report | Good | Uterine sarcoma (1) | SPECC1L–NTRK3 fusion | Larotrectinib | CR 15+ months |
| Recine, 2021 [53] | Case report | Good | Spindle cell neoplasm (1) | TPM4–NTRK1 fusion | Larotrectinib | PR 19+ months |
| Seligson, 2021 [54] | Case report | Good | Small cell round tumour (1) | EWSR1–NFATc2 fusion | Everolimus + surgery + non-TT | SD 47 months |
| Seol, 2019 [15] | Prospective cohort study | Good | Advanced tumours | |||
| Uterine sarcoma (1) | AKT3 amplification | Everolimus | PR 5 months | |||
| Somaiah, 2018 [37] | Cohort study | Good | LPS (8) | MDM2 amplification | MDM2 inhibitor | Median time to progression: 23 months (95%-CI: 10–83 months) |
| Subbiah, 2015 [40] | Case report | Fair | IMS (1) | DCTN1–ALK fusion | Crizotinib + pazopanib | PR 6+ months |
| Subbiah, 2020 [55] | Cohort study | Good | Advanced tumours Clear cell sarcoma (1) | Placental cadherin | 90Y-FF-21101 mAb 25 mCi/m2 | SD 50 weeks |
| Valenciaga, 2021 [56] | Case report | Good | Pleomorphic LPS (1) | IQGAP–NTRK3 fusion | Entrectinib → Pazopanib + radiation → Larotrectinib | PD 4 cycles → PD 3 months → SD 18+ months |
| Walsh, 2021 [57] | Case report | Fair | IMT (1) | LRRFIP1–ALK fusion | Alectinib | PR 19 months |
| Weidenbusch, 2018 [39] | Cohort study | Poor | Paediatric sarcoma | |||
| RMS (1) | MET and FGFR1 mutation | Crizotinib + ponatinib | SD 7 months | |||
| SS (1) | FGFR1 and EGFR mutation | Ponatinib + gefitinib | PD | |||
| Wu, 2021 [58] | Case report | Good | Primary pulmonary artery sarcoma (1) | Loss of ATM and H2AX | Olaparib | PR 2 months |
| Yang, 2018 [38] | Case report | Good | Myofibroblastic sarcoma (1) | ALK mutation | Crizotinib + bevacizumab | PFS 3 months |
| Zhou, 2018 [34] | Case report | Good | UPS (1) | LMNA–NTRK1 fusion | Crizotinib | Near-CR 18+ months |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Coffin, P.; Brahmi, M.; Vanacker, H.; Eberst, L.; Tredan, O.; Attignon, V.; Pissaloux, D.; Sohier, E.; Cassier, P.; Garin, G.; et al. Therapeutic relevance of molecular screening program in patients with metastatic sarcoma: Analysis from the ProfiLER 01 trial. Transl. Oncol. 2020, 13, 100870. [Google Scholar] [CrossRef]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- The European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii102–iii112. [Google Scholar] [CrossRef]
- Singhi, E.K.; Moore, D.C.; Muslimani, A. Metastatic Soft Tissue Sarcomas: A Review of Treatment and New Pharmacotherapies. Pharm. Ther. 2018, 43, 410–429. [Google Scholar]
- Linch, M.; Miah, A.B.; Thway, K.; Judson, I.R.; Benson, C. Systemic treatment of soft-tissue sarcoma—Gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014, 11, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Available online: https://www.cbioportal.org/study/summary?id=sarc_tcga_pub (accessed on 12 March 2021).
- He, M.; Abro, B.; Kaushal, M.; Chen, L.; Chen, T.; Gondim, M.; Yan, W.; Neidich, J.; Dehner, L.P.; Pfeifer, J.D. Tumor mutation burden and checkpoint immunotherapy markers in primary and metastatic synovial sarcoma. Hum. Pathol. 2020, 100, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Nowak, J.A.; Nathenson, M.J.; Thornton, K.; Wagner, A.J.; Johnson, J.M.; Albrayak, A.; George, S.; Sholl, L.M. Characteristics of mismatch repair deficiency in sarcomas. Mod. Pathol. 2019, 32, 977–987. [Google Scholar] [CrossRef]
- Groisberg, R.; Hong, D.S.; Holla, V.; Janku, F.; Piha-Paul, S.; Ravi, V.; Benjamin, R.; Kumar Patel, S.; Somaiah, N.; Conley, A.; et al. Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas. Oncotarget 2017, 8, 39254–39267. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Kang, H.G.; Park, S.Y.; Yu, J.Y.; Lee, E.Y.; Oh, S.E.; Kim, Y.H.; Yun, T.; Park, C.; et al. Integrated molecular characterization of adult soft tissue sarcoma for therapeutic targets. BMC Med. Genet. 2018, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Dembla, V.; Somaiah, N.; Barata, P.; Hess, K.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Piha-Paul, S.A.; Subbiah, V.; et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson phase 1 clinic. Oncotarget 2018, 9, 33232–33243. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Allen, J.; Hecht, J.; Killian, J.; Ngo, N.; Edgerly, C.; Severson, E.; Ali, S.; Erlich, R.; Ramkissoon, S.; et al. SMARCA4 inactivation defines a subset of undifferentiated uterine sarcomas with rhabdoid and small cell features and germline mutation association. Int. J. Gynecol. Cancer 2019, 29, A42. [Google Scholar] [CrossRef]
- Seol, Y.M.; Kwon, C.H.; Lee, S.J.; Lee, S.J.; Choi, Y.; Choi, Y.J.; Kim, H.; Park, D.Y. A Pilot Prospective Study of Refractory Solid Tumor Patients for NGS-Based Targeted Anticancer Therapy. Transl. Oncol. 2019, 12, 301–307. [Google Scholar] [CrossRef]
- Lin, D.I.; Hemmerich, A.; Edgerly, C.; Duncan, D.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; Connor, Y.D.; Shea, M.; Hecht, J.L.; et al. Genomic profiling of BCOR-rearranged uterine sarcomas reveals novel gene fusion partners, frequent CDK4 amplification and CDKN2A loss. Gynecol. Oncol. 2020, 157, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Madison, R.; Fridman, E.; Mangray, S.; Carneiro, B.A.; Lu, S.; Cooke, M.; Bratslavsky, G.; Webster, J.; Ross, J.S.; et al. Comprehensive Genomic Profiling of Adult Renal Sarcomas Provides Insight into Disease Biology and Opportunities for Targeted Therapies. Eur. Urol. Oncol. 2019, 4, 282–288. [Google Scholar] [CrossRef]
- Lucchesi, C.; Khalifa, E.; Laizet, Y.; Soubeyran, I.; Mathoulin-Pelissier, S.; Chomienne, C.; Italiano, A. Targetable Alterations in Adult Patients with Soft-Tissue Sarcomas: Insights for Personalized Therapy. JAMA Oncol. 2018, 4, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Gusho, C.A.; Weiss, M.C.; Lee, L.; Gitelis, S.; Blank, A.T.; Wang, D.; Batus, M. The clinical utility of next-generation sequencing for bone and soft tissue sarcoma. Acta Oncol. 2022, 61, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Brinch, C.M.; Aggerholm-Pedersen, N.; Hogdall, E.; Krarup-Hansen, A. Medical oncological treatment for patients with Gastrointestinal Stromal Tumor (GIST)—A systematic review. Crit. Rev. Oncol. Hematol. 2022, 172, 103650. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Safwat, A.; Boysen, A.; Lucke, A.; Rossen, P. Pazopanib in metastatic osteosarcoma: Significant clinical response in three consecutive patients. Acta Oncol. 2014, 53, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Alcindor, T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015, 54, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Kollar, A.; Jones, R.L.; Stacchiotti, S.; Gelderblom, H.; Guida, M.; Grignani, G.; Steeghs, N.; Safwat, A.; Katz, D.; Duffaud, F.; et al. Pazopanib in advanced vascular sarcomas: An EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hirbe, A.C.; Eulo, V.; Moon, C.I.; Luo, J.; Myles, S.; Seetharam, M.; Toeniskoetter, J.; Kershner, T.; Haarberg, S.; Agulnik, M.; et al. A phase II study of pazopanib as front-line therapy in patients with non-resectable or metastatic soft-tissue sarcomas who are not candidates for chemotherapy. Eur. J. Cancer 2020, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Kawai, A.; Goto, T.; Hiraga, H.; Ozaki, T.; Tsuchiya, H.; Nakayama, R.; Naka, N.; Matsumoto, Y.; Kobayashi, E.; et al. Phase II trial of pazopanib in patients with metastatic or unresectable chemoresistant sarcomas: A Japanese Musculoskeletal Oncology Group study. Cancer Sci. 2020, 111, 3303–3312. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Rossen, P.; Rose, H.; Safwat, A. Pazopanib in the Treatment of Bone Sarcomas: Clinical Experience. Transl. Oncol. 2020, 13, 295–299. [Google Scholar] [CrossRef]
- Brodowicz, T.; Liegl-Atzwager, B.; Tresch, E.; Taieb, S.; Kramar, A.; Gruenwald, V.; Vanseymortier, M.; Clisant, S.; Blay, J.Y.; Le Cesne, A.; et al. Study protocol of REGOSARC trial: Activity and safety of regorafenib in advanced soft tissue sarcoma: A multinational, randomized, placebo-controlled, phase II trial. BMC Cancer 2015, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet.Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Forde, G.K.; Tewari, D. Targeted Treatment of a Rare Vaginal Sarcoma with an Anaplastic Lymphoma Kinase Inhibitor. Obstet. Gynecol. 2016, 127, 222–225. [Google Scholar] [CrossRef]
- Kinne, N.; Hodeib, M.; Kashani, N.A.; Tewari, D. Targeted Treatment of a Rare Vaginal Sarcoma with an Anaplastic Lymphoma Kinase Inhibitor. Obstet. Gynecol. 2019, 134, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Jiang, D.; Luan, L.; Zhou, Y.; Hou, Y. Unclassified mesenchymal sarcoma with NTRK1-KHDRBS1 gene fusion: A case report of long-term tumor-free survival with crizotinib treatment. World J. Surg. Oncol. 2021, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schäfer, R.; Li, T.; Fang, M.; Liu, L. A primary undifferentiated pleomorphic sarcoma of the lumbosacral region harboring a LMNA-NTRK1 gene fusion with durable clinical response to crizotinib: A case report. BMC Cancer 2018, 18, 842. [Google Scholar] [CrossRef]
- Rabban, J.T.; Devine, W.P.; Sangoi, A.R.; Poder, L.; Alvarez, E.; Davis, J.L.; Rudzinski, E.; Garg, K.; Bean, G.R. NTRK fusion cervical sarcoma: A report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology 2020, 77, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Thompson, L.D.R.; Tafe, L.J.; Jo, V.Y.; Neyaz, A.; Divakar, P.; Paydarfar, J.A.; Pastel, D.A.; Shirai, K.; John, I.; et al. Clinicopathologic and genomic characterization of inflammatory myofibroblastic tumors of the head and neck highlighting a novel fusion and potential diagnostic pitfall. Am. J. Surg. Pathol. 2021, 45, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Beird, H.C.; Barbo, A.; Song, J.; Mills Shaw, K.R.; Wang, W.L.; Eterovic, K.; Chen, K.; Lazar, A.; Conley, A.P.; et al. Targeted next generation sequencing of well-differentiated/ dedifferentiated liposarcoma reveals novel gene amplifications and mutations. Oncotarget 2018, 9, 19891–19899. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Tang, H.; Zhao, J.; Zhao, D.; Yang, S.; Wang, Q. PET-CT evaluation of the curative effect of crizotinib on malignant myofibroblastoma with rare mutation of ALK R401: A case report and literature review. OncoTargets Ther. 2018, 11, 1921–1927. [Google Scholar] [CrossRef]
- Weidenbusch, B.; Richter, G.H.S.; Kesper, M.S.; Guggemoos, M.; Gall, K.; Prexler, C.; Kazantsev, I.; Sipol, A.; Lindner, L.; Nathrath, M.; et al. Transcriptome based individualized therapy of refractory pediatric sarcomas: Feasibility, tolerability and efficacy. Oncotarget 2018, 9, 20747–20760. [Google Scholar] [CrossRef]
- Subbiah, V.; McMahon, C.; Patel, S.; Zinner, R.; Silva, E.G.; Elvin, J.A.; Subbiah, I.M.; Ohaji, C.; Ganeshan, D.M.; Anand, D.; et al. STUMP un “stumped”: Anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J. Hematol. Oncol. 2015, 8, 66. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Murphy, S.J.; Harris, F.R.; Robinson, S.I.; Marks, R.S.; Johnson, S.H.; Smadbeck, J.B.; Halling, G.C.; Yi, E.S.; Wigle, D.; et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann. Oncol. 2016, 27, 2111–2117. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, Q.; Huang, Y.; Zhang, Y.; Gan, X.; Liu, S.; Yue, Z.; Wei, Y. A novel TJP1-ROS1 fusion in malignant peripheral nerve sheath tumor responding to crizotinib: A case report. Medicine 2020, 99, e20725. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kurasaki, K.; Ikeda, S.; Kurzrock, R. Rare Tumor Clinic: The University of California San Diego Moores Cancer Center Experience with a Precision Therapy Approach. Oncologist 2018, 23, 171–178. [Google Scholar] [CrossRef]
- Kyi, C.; Friedman, C.F.; Mueller, J.J.; Benayed, R.; Ladanyi, M.; Arcila, M.; Yang, S.R.; Hensley, M.L.; Chiang, S. Uterine mesenchymal tumors harboring ALK fusions and response to ALK-targeted therapy. Gynecol. Oncol. Rep. 2021, 37, 100852. [Google Scholar] [CrossRef]
- Giraudet, A.L.; Cassier, P.A.; Iwao-Fukukawa, C.; Garin, G.; Badel, J.N.; Kryza, D.; Chabaud, S.; Gilles-Afchain, L.; Clapisson, G.; Desuzinges, C.; et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer 2018, 18, 646. [Google Scholar] [CrossRef] [PubMed]
- Brian Dalton, W.; Forde, P.M.; Kang, H.; Connolly, R.M.; Stearns, V.; Gocke, C.D.; Eshleman, J.R.; Axilbund, J.; Petry, D.; Geoghegan, C.; et al. Personalized medicine in the oncology clinic: Implementation and outcomes of the Johns Hopkins molecular tumor board. JCO Precis. Oncol. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Walther, Z.; Sklar, J.L.; Bindra, R.S.; Petrylak, D.P.; Eder, J.P.; Goldberg, S.B. Yale Cancer Center Precision Medicine Tumor Board: Two patients, one targeted therapy, different outcomes. Lancet Oncol. 2018, 19, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Elvin, J.A.; Gay, L.M.; Ort, R.; Shuluk, J.; Long, J.; Shelley, L.; Lee, R.; Chalmers, Z.R.; Frampton, G.M.; Ali, S.M.; et al. Clinical Benefit in Response to Palbociclib Treatment in Refractory Uterine Leiomyosarcomas with a Common CDKN2A Alteration. Oncologist 2017, 22, 416–421. [Google Scholar] [CrossRef]
- Groisberg, R.; Roszik, J.; Conley, A.P.; Lazar, A.J.; Portal, D.E.; Hong, D.S.; Naing, A.; Herzog, C.E.; Somaiah, N.; Zarzour, M.A.; et al. Genomics, morphoproteomics, and treatment patterns of patients with alveolar soft part sarcoma and response to multiple experimental therapies. Mol. Cancer Ther. 2020, 19, 1165–1172. [Google Scholar] [CrossRef]
- Harttrampf, A.C.; Lacroix, L.; Deloger, M.; Deschamps, F.; Puget, S.; Auger, N.; Vielh, P.; Varlet, P.; Balogh, Z.; Abbou, S.; et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in pediatric patients: A single-institutional prospective molecular stratification trial. Clin. Cancer Res. 2017, 23, 6101–6112. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Hong, L.; Yang, P. Successful treatment of angiosarcoma of the scalp with apatinib: A case report. OncoTargets Ther. 2016, 9, 4989–4992. [Google Scholar] [CrossRef]
- Jin, G.; Wang, C.; Jia, D.; Qian, W.; Yin, C.; Wang, D.; Yang, Q.; Li, T.; Zheng, A. Next Generation Sequencing Reveals Pathogenic and Actionable Genetic Alterations of Soft Tissue Sarcoma in Chinese Patients: A Single Center Experience. Technol. Cancer Res. Treat. 2021, 20, 15330338211068964. [Google Scholar] [CrossRef] [PubMed]
- Recine, F.; De Vita, A.; Fausti, V.; Pieri, F.; Bongiovanni, A.; Franchini, E.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Matteucci, F.; et al. Case Report: Adult NTRK-Rearranged Spindle Cell Neoplasm: Early Tumor Shrinkage in a Case with Bone and Visceral Metastases Treated with Targeted Therapy. Front. Oncol. 2021, 11, 5578. [Google Scholar] [CrossRef] [PubMed]
- Seligson, N.D.; Maradiaga, R.D.; Stets, C.M.; Katzenstein, H.M.; Millis, S.Z.; Rogers, A.; Hays, J.L.; Chen, J.L. Multiscale-omic assessment of EWSR1-NFATc2 fusion positive sarcomas identifies the mTOR pathway as a potential therapeutic target. Npj Precis. Oncol. 2021, 5, 43. [Google Scholar] [CrossRef]
- Subbiah, V.; Erwin, W.; Mawlawi, O.; McCoy, A.; Wages, D.; Wheeler, C.; Gonzalez-Lepera, C.; Liu, H.; Macapinlac, H.; Meric-Bernstam, F.; et al. Phase I Study of P-cadherin-targeted Radioimmunotherapy with (90)Y-FF-21101 Monoclonal Antibody in Solid Tumors. Clin. Cancer Res. 2020, 26, 5830–5842. [Google Scholar] [CrossRef]
- Valenciaga, A.; Iwenofu, O.H.; Tinoco, G. Larotrectinib in a Patient with Advanced Pleomorphic Liposarcoma of the Uterus. J. Natl. Compr. Canc. Netw. 2021, 19, 775–779. [Google Scholar] [CrossRef]
- Walsh, E.M.; Xing, D.; Lippitt, M.H.; Fader, A.N.; Wethington, S.L.; Meyer, C.F.; Gaillard, S.L. Molecular tumor board guides successful treatment of a rare, locally aggressive, uterine mesenchymal neoplasm. JCO Precis. Oncol. 2021, 5, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Ng, C.T.; Tan, K.T. Transient response of olaparib on pulmonary artery sarcoma harboring multiple homologous recombinant repair gene alterations. J. Pers. Med. 2021, 11, 357. [Google Scholar] [CrossRef]
| Database | Search Strings | Number of Results |
|---|---|---|
| PubMed | (“Sarcoma”[MeSH Terms] OR “soft tissue sarcoma*”[Text Word] OR “soft tissue neoplasm*”[Text Word]) AND (“adult*”[Text Word] OR “Adult”[MeSH Terms]) AND (“Molecular Targeted Therapy”[MeSH Terms] OR “Immunotherapy”[MeSH Terms] OR “molecular targeted therapy*”[Text Word] OR “immunotherapy*”[Text Word] OR “targeted therapy*”[Text Word]) | 846 |
| Embase | (‘sarcoma’/exp OR sarcoma*:ab,kw,ti OR ‘soft tissue sarcoma*’:ab,kw,ti OR ‘soft tissue neoplasm*’:ab,kw,ti) AND (‘adult’/exp OR adult*:ab,kw,ti) AND (‘molecularly targeted therapy’/exp OR ‘molecularly targeted therapy*’:ab,kw,ti OR ‘immunotherapy’/exp OR immunotherapy*:ab,kw,ti OR ‘targeted therapy*’:ab,kw,ti) AND [humans]/lim AND [english]/lim | 3150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riskjell, A.I.; Mäkinen, V.-N.; Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N. Targeted Treatment of Soft-Tissue Sarcoma. J. Pers. Med. 2023, 13, 730. https://doi.org/10.3390/jpm13050730
Riskjell AI, Mäkinen V-N, Sandfeld-Paulsen B, Aggerholm-Pedersen N. Targeted Treatment of Soft-Tissue Sarcoma. Journal of Personalized Medicine. 2023; 13(5):730. https://doi.org/10.3390/jpm13050730
Chicago/Turabian StyleRiskjell, Anne Iren, Vivi-Nelli Mäkinen, Birgitte Sandfeld-Paulsen, and Ninna Aggerholm-Pedersen. 2023. "Targeted Treatment of Soft-Tissue Sarcoma" Journal of Personalized Medicine 13, no. 5: 730. https://doi.org/10.3390/jpm13050730
APA StyleRiskjell, A. I., Mäkinen, V.-N., Sandfeld-Paulsen, B., & Aggerholm-Pedersen, N. (2023). Targeted Treatment of Soft-Tissue Sarcoma. Journal of Personalized Medicine, 13(5), 730. https://doi.org/10.3390/jpm13050730






