Abstract
Background: Soft-tissue sarcoma (STS) is a heterogeneous group of sarcomas with a low incidence. The treatment of advanced disease is poor, and mortality is high. We aimed to generate an overview of the clinical experiences with targeted treatments based on a pre-specified target in patients with STS. Methods: A systematic literature search was conducted in PubMed and Embase databases. The programs ENDNOTE and COVIDENCE were used for data management. The literature was screened to assess the article’s eligibility for inclusion. Results: Twenty-eight targeted agents were used to treat 80 patients with advanced STS and a known pre-specified genetic alteration. MDM2 inhibitors were the most-studied drug (n = 19), followed by crizotinib (n = 9), ceritinib (n = 8), and 90Y-OTSA (n = 8). All patients treated with the MDM2 inhibitor achieved a treatment response of stable disease (SD) or better with a treatment duration of 4 to 83 months. For the remaining drugs, a more mixed response was observed. The evidence is low because most studies were case reports or cohort studies, where only a few STS patients were included. Conclusions: Many targeted agents can precisely target specific genetic alterations in advanced STS. The MDM2 inhibitor has shown promising results.
1. Introduction
Sarcomas are neoplasms originating from the connective tissue. With more than eighty histological subtypes, sarcomas are highly heterogenous and rare, representing only about 1–2% of all adult cancers [1,2]. Sarcomas are divided into soft-tissue sarcoma (STS), representing approximately 84% of sarcoma patients, and sarcomas from bone and cartilage, representing approximately 16% of the patients [3]. The standard treatment of localised disease is surgery, with or without radiotherapy [4]. Despite a curative-intended therapy, the prognosis is grave as 25% of the patients will develop metastatic disease. These patients are primarily treated with chemotherapy [5,6]. However, the response to chemotherapy is low, and the mortality is high [2]. Therefore, the need for improved treatment for patients with advanced STS is needed.
Over the past decades, multiple genetic alterations in cancer have been identified. This has revolutionised the treatment by enabling targeted treatment to precisely inhibit the growth and progression of cancer cells [7].
STS is driven primarily by a fusion of genes rather than mutations. However, several studies have shown some genetic alterations in STS. The Cancer Genome Atlas (TCGA) described the molecular landscape of 206 adult STS, representing six subtypes of STS, including synovial sarcoma (SS), liposarcoma (LPS), leiomyosarcoma (LMS), malignant peripheral nerve sheath sarcoma (MPNST), myxofibrosarcoma, and undifferentiated sarcoma. They found that most sarcomas are characterised by copy number alterations (CNAs) and a low tumour mutation burden (TMB) [8]. TMB is usually categorised into three categories: low (1–5 mutations/Mb), intermediate (6–19 mutations/Mb), and high (≥20 mutations/Mb) [9]. The average TMB in the TCGA was low for sarcoma, with an average of 1.06 mutations/Mb. Other mutations represent a few highly recurrent genes. The most frequently mutated genes in the database were TP53 (n = 69), ATRX (n = 31), RB1 (n = 18), PCLO (n = 9), FAT1 (n = 6), NF1 (n = 6), PRKDC (n = 6), and LRP1B (n = 6). The most frequent amplifications were seen in MDM2 (n = 46), FRS2 (n = 45), CDK4 (n = 44), HMG2A (n = 36), and PTPRB (n = 33). The most frequent deletions were observed in CDKN2A (n = 22), CDKN2B (n = 22), RB1 (n = 22), CYSLTR2 (n = 18), and TP53 (n = 16) [8]. In a study investigating the mismatch repair (MMR) status of 304 sarcomas, seven were found to be MMR-deficient. MMR-deficient sarcomas had a significantly higher TMB than MMR-proficient ones, with an average of 16.95 mutations/Mb and 4.56 mutations/Mb, respectively [10].
Arnaud-Coffin et al. investigated 158 patients with advanced STS by genetic profiling. They found 289 relevant genetic alterations in 149 genes. The most frequent alterations were TP53 (n = 36), RB1 (n = 22), CDKN2A (n = 17), CDK4 (n = 9), MDM2 (n = 8), and PTEN (n = 7). The alterations in CDK4 and MDM2 were amplifications only [2]. In a study including 102 sarcoma patients, the most frequent alterations were also TP53 (n = 32), CDK4 (n = 24), MDM2 (n = 22), RB1 (n = 19), and CDKN2A/B (n = 14) [11]. In a study investigating the molecular characterisation of fourteen adult STS, the most frequently altered genes were FRGB1 (n = 8) and CDC27 (n = 6). TP53, ARTX, and PTEN were mutated in three cases. The median TMB was low (2.38 mutations/Mb) [12]. Dembla et al. found the prevalence of MDM2 amplification in 13/33 sarcoma patients [13]. Lin et al. included 301 with uterine sarcomas representing many subtypes of STS. Here, they found that nineteen were SMARC4-deficient. In the SMARC4-deficient cohort, they performed next-generation sequencing (NGS) on sixteen patients. The average TMB was low (1.7 mutations/Mb), and they found mutations in TP53 (n = 2), RB1 (n = 1), CTNNB1 (n = 1), and ZNF703 (n = 1) [14]. Seol et al. also analysed five patients with uterine sarcoma with NGS. In one patient, they found amplifications of AKT3, BRAF, and EGFR. Another patient had an amplification of PDGFRB [15]. Li et al. investigated forty BCOR-rearranged uterine sarcomas. Among these patients, thirty-eight had ZC3H7B–BCOR fusion. They also found amplifications of MDM2 (n = 18), FRS2 (n = 16), CKD4 (n = 15), PDGFRA (n = 3), KDR (n = 2), ERBB3 (n = 2), and KIT (n = 1). Loss of CDKN2A and CDKN2B were observed in eleven and seven cases, respectively. They also found inactivating mutations in TP53 (n = 4), PTCH1 (n = 2), NF1 (n = 2), and NF2 (n = 1). In addition to BCOR, other rearrangements were in the HMGA2 and NCOR2 genes, with six and two cases, respectively. Thirty-nine patients had a low TMB, and one had an intermediate. In addition to the BCOR cohort, they also investigated a cohort consisting of fifteen patients with BCOR internal tandem duplication. Here, none of the cases had MDM2 and CDK4 amplifications. Three of the cases had a CDKN2A/B loss and mutations in STAG2 (n = 2), PASK (n = 2), and ARID1A (n = 2) [16]. Thirteen patients with renal sarcoma had a low TMB with a mean of 3.5 mutations/Mb. Amplifications of KIT and PDGFRA were observed in four patients. Three cases experienced a loss of CDKN2A/B. Genetic alterations were also found in TP53 (n = 4), NF1 (n = 3), and MLL2 (n = 2). One case had a fusion of STAT6–NAB2 [17].
In STS, the frequency of genetic alterations is between 84 and 91%, with the most frequently altered genes being TP53, ATRX, RB1, PTEN, MDM2, CDK4, and CDKN2A/B [2,8,11,13,16,17,18,19]. Different genetic alterations can enable targeted therapy in patients suffering from advanced STS. However, while much is currently known about the genetic landscape of sarcoma, no overview covering treatment options against specific genetic alterations in STS exists. This systematic review aims to generate an overview of the clinical experiences with targeted treatments based on a pre-specified target in patients with STS. This study focuses on the outcome after targeted treatment of rare genetic alterations in sarcoma patients, not the genetic alteration by themselves or already effective treatments. Genetic testing is primarily performed in sarcoma patients with locally advanced or metastatic diseases treated with known standard palliative treatment. The studies included in this systematic review were selected for treatment given based on the results from comprehensive genetic testing.
2. Materials and Methods
2.1. Data Sources and Search Strings
A systematic review of the existing literature investigating the targeted treatment of adult STS was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [20]. A comprehensive literature search in the medical databases PubMed and Embase was conducted on 1 April 2022. The following filters were used in both databases: “not animals” and “English”. There was no time restriction. The search strings used are presented in Table 1.

Table 1.
Presentation of the search strings.
2.2. Study Selection
The inclusion criteria were as follows: (I) original data, (II) the patients had to have a proven pathological STS with a specific genetic alteration prior to therapy with a targeted drug, (III) treatment outcome of one or several targeted agents had to be presented, (IV) if a study also included other types of cancer, the treatment outcome on STS had to be presented separately, (V) the patients had to be older than 15 years old, (VI) if a study included both paediatric and adult STS, the treatment outcome regarding adult STS had to be presented separately. Exclusion criteria were as follows: (I) non-English articles, (II) conference abstracts, (III) animal or in vitro studies, (IV) bone or cartilage sarcomas, hemopoietic sarcomas, and sarcomatoid tumours, (V) results only presented in figures, (VI) studies regarding immunotherapy. Patients with gastrointestinal stomal tumours (GISTs) treated with tyrosine kinase inhibitors (TKIs) were not included in the systemic review, even though they are treated with TKIs targeting different tyrosine kinases. The evidence for this treatment is well known, and for a systematic review of treating GIST, we referred to a systematic review published by Brinch et al. [21]. The multi-targeted drug pazopanib, a tyrosine kinase inhibitor targeting VEGFR, a platelet-derived growth factor receptor, and a c-kit were not included, as the treatment is given to many sarcoma patients with genetic testing with good clinical responses [22,23,24,25,26,27,28]. The same is true for regorafenib [29,30].
2.3. Data Extraction and Quality Assessment
All titles and abstracts were screened to identify eligible articles. One hundred studies were randomly selected and screened independently by all four authors to validate the abovementioned in- and exclusion criteria. Any disagreement was resolved by consensus. Two of the authors (AIR and VNM) screened the rest of the titles and abstracts. In the case of disagreement, conflicts were resolved by all four authors.
After the identification of eligible full texts, ten randomly selected articles were read by three authors (AIR, BSP, and NAP) and subsequently included or excluded. The rest were included or excluded through full-text reading by one author (AIR) and, in the case of doubt, discussed by the other authors until a consensus was reached. Covidence (covidence.org) and Endnote (Clarivate Analytics) were used for duplicate and reference management during the inclusion and exclusion processes. The protocol was submitted to the PROSPERO database (CRD42021252341).
One author (AIR) performed data extraction, which subsequently was checked by the other authors. Data extraction included the first author’s name, year of publishing, study design, genetic alteration that served as a target, type and/or name of the targeted drug, population, and treatment outcome. Studies included in this systematic review were quality scored by all authors using the Quality Assessment Tools for Case Series Studies and the Quality Assessment Tools for Observational Cohort and Cross-Sectional Studies, National Institute of Health, USA (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 1 February 2022). The studies were rated “good”, “fair”, or “poor” according to the estimated risk of bias. All authors performed the quality assessment, and any disagreements were solved by consensus. Due to the heterogeneity of the studies and the many different outcomes they used, a meta-analysis could not be performed.
3. Results
3.1. Study Selection
A total of 3996 titles and abstracts were identified using the two search strings presented in Table 1. After duplication screening, 493 duplicates were removed, resulting in 3503 unique hits. All titles and abstracts were screened, and subsequently, 169 full texts were read, returning 31 articles that met the abovementioned inclusion and exclusion criteria and, thus, were included in this systematic review. The inclusion and exclusion process is presented in Figure 1.
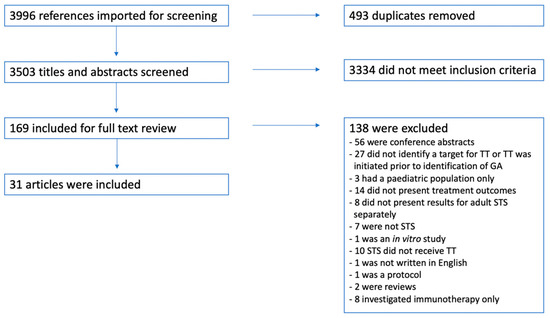
Figure 1.
A flow chart presenting the in- and exclusion process. Abbreviations: GA: genetic alteration, STS: soft-tissue sarcoma, and TT: targeted treatment.
3.2. Study Description and Quality Assessment
Study characteristics for all included studies are presented in Table 2. All studies were either case reports (n = 17) or cohort studies (n = 14). In addition, an update of a case report was identified, and the sum of the two identified case reports was included [31,32]. The articles were published between 2015 and 2021. Twelve case reports were rated good, and the last five were rated as fair. Eleven cohort studies were rated good, two fair, and one poor.
In total, 80 patients representing 30 different histological subtypes were treated with targeted therapy based on a pre-specified target. Twenty-eight different drugs were used, targeting forty-one different targets. Fourteen patients received two or more different targeted agents in the same treatment course. Thirteen of the drugs were given only to one patient. The treatment effect on patients with advanced STS was measured by nine different treatment outcomes: complete response (CR), median time to progression, no evidence of disease (NED), overall survival (OS), partial response (PR), progression-free survival (PFS), progressive disease (PD), relapse-free survival, and stable disease (SD). In total, seven patients achieved CR, near CR, or NED. The patients achieving CR were treated as follows: Three patients received crizotinib targeting an NTRK1–KHDRBS1 fusion, ALK rearrangements, or an LMNA–NTRK1 fusion [32,33,34]; one patient received the MDM2 inhibitor, targeting an MDM2 amplification [11], and one patient received larotrectinib against a SPECC1L–NTRK3 fusion [35]. The two patients achieving NED received crizotinib against ALK expression and a SLC12A1–ROS1 fusion, respectively [36]. Only the MDM2 inhibitor, crizotinib, ceritinib, and 90Y-OTSA were given to eight or more patients.
3.2.1. MDM2 Inhibitors
MDM2 inhibitors were given to 19 patients. All patients receiving MDM2 inhibitors were patients with liposarcoma and MDM2 amplification; furthermore, all patients had a treatment effect of SD or better with a treatment duration ranging from 4 to 83 months [11,13,37].
3.2.2. Crizotinib
A total of 15 patients received crizotonib. The patients receiving crizotinib had NTRK1 fusions (n = 2), ALK rearrangements (n = 9), ROS1 mutations (n = 3), or MET mutations (n = 1) and achieved varying responses from PD to CR with a treatment duration ranging from three months to two years [11,32,34,38,39,40,41,42].
3.2.3. Ceritinib
Ceritinib was given to eight patients. The patients receiving ceritinib had ALK rearrangements (n = 6), ROS1 amplifications (n = 1), or IGFR1 amplifications (n = 1) and achieved varying responses from PD to PR with a treatment duration of up to 24 months [11,41,43,44].
3.2.4. 90Y-OTSA
Eight patients with FZD10 expression received the targeted radioactive drug 90Y-OTSA. Three patients received 370 MBq of these; one patient had SD, and two patients PD. Five patients received 1100 MBq; two had SD, and three had PD. One of the patients with SD receiving 1100 MB1 90Y-OTSA also received a second injection that resulted in PFS for 21.4 weeks [45].

Table 2.
Study characteristics.
Table 2.
Study characteristics.
| First Author, Year | Study Design | Quality Assessment | Histological Subtype (n) | Genetic Alteration | Targeted Agent | Treatment Outcome |
|---|---|---|---|---|---|---|
| Arnaud-Coffin, 2020 [2] | Prospective cohort study | Good | Advanced STS | (PFS/OS) | ||
| LMS (1) | AKT2 amplification | Everolimus | 2.6/10.9 months | |||
| MPNST (1) | ERBB2 mutation | Lapatinib | 1.9/3.8 months | |||
| Angiosarcoma (1) | FLT4 mutation | Pazopanib | 3.1/10.7 months | |||
| UPS (1) | AKT2 deletion | Everolimus | 1.4/4.1 months | |||
| GIST (3) | CDKN2A deletion | Palbociclib | 0.8/4.9, 0.9/2.6, 3.7/22.9 months | |||
| Brian Dalton, 2017 [46] | Cohort study | Good | Advanced cancer | |||
| MPNST (1) | EGFR duplication | Afatinib | PD 2 months | |||
| RMS (1) | FGFR1 mutation | Pazopanib | PD 4 months | |||
| Cecchini, 2018 [47] | Case report | Fair | High-grade sarcoma (1) | ATM frameshift mutation | Olaparib | PD 2 months |
| Chen, 2021 [33] | Case report | Good | Mesenchymal sarcoma (1) | NTRK1–KHDRBS1 fusion | Crizotinib | CR 40+ months |
| Dembla, 2018 [13] | Retrospective cohort study | Fair | LPS (6) | MDM2 amplification | MDM2 inhibitor | 3 had PR, 2 had SD (15.7 and 4.7 months, respectively) 1 n/a |
| Elvin, 2017 [48] | Case report | Good | LMS (1) | CDKN2A deletion | Palbociclib | SD 4 months, radiological progression at 8 months |
| Forde, 2016 [31] Kinne, 2019 [32] | Case report Update | Fair | IMS (1) | ALKrearrangements | Crizotinib | CR 3 months, CR 164 weeks |
| Giraudet, 2018 [45] | Phase 1 cohort study | Good | SS (8) | FZD10 | 90Y-OTSA-101 | |
| 3 received 370 MBq of 90Y | 1 had SD, 2 had PD | |||||
| 5 received 1110 MBq of 90Y | 2 had SD, 3 had PD 1 with SD received a second injection resulting in PFS for 21.4 weeks | |||||
| Groisberg, 2017 [11] | Cohort study | Good | Advanced sarcoma | |||
| Gliosarcoma (1) | BRAF V600E | Vemurafenib | PR 16 months | |||
| DDLPS (1) | ROS1 amplification | Ceritinib | SD 5 months | |||
| DDLPS (1) | MDM2 amplification | MDM2 inhibitor | PR 3 cycles | |||
| GIST (1) | KIT +AKT Amplification | Imatinib, sunitinib, regorafenib, AKT inhibitor | PD x3 | |||
| LMS (2) | ROS1 mutation | Pazopanib +crizotinib | PR 22 cycles, SD 6 months, PD | |||
| LMS (1) | PTEN deletion | PI3K inhibitor | PD | |||
| Pleomorphic sarcoma (1) | MEMO1–ALK fusion | Ceritinib | PD after 4 cycles | |||
| Myxoid LPS (1) | AKT1 mutation | AKT inhibitor | SD 1 cycle | |||
| Spindle cell sarcoma (1) | KIAA1549–BRAF fusion | Sorafenib+ bevacizumab + temsirolimus | SD 11 cycles | |||
| WDLPS (4) | MDM2 amplification | MDM2 inhibitor | SD 8 cycles, CR, SD 2 cycles, SD 23 months | |||
| Groisberg, 2020 [49] | Cohort study | Fair | Alveolar soft part sarcoma (1) | HGF amplification | Pazopanib + vorinostat | SD 28 months |
| Harttrampf, 2017 [50] | Prospective cohort study | Good | Paediatric advanced tumours Epithelioid sarcoma (1) | SMARCB1 deletion | Tazemetostat | PD 2 months |
| Ji, 2016 [51] | Case report | Good | Angiosarcoma (1) | VEGFR2 | Apatinib | PFS 12 months |
| Jin, 2021 [52] | Cohort study | Good | STS | |||
| Inflammatory myofibroblastoma (1) | MAP2K1 | Trametinib | RFS: 2 months | |||
| LPS (1) | CDK4 | Palbociclib | RFS: 4 months | |||
| Fibrosarcoma (1) | COL1A1–PDGFB fusion | Imatinib | RFS: 10 months | |||
| Clear cell sarcoma (1) | BRAF V600E | Vemurafenib | RFS: 21 months | |||
| Kato, 2018 [43] | Cohort study | Good | Advanced cancer | |||
| Desmoid tumour (1) | CTNNB1 mutation | Sorafenib + sulindac | SD, PFS 9.1+ months | |||
| ESS (1) | CDKN2A mutations and FRS2 amplification | Palbociclib + lenvatinib + anastrozole + doxorubicin | SD, PFS 3.6+ months | |||
| Myxofibrosarcoma (1) | IGFR1 amplification | Ceritinib | PD, PFS 1.8+ months | |||
| Kerr, 2021 [36] | Cohort study | Good | IMT (2) | ALK+ SLC12A1–ROS1 fusion | Crizotinib Crizotinib+ surgery | NED: 1.8 years NED: 2 years |
| Kyi, 2021 [44] | Case report | Good | IMT (1) | FN1–ALK fusion | Crizotinib→ ceritinib | SD 4 months→ SD 6 months |
| IMT (1) | TNS1–ALK fusion | Crizotinib→ Alectinib→ Ceritinib → Lorlatinib | SD 3 months → SD 12 months → SD 2 months → PD 1 month | |||
| Myofibroblastic sarcoma (1) | LBH–ALK fusion | Crizotinib→ Ceritinib | SD 30 months → SD 6 months | |||
| IMT (1) | IGFBP5–ALK fusion | Ceritinib | PR 24+ months | |||
| Li, 2020 [42] | Case report | Fair | MPNST (1) | TJP1–ROS1 fusion | Crizotinib | SD 2 months, PD after 4 months |
| Mansfield, 2016 [41] | Case report | Good | IMS (1) | ALK+ | Crizotinib → Ceritinib + surgery | PR 8 months→ progression → PR 18 months |
| Rabban, 2020 [35] | Case report | Good | Uterine sarcoma (1) | SPECC1L–NTRK3 fusion | Larotrectinib | CR 15+ months |
| Recine, 2021 [53] | Case report | Good | Spindle cell neoplasm (1) | TPM4–NTRK1 fusion | Larotrectinib | PR 19+ months |
| Seligson, 2021 [54] | Case report | Good | Small cell round tumour (1) | EWSR1–NFATc2 fusion | Everolimus + surgery + non-TT | SD 47 months |
| Seol, 2019 [15] | Prospective cohort study | Good | Advanced tumours | |||
| Uterine sarcoma (1) | AKT3 amplification | Everolimus | PR 5 months | |||
| Somaiah, 2018 [37] | Cohort study | Good | LPS (8) | MDM2 amplification | MDM2 inhibitor | Median time to progression: 23 months (95%-CI: 10–83 months) |
| Subbiah, 2015 [40] | Case report | Fair | IMS (1) | DCTN1–ALK fusion | Crizotinib + pazopanib | PR 6+ months |
| Subbiah, 2020 [55] | Cohort study | Good | Advanced tumours Clear cell sarcoma (1) | Placental cadherin | 90Y-FF-21101 mAb 25 mCi/m2 | SD 50 weeks |
| Valenciaga, 2021 [56] | Case report | Good | Pleomorphic LPS (1) | IQGAP–NTRK3 fusion | Entrectinib → Pazopanib + radiation → Larotrectinib | PD 4 cycles → PD 3 months → SD 18+ months |
| Walsh, 2021 [57] | Case report | Fair | IMT (1) | LRRFIP1–ALK fusion | Alectinib | PR 19 months |
| Weidenbusch, 2018 [39] | Cohort study | Poor | Paediatric sarcoma | |||
| RMS (1) | MET and FGFR1 mutation | Crizotinib + ponatinib | SD 7 months | |||
| SS (1) | FGFR1 and EGFR mutation | Ponatinib + gefitinib | PD | |||
| Wu, 2021 [58] | Case report | Good | Primary pulmonary artery sarcoma (1) | Loss of ATM and H2AX | Olaparib | PR 2 months |
| Yang, 2018 [38] | Case report | Good | Myofibroblastic sarcoma (1) | ALK mutation | Crizotinib + bevacizumab | PFS 3 months |
| Zhou, 2018 [34] | Case report | Good | UPS (1) | LMNA–NTRK1 fusion | Crizotinib | Near-CR 18+ months |
Abbreviations: CR: complete response, DDLPS: dedifferentiated liposarcoma, ESS: endometrial stromal sarcoma, GIST: gastrointestinal stromal tumour, IMS: inflammatory myofibroblastic sarcoma, IMT: inflammatory myofibroblastic tumour, LMS: leiomyosarcoma, LPS: liposarcoma, mAb: monoclonal antibody, MPNST: malignant peripheral nerve sheath tumour, n/a: not applicable, NED: no evidence of disease, OS: overall survival, PD: progressive disease, PFS: progression-free survival, PR: partial response, RFS: relapse-free survival, RMS: rhabdomyosarcoma, SD: stable disease, SS: synovial sarcoma, TT: targeted treatment, UPS: undifferentiated pleomorphic sarcoma, WDLPS: well-differentiated liposarcoma.
4. Discussion
Advanced sarcoma is a rare but severe disease, and better treatment options are needed. We have conducted a systematic review to cover targeted treatment for patients suffering from advanced STS with a known genetic alteration.
The use of targeted treatment in STS is still on an individual and experimental level. Most of the studies included in this systematic review were case reports or cohort studies where only a few individuals suffering from advanced STS were included. Summarised, 80 patients received 28 different targeted drugs. Thirty different subtypes of STS received targeted treatment, and among them, forty-one different genetic alterations were identified. Thus, several targeted agents were administered to patients with different kinds of advanced STS but with highly variable results.
Several studies have shown that there are many different genetic alterations in STS. Groisberg et al. found that 93 out of 102 advanced STS patients had at least one genetic alteration, where the most frequent were found in TP53, CDK4, and MDM2, and 61% had a potentially targetable alteration. However, only 16% of the patients received a targeted drug [11]. Similar observations were found by Lucchesi et al., where 84% of the 584 included STS patients enharboured a genetic alteration. Furthermore, the most frequent mutations were observed in TP53, MDM2, and CDK4, and 41% of the patients had at least one potentially targetable alteration [18]. Gusho et al. also found a high frequency of genetic alterations with at least 1 genetic alteration in 122 out of 136 samples. The most frequent mutations observed were in TP53, CDKN2A/B, and RB1, and 47% had a potentially targetable mutation [19].
In this review, all patients receiving MDM2 inhibitors had liposarcomas, with a treatment response of SD or better, including one CR [11,13,37]. MDM2 amplification is a common genetic alteration in STS. Three studies have shown a 5%, 22%, and 40% prevalence of STS [2,11,13]. In specific subtypes of STS, the prevalence is even higher. In well-differentiated and dedifferentiated liposarcoma, the amplification of 12q13-15 is common, resulting in the amplification of the MDM2 gene. Studies have shown that over 90% of these sarcomas have MDM2 amplification [13,37]. Because of the high prevalence of MDM2 alterations in STS, especially in liposarcoma, and the reports with high clinical effects, targeting this specific alteration must be considered.
Crizotinib was given to patients with mutations in ALK, ROS1, MET, or NTRK1. There were varying treatment responses, but three out of nine patients had CR or near-CR, and two patients had NED; therefore, they had an overall survival benefit. One of the patients with CR had ALK gene rearrangements, and the other had NTRK1–KHDRBS1 fusion. The patient with a near-CR response had an LMNA–NTRK1 fusion. The patients with NED had high ALK expression and a SLC12A1–ROS1 fusion [32,33,34,36].
Owing to the aim of this study, only patients receiving treatments targeting a known specific target were included. Therefore, only seven of the included patients received pazopanib, a multi-target tyrosine kinase inhibitor; however, the drug is given to far more patients suffering from advanced STS. Even though studies exist on pazopanib in STS, these studies were not included as they did not have a specific pathological proven target before treatment with pazopanib. Many of these studies showed a treatment effect on STS without prior identification of a specific genetic alteration.
MDM2 inhibitors, crizotinib, ceritinib, and 90Y-OTSA were given to most patients. However, the population receiving targeted therapy is so tiny that it is difficult to conclude the overall survival effect of the targeted agents. Furthermore, nine different treatment outcomes were used to measure the effectiveness of the drugs, making it challenging to conduct a meta-analysis to investigate the potential survival effect of agents on advanced STS.
Most of the included patients were previously treated with curative-intended treatments, and many had one or more treatment courses with chemotherapy. Furthermore, targeted agents were tried as a last resort for patients with advanced STS. Maybe, if the targeted therapy had been introduced to the patients before relapse occurred, the patients might have had a more substantial benefit from the treatment and more prolonged survival. It would have been interesting to compare targeted treatment against chemotherapy as the first-line treatment for advanced STS to see if there is a difference. Larger studies are needed to investigate the potential effect of targeted therapy on STS, for example, case/control studies, where targeted treatment is compared to chemotherapy and ultimately randomised controlled trials.
The strengths of this systematic review are the comprehensive systematic review of the available literature on targeted treatment of STS in two major medical databases. Broad search strings and in- and exclusion criteria were used to avoid missing relevant articles. Two authors screened the literature, and all authors validated the in- and exclusion criteria. Nonetheless, this review also has its limitations. All the studies included are either case reports or cohort studies, where only a few patients have advanced STS. The studies used many different measures of outcome. One of the exclusion criteria was non-English articles, and it is possible that other studies concerning the same topic were written in other languages. Thus, language bias cannot be excluded.
5. Conclusions
During the last decades, many genetic alterations in STS have been identified, enabling the use of targeted treatment. This systematic review revealed many studies regarding genetic alterations and targeted treatment in advanced STS. Twenty-eight targeted agents have currently been tried in advanced STS. However, most articles are case reports and cohort studies representing only a few patients with advanced STS. Studies comparing targeted treatment to chemotherapy in case/control studies and ultimately randomised controlled trials can make it easier to investigate the potential survival benefit that targeted agents can provide to patients suffering from advanced STS.
Author Contributions
Conceptualisation, A.I.R., V.-N.M., B.S.-P. and N.A.-P.; methodology, A.I.R., V.-N.M., B.S.-P. and N.A.-P.; validation, A.I.R., V.-N.M., B.S.-P. and N.A.-P.; resources, N.A.-P.; writing—original draft preparation, A.I.R.; writing—review and editing, A.I.R., V.-N.M., B.S.-P. and N.A.-P.; supervision, B.S.-P. and N.A.-P.; funding acquisition, N.A.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a generous grant from the Danish Cancer Society, number R248-Ai4683.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Coffin, P.; Brahmi, M.; Vanacker, H.; Eberst, L.; Tredan, O.; Attignon, V.; Pissaloux, D.; Sohier, E.; Cassier, P.; Garin, G.; et al. Therapeutic relevance of molecular screening program in patients with metastatic sarcoma: Analysis from the ProfiLER 01 trial. Transl. Oncol. 2020, 13, 100870. [Google Scholar] [CrossRef]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- The European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii102–iii112. [Google Scholar] [CrossRef]
- Singhi, E.K.; Moore, D.C.; Muslimani, A. Metastatic Soft Tissue Sarcomas: A Review of Treatment and New Pharmacotherapies. Pharm. Ther. 2018, 43, 410–429. [Google Scholar]
- Linch, M.; Miah, A.B.; Thway, K.; Judson, I.R.; Benson, C. Systemic treatment of soft-tissue sarcoma—Gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014, 11, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Available online: https://www.cbioportal.org/study/summary?id=sarc_tcga_pub (accessed on 12 March 2021).
- He, M.; Abro, B.; Kaushal, M.; Chen, L.; Chen, T.; Gondim, M.; Yan, W.; Neidich, J.; Dehner, L.P.; Pfeifer, J.D. Tumor mutation burden and checkpoint immunotherapy markers in primary and metastatic synovial sarcoma. Hum. Pathol. 2020, 100, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Nowak, J.A.; Nathenson, M.J.; Thornton, K.; Wagner, A.J.; Johnson, J.M.; Albrayak, A.; George, S.; Sholl, L.M. Characteristics of mismatch repair deficiency in sarcomas. Mod. Pathol. 2019, 32, 977–987. [Google Scholar] [CrossRef]
- Groisberg, R.; Hong, D.S.; Holla, V.; Janku, F.; Piha-Paul, S.; Ravi, V.; Benjamin, R.; Kumar Patel, S.; Somaiah, N.; Conley, A.; et al. Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas. Oncotarget 2017, 8, 39254–39267. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Kang, H.G.; Park, S.Y.; Yu, J.Y.; Lee, E.Y.; Oh, S.E.; Kim, Y.H.; Yun, T.; Park, C.; et al. Integrated molecular characterization of adult soft tissue sarcoma for therapeutic targets. BMC Med. Genet. 2018, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Dembla, V.; Somaiah, N.; Barata, P.; Hess, K.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Piha-Paul, S.A.; Subbiah, V.; et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson phase 1 clinic. Oncotarget 2018, 9, 33232–33243. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Allen, J.; Hecht, J.; Killian, J.; Ngo, N.; Edgerly, C.; Severson, E.; Ali, S.; Erlich, R.; Ramkissoon, S.; et al. SMARCA4 inactivation defines a subset of undifferentiated uterine sarcomas with rhabdoid and small cell features and germline mutation association. Int. J. Gynecol. Cancer 2019, 29, A42. [Google Scholar] [CrossRef]
- Seol, Y.M.; Kwon, C.H.; Lee, S.J.; Lee, S.J.; Choi, Y.; Choi, Y.J.; Kim, H.; Park, D.Y. A Pilot Prospective Study of Refractory Solid Tumor Patients for NGS-Based Targeted Anticancer Therapy. Transl. Oncol. 2019, 12, 301–307. [Google Scholar] [CrossRef]
- Lin, D.I.; Hemmerich, A.; Edgerly, C.; Duncan, D.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; Connor, Y.D.; Shea, M.; Hecht, J.L.; et al. Genomic profiling of BCOR-rearranged uterine sarcomas reveals novel gene fusion partners, frequent CDK4 amplification and CDKN2A loss. Gynecol. Oncol. 2020, 157, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Madison, R.; Fridman, E.; Mangray, S.; Carneiro, B.A.; Lu, S.; Cooke, M.; Bratslavsky, G.; Webster, J.; Ross, J.S.; et al. Comprehensive Genomic Profiling of Adult Renal Sarcomas Provides Insight into Disease Biology and Opportunities for Targeted Therapies. Eur. Urol. Oncol. 2019, 4, 282–288. [Google Scholar] [CrossRef]
- Lucchesi, C.; Khalifa, E.; Laizet, Y.; Soubeyran, I.; Mathoulin-Pelissier, S.; Chomienne, C.; Italiano, A. Targetable Alterations in Adult Patients with Soft-Tissue Sarcomas: Insights for Personalized Therapy. JAMA Oncol. 2018, 4, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Gusho, C.A.; Weiss, M.C.; Lee, L.; Gitelis, S.; Blank, A.T.; Wang, D.; Batus, M. The clinical utility of next-generation sequencing for bone and soft tissue sarcoma. Acta Oncol. 2022, 61, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Brinch, C.M.; Aggerholm-Pedersen, N.; Hogdall, E.; Krarup-Hansen, A. Medical oncological treatment for patients with Gastrointestinal Stromal Tumor (GIST)—A systematic review. Crit. Rev. Oncol. Hematol. 2022, 172, 103650. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Safwat, A.; Boysen, A.; Lucke, A.; Rossen, P. Pazopanib in metastatic osteosarcoma: Significant clinical response in three consecutive patients. Acta Oncol. 2014, 53, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Alcindor, T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015, 54, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Kollar, A.; Jones, R.L.; Stacchiotti, S.; Gelderblom, H.; Guida, M.; Grignani, G.; Steeghs, N.; Safwat, A.; Katz, D.; Duffaud, F.; et al. Pazopanib in advanced vascular sarcomas: An EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hirbe, A.C.; Eulo, V.; Moon, C.I.; Luo, J.; Myles, S.; Seetharam, M.; Toeniskoetter, J.; Kershner, T.; Haarberg, S.; Agulnik, M.; et al. A phase II study of pazopanib as front-line therapy in patients with non-resectable or metastatic soft-tissue sarcomas who are not candidates for chemotherapy. Eur. J. Cancer 2020, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Kawai, A.; Goto, T.; Hiraga, H.; Ozaki, T.; Tsuchiya, H.; Nakayama, R.; Naka, N.; Matsumoto, Y.; Kobayashi, E.; et al. Phase II trial of pazopanib in patients with metastatic or unresectable chemoresistant sarcomas: A Japanese Musculoskeletal Oncology Group study. Cancer Sci. 2020, 111, 3303–3312. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Rossen, P.; Rose, H.; Safwat, A. Pazopanib in the Treatment of Bone Sarcomas: Clinical Experience. Transl. Oncol. 2020, 13, 295–299. [Google Scholar] [CrossRef]
- Brodowicz, T.; Liegl-Atzwager, B.; Tresch, E.; Taieb, S.; Kramar, A.; Gruenwald, V.; Vanseymortier, M.; Clisant, S.; Blay, J.Y.; Le Cesne, A.; et al. Study protocol of REGOSARC trial: Activity and safety of regorafenib in advanced soft tissue sarcoma: A multinational, randomized, placebo-controlled, phase II trial. BMC Cancer 2015, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet.Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Forde, G.K.; Tewari, D. Targeted Treatment of a Rare Vaginal Sarcoma with an Anaplastic Lymphoma Kinase Inhibitor. Obstet. Gynecol. 2016, 127, 222–225. [Google Scholar] [CrossRef]
- Kinne, N.; Hodeib, M.; Kashani, N.A.; Tewari, D. Targeted Treatment of a Rare Vaginal Sarcoma with an Anaplastic Lymphoma Kinase Inhibitor. Obstet. Gynecol. 2019, 134, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Jiang, D.; Luan, L.; Zhou, Y.; Hou, Y. Unclassified mesenchymal sarcoma with NTRK1-KHDRBS1 gene fusion: A case report of long-term tumor-free survival with crizotinib treatment. World J. Surg. Oncol. 2021, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schäfer, R.; Li, T.; Fang, M.; Liu, L. A primary undifferentiated pleomorphic sarcoma of the lumbosacral region harboring a LMNA-NTRK1 gene fusion with durable clinical response to crizotinib: A case report. BMC Cancer 2018, 18, 842. [Google Scholar] [CrossRef]
- Rabban, J.T.; Devine, W.P.; Sangoi, A.R.; Poder, L.; Alvarez, E.; Davis, J.L.; Rudzinski, E.; Garg, K.; Bean, G.R. NTRK fusion cervical sarcoma: A report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology 2020, 77, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Thompson, L.D.R.; Tafe, L.J.; Jo, V.Y.; Neyaz, A.; Divakar, P.; Paydarfar, J.A.; Pastel, D.A.; Shirai, K.; John, I.; et al. Clinicopathologic and genomic characterization of inflammatory myofibroblastic tumors of the head and neck highlighting a novel fusion and potential diagnostic pitfall. Am. J. Surg. Pathol. 2021, 45, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Beird, H.C.; Barbo, A.; Song, J.; Mills Shaw, K.R.; Wang, W.L.; Eterovic, K.; Chen, K.; Lazar, A.; Conley, A.P.; et al. Targeted next generation sequencing of well-differentiated/ dedifferentiated liposarcoma reveals novel gene amplifications and mutations. Oncotarget 2018, 9, 19891–19899. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Tang, H.; Zhao, J.; Zhao, D.; Yang, S.; Wang, Q. PET-CT evaluation of the curative effect of crizotinib on malignant myofibroblastoma with rare mutation of ALK R401: A case report and literature review. OncoTargets Ther. 2018, 11, 1921–1927. [Google Scholar] [CrossRef]
- Weidenbusch, B.; Richter, G.H.S.; Kesper, M.S.; Guggemoos, M.; Gall, K.; Prexler, C.; Kazantsev, I.; Sipol, A.; Lindner, L.; Nathrath, M.; et al. Transcriptome based individualized therapy of refractory pediatric sarcomas: Feasibility, tolerability and efficacy. Oncotarget 2018, 9, 20747–20760. [Google Scholar] [CrossRef]
- Subbiah, V.; McMahon, C.; Patel, S.; Zinner, R.; Silva, E.G.; Elvin, J.A.; Subbiah, I.M.; Ohaji, C.; Ganeshan, D.M.; Anand, D.; et al. STUMP un “stumped”: Anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J. Hematol. Oncol. 2015, 8, 66. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Murphy, S.J.; Harris, F.R.; Robinson, S.I.; Marks, R.S.; Johnson, S.H.; Smadbeck, J.B.; Halling, G.C.; Yi, E.S.; Wigle, D.; et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann. Oncol. 2016, 27, 2111–2117. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, Q.; Huang, Y.; Zhang, Y.; Gan, X.; Liu, S.; Yue, Z.; Wei, Y. A novel TJP1-ROS1 fusion in malignant peripheral nerve sheath tumor responding to crizotinib: A case report. Medicine 2020, 99, e20725. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kurasaki, K.; Ikeda, S.; Kurzrock, R. Rare Tumor Clinic: The University of California San Diego Moores Cancer Center Experience with a Precision Therapy Approach. Oncologist 2018, 23, 171–178. [Google Scholar] [CrossRef]
- Kyi, C.; Friedman, C.F.; Mueller, J.J.; Benayed, R.; Ladanyi, M.; Arcila, M.; Yang, S.R.; Hensley, M.L.; Chiang, S. Uterine mesenchymal tumors harboring ALK fusions and response to ALK-targeted therapy. Gynecol. Oncol. Rep. 2021, 37, 100852. [Google Scholar] [CrossRef]
- Giraudet, A.L.; Cassier, P.A.; Iwao-Fukukawa, C.; Garin, G.; Badel, J.N.; Kryza, D.; Chabaud, S.; Gilles-Afchain, L.; Clapisson, G.; Desuzinges, C.; et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer 2018, 18, 646. [Google Scholar] [CrossRef] [PubMed]
- Brian Dalton, W.; Forde, P.M.; Kang, H.; Connolly, R.M.; Stearns, V.; Gocke, C.D.; Eshleman, J.R.; Axilbund, J.; Petry, D.; Geoghegan, C.; et al. Personalized medicine in the oncology clinic: Implementation and outcomes of the Johns Hopkins molecular tumor board. JCO Precis. Oncol. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Walther, Z.; Sklar, J.L.; Bindra, R.S.; Petrylak, D.P.; Eder, J.P.; Goldberg, S.B. Yale Cancer Center Precision Medicine Tumor Board: Two patients, one targeted therapy, different outcomes. Lancet Oncol. 2018, 19, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Elvin, J.A.; Gay, L.M.; Ort, R.; Shuluk, J.; Long, J.; Shelley, L.; Lee, R.; Chalmers, Z.R.; Frampton, G.M.; Ali, S.M.; et al. Clinical Benefit in Response to Palbociclib Treatment in Refractory Uterine Leiomyosarcomas with a Common CDKN2A Alteration. Oncologist 2017, 22, 416–421. [Google Scholar] [CrossRef]
- Groisberg, R.; Roszik, J.; Conley, A.P.; Lazar, A.J.; Portal, D.E.; Hong, D.S.; Naing, A.; Herzog, C.E.; Somaiah, N.; Zarzour, M.A.; et al. Genomics, morphoproteomics, and treatment patterns of patients with alveolar soft part sarcoma and response to multiple experimental therapies. Mol. Cancer Ther. 2020, 19, 1165–1172. [Google Scholar] [CrossRef]
- Harttrampf, A.C.; Lacroix, L.; Deloger, M.; Deschamps, F.; Puget, S.; Auger, N.; Vielh, P.; Varlet, P.; Balogh, Z.; Abbou, S.; et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in pediatric patients: A single-institutional prospective molecular stratification trial. Clin. Cancer Res. 2017, 23, 6101–6112. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Hong, L.; Yang, P. Successful treatment of angiosarcoma of the scalp with apatinib: A case report. OncoTargets Ther. 2016, 9, 4989–4992. [Google Scholar] [CrossRef]
- Jin, G.; Wang, C.; Jia, D.; Qian, W.; Yin, C.; Wang, D.; Yang, Q.; Li, T.; Zheng, A. Next Generation Sequencing Reveals Pathogenic and Actionable Genetic Alterations of Soft Tissue Sarcoma in Chinese Patients: A Single Center Experience. Technol. Cancer Res. Treat. 2021, 20, 15330338211068964. [Google Scholar] [CrossRef] [PubMed]
- Recine, F.; De Vita, A.; Fausti, V.; Pieri, F.; Bongiovanni, A.; Franchini, E.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Matteucci, F.; et al. Case Report: Adult NTRK-Rearranged Spindle Cell Neoplasm: Early Tumor Shrinkage in a Case with Bone and Visceral Metastases Treated with Targeted Therapy. Front. Oncol. 2021, 11, 5578. [Google Scholar] [CrossRef] [PubMed]
- Seligson, N.D.; Maradiaga, R.D.; Stets, C.M.; Katzenstein, H.M.; Millis, S.Z.; Rogers, A.; Hays, J.L.; Chen, J.L. Multiscale-omic assessment of EWSR1-NFATc2 fusion positive sarcomas identifies the mTOR pathway as a potential therapeutic target. Npj Precis. Oncol. 2021, 5, 43. [Google Scholar] [CrossRef]
- Subbiah, V.; Erwin, W.; Mawlawi, O.; McCoy, A.; Wages, D.; Wheeler, C.; Gonzalez-Lepera, C.; Liu, H.; Macapinlac, H.; Meric-Bernstam, F.; et al. Phase I Study of P-cadherin-targeted Radioimmunotherapy with (90)Y-FF-21101 Monoclonal Antibody in Solid Tumors. Clin. Cancer Res. 2020, 26, 5830–5842. [Google Scholar] [CrossRef]
- Valenciaga, A.; Iwenofu, O.H.; Tinoco, G. Larotrectinib in a Patient with Advanced Pleomorphic Liposarcoma of the Uterus. J. Natl. Compr. Canc. Netw. 2021, 19, 775–779. [Google Scholar] [CrossRef]
- Walsh, E.M.; Xing, D.; Lippitt, M.H.; Fader, A.N.; Wethington, S.L.; Meyer, C.F.; Gaillard, S.L. Molecular tumor board guides successful treatment of a rare, locally aggressive, uterine mesenchymal neoplasm. JCO Precis. Oncol. 2021, 5, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Ng, C.T.; Tan, K.T. Transient response of olaparib on pulmonary artery sarcoma harboring multiple homologous recombinant repair gene alterations. J. Pers. Med. 2021, 11, 357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).