The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
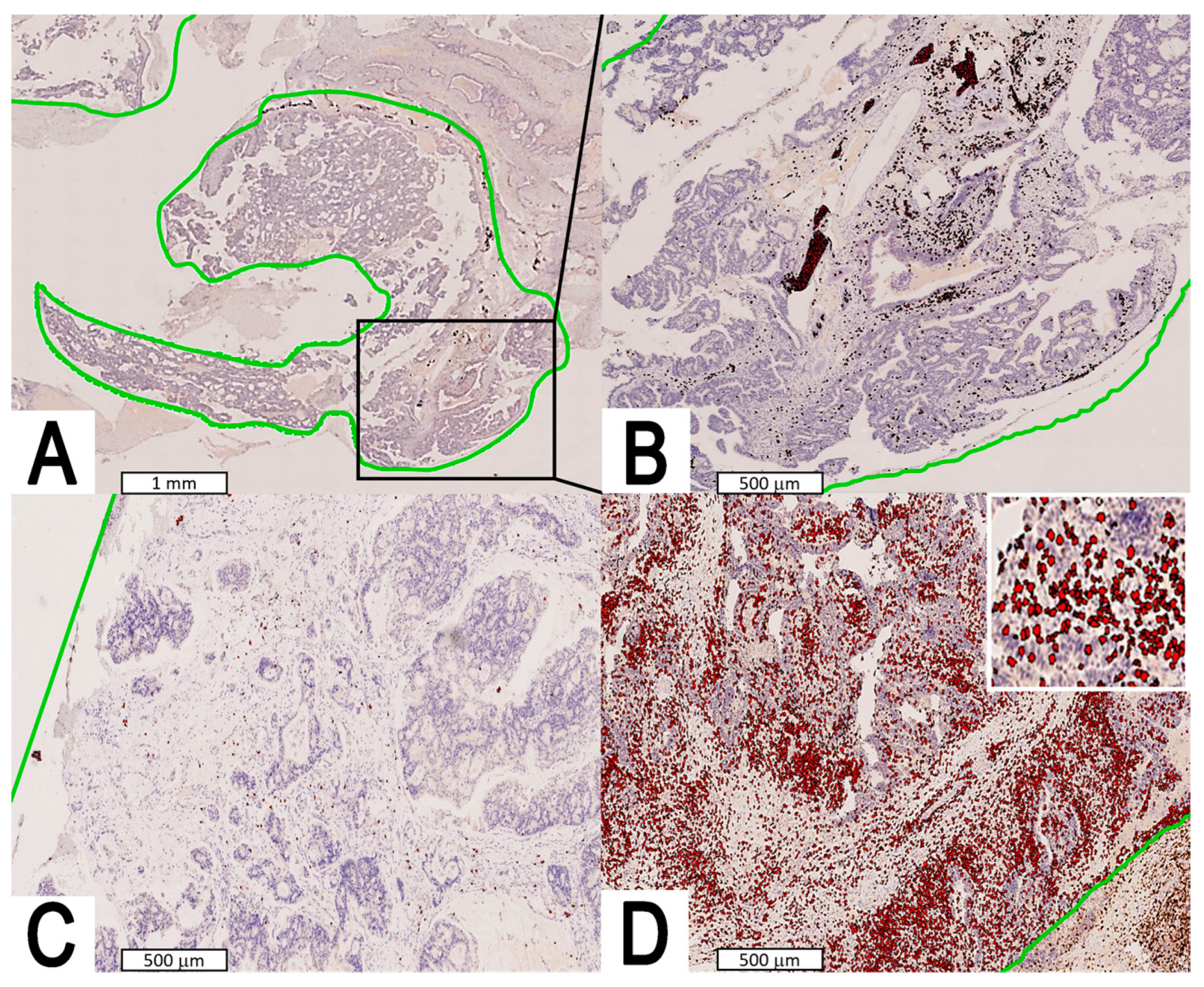
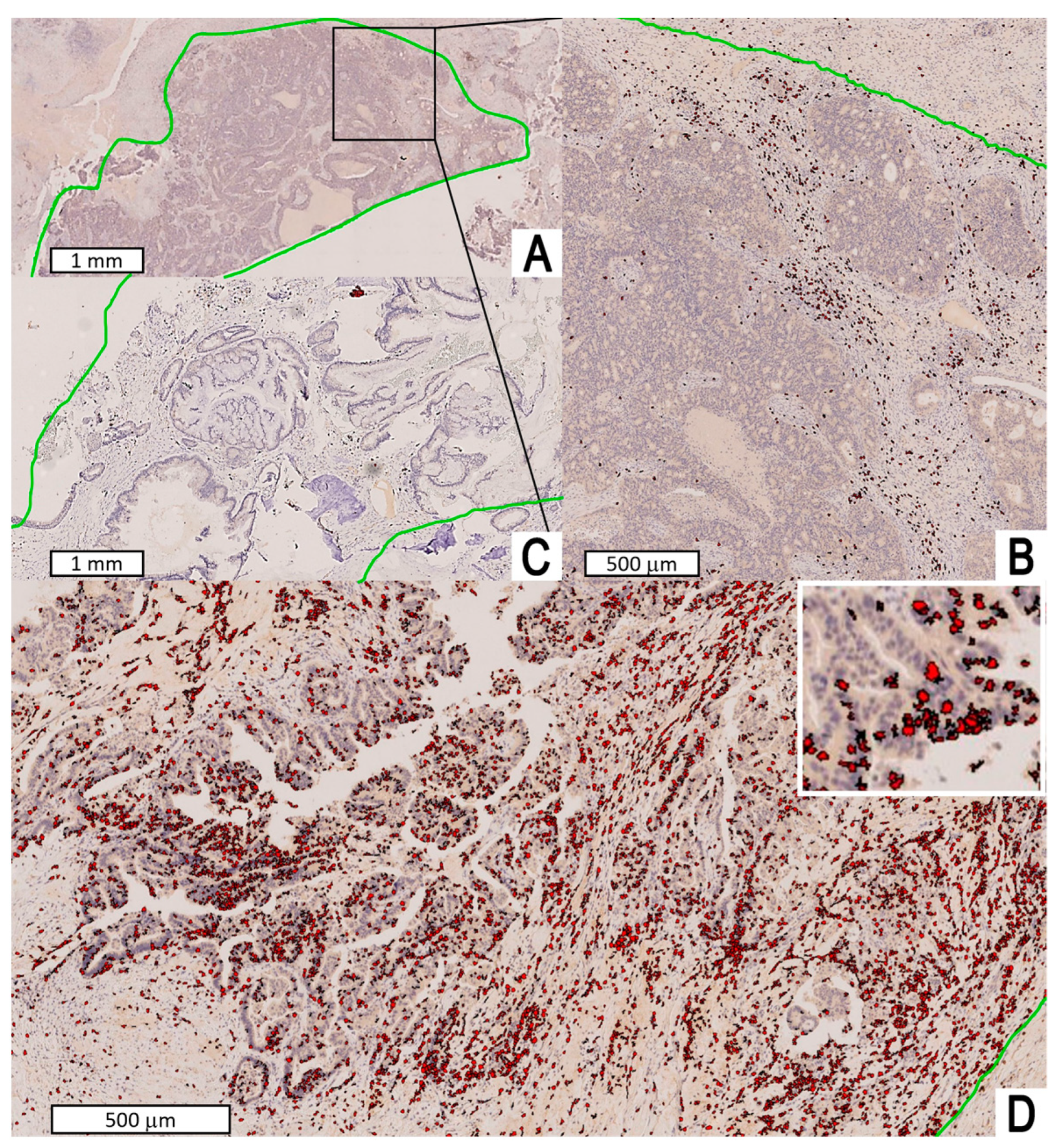
2.2. Staging, Histopathology, Immunohistochemistry, and Image Analysis
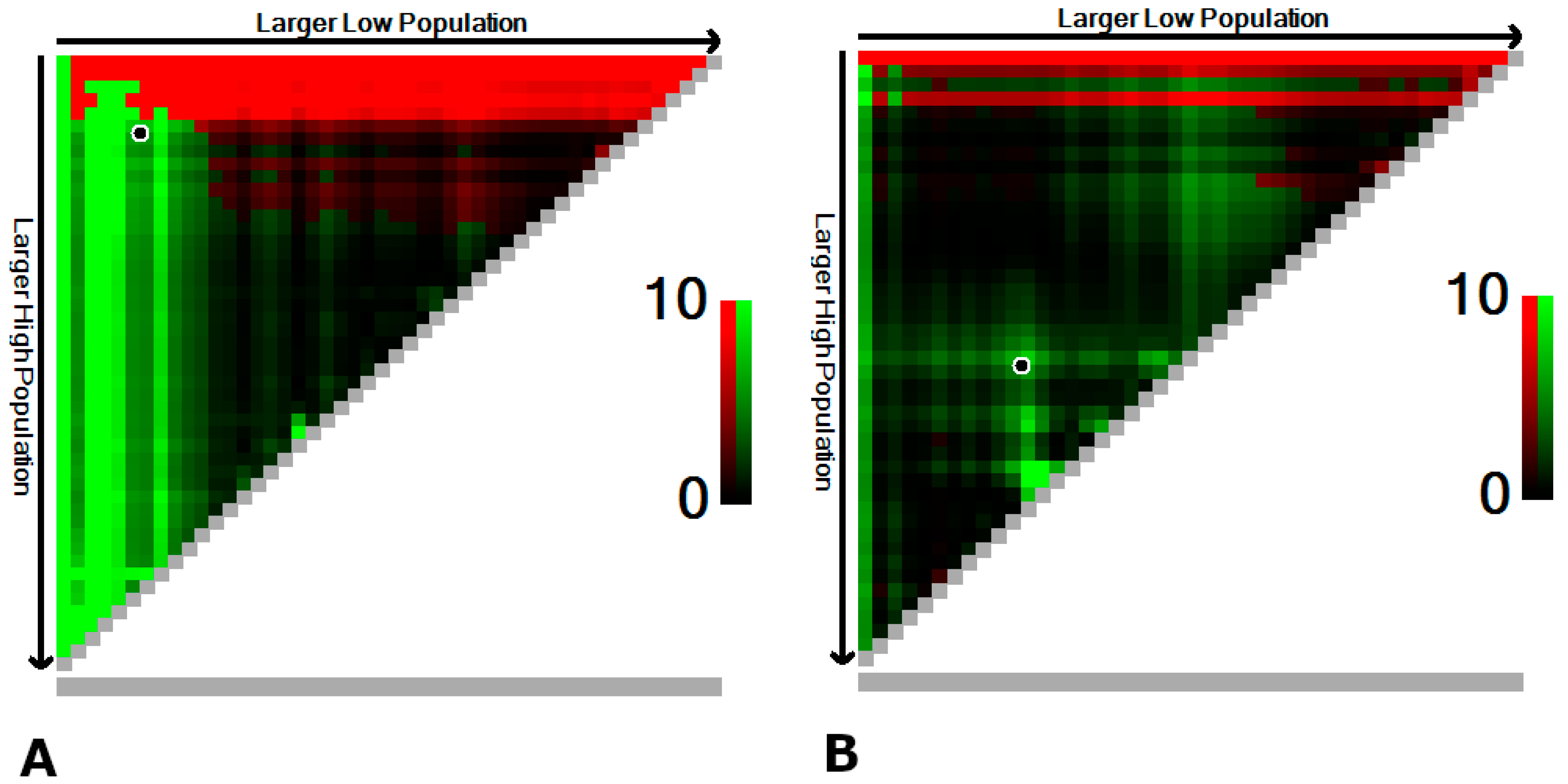
2.3. Statistical Analysis
3. Results
3.1. Cohort Description
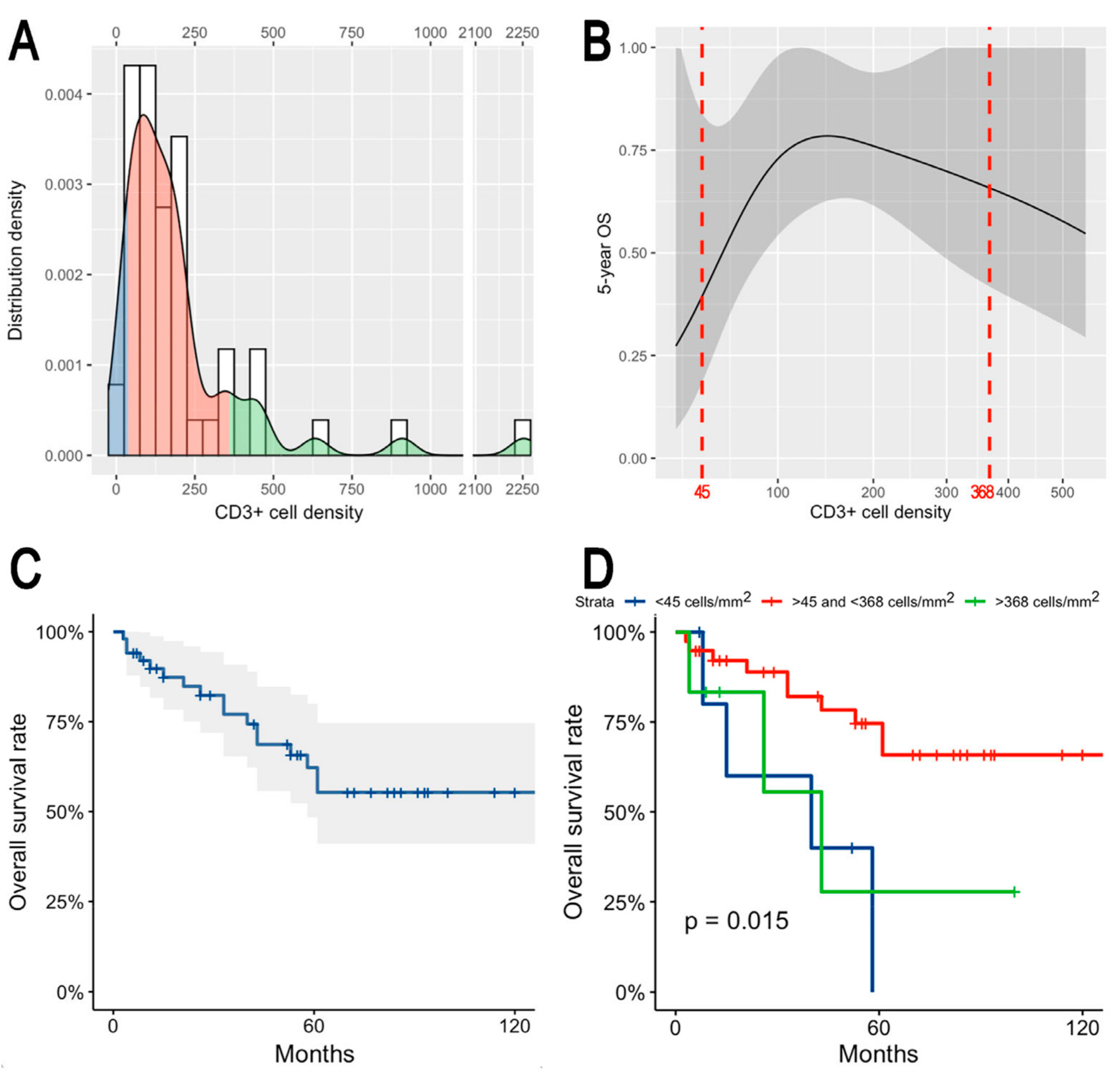
3.2. CD3+ and CD8+ Tumor-Infiltrating Lymphocytes Density
3.3. Prognostic Effect of Tumor-Infiltrating Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llorente, J.L.; López, F.; Suárez, C.; Hermsen, M.A. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat. Rev. Clin. Oncol. 2014, 11, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Bossi, P.; Mattavelli, D.; Ardighieri, L.; Nicolai, P. Management of sinonasal adenocarcinomas with anterior skull base extension. J. Neurooncol. 2020, 150, 405–417. [Google Scholar] [CrossRef]
- Rampinelli, V.; Ferrari, M.; Nicolai, P. Intestinal-type adenocarcinoma of the sinonasal tract: An update. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 115–121. [Google Scholar] [CrossRef]
- Leivo, I.; Holmila, R.; Luce, D.; Steiniche, T.; Dictor, M.; Heikkilä, P.; Husgafvel-pursiainen, K.; Wolff, H. Occurrence of Sinonasal Intestinal-Type Adenocarcinoma and Non-Intestinal-Type Adenocarcinoma in Two Countries with Different Patterns of Wood Dust Exposure. Cancers 2021, 13, 5245. [Google Scholar] [CrossRef]
- Alpuim Costa, D.; Monteiro, A.; André, T.; Esteves, S.; Sargento, I.; Ferreira, M.; Alexandre, T.; Clara, A.; Freire, J.; Moreira, A. A Potential Link between Prolonged Cork Exposure and Intestinal-Type Sinonasal Adenocarcinoma—Special Findings of a Retrospective Cohort Analysis. Front. Oncol. 2020, 10, 565036. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Orlandi, E.; Bossi, P. Sinonasal cancers treatments: State of the art. Curr. Opin. Oncol. 2021, 33, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cazzador, D.; Taboni, S.; Trimarchi, M.V.; Emanuelli, E.; Nicolai, P. When is a multidisciplinary surgical approach required in sinonasal tumours with cranial involvement? Acta Otorhinolaryngol. Ital. 2021, 41, S3–S17. [Google Scholar] [CrossRef]
- Barnes, L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am. J. Surg. Pathol. 1986, 10, 192–202. [Google Scholar] [CrossRef]
- Kleinsasser, O.; Schroeder, H.G. The pathology and clinical picture of adenocarcinoma of the nose after wood dust exposure. Strahlenther. Onkol. 1989, 165, 437–440. [Google Scholar]
- Donhuijsen, K.; Kollecker, I.; Petersen, P.; Gaßler, N.; Schulze, J.; Schroeder, H.G. Metastatic behaviour of sinonasal adenocarcinomas of the intestinal type (ITAC). Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 649–654. [Google Scholar] [CrossRef]
- Camp, S.; Van Gerven, L.; Poorten, V.V.; Nuyts, S.; Hermans, R.; Hauben, E.; Jorissen, M. Long-term follow-up of 123 patients with adenocarcinoma of the sinonasal tract treated with endoscopic resection and postoperative radiation therapy. Head Neck 2016, 38, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mattavelli, D.; Tomasoni, M.; Raffetti, E.; Bossi, P.; Schreiber, A.; Orlandi, E.; Taboni, S.; Rampinelli, V.; Gualtieri, T.; et al. The MUSES*: A prognostic study on 1360 patients with sinonasal cancer undergoing endoscopic surgery-based treatment. *MUlti-institutional collaborative Study on Endoscopically treated Sinonasal cancers. Eur. J. Cancer 2022, 171, 161–182. [Google Scholar] [CrossRef]
- Franchi, A.; Gallo, O.; Santucci, M. Clinical relevance of the histological classification of sinonasal intestinal-type adenocarcinomas. Hum. Pathol. 1999, 30, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Hadrup, S.; Donia, M.; Thor Straten, P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013, 6, 123–133. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; Alessandrini, L.; Fasanaro, E.; Gaudioso, P.; Carli, A.; Nicolai, P.; Marioni, G. Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer. Ann. Diagn. Pathol. 2021, 50, 151657. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Alessandrini, L.; Franz, L.; Ottaviano, G.; Ghi, M.G.; Lanza, C.; Blandamura, S.; Marioni, G. Prognostic role of programmed death ligand 1 (PD-L1) and the immune microenvironment in laryngeal carcinoma. Oral Oncol. 2020, 108, 104836. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- García-Marín, R.; Reda, S.; Riobello, C.; Cabal, V.N.; Suárez-Fernández, L.; Vivanco, B.; López, F.; Llorente, J.L.; Hermsen, M.A. CD8+ tumour-infiltrating lymphocytes and tumour microenvironment immune types as biomarkers for immunotherapy in sinonasal intestinal-type adenocarcinoma. Vaccines 2020, 8, 202. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar]
- Union for International Cancer Control (UICC). Tumor Node Metastasis (TNM) Classification of Malignant Tumours; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; ISBN 978-1-119-26356-2. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J.; International Agency for Research on Cancer. WHO Classification of Head and Neck Tumours; WHO: Geneva, Switzerland, 2017; ISBN 9283224388. [Google Scholar]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Ioppi, A.; Schreiber, A.; Gualtieri, T.; Mattavelli, D.; Rampinelli, V.; Taboni, S.; Tomasoni, M.; Bossi, P.; Deganello, A.; et al. Malignant tumors of the maxillary sinus: Prognostic impact of neurovascular invasion in a series of 138 patients. Oral Oncol. 2020, 106, 104672. [Google Scholar] [CrossRef]
- Shaban, M.; Raza, S.E.A.; Hassan, M.; Jamshed, A.; Mushtaq, S.; Loya, A.; Batis, N.; Brooks, J.; Nankivell, P.; Sharma, N.; et al. A digital score of tumour-associated stroma infiltrating lymphocytes predicts survival in head and neck squamous cell carcinoma. J. Pathol. 2022, 256, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Cabanero, M.; Hyrcza, M.; Butler, M.; Liu, F.F.; Hansen, A.; Huang, S.H.; Tsao, M.S.; Song, Y.; Lu, L.; et al. Computer-assisted image analysis of the tumor microenvironment on an oral tongue squamous cell carcinoma tissue microarray. Clin. Transl. Radiat. Oncol. 2019, 17, 32–39. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Kirilovsky, A.; Marliot, F.; El Sissy, C.; Haicheur, N.; Galon, J.; Pagès, F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int. Immunol. 2016, 28, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Riobello, C.; Vivanco, B.; Reda, S.; López-Hernández, A.; García-Inclán, C.; Potes-Ares, S.; Cabal, V.N.; López, F.; Llorente, J.L.; Hermsen, M.A. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck 2018, 40, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Turri-Zanoni, M.; Battaglia, P.; Lambertoni, A.; Giovannardi, M.; Schreiber, A.; Volpi, L.; Bolzoni-Villaret, A.; Lombardi, D.; Bignami, M.; Magnoli, F.; et al. Treatment strategies for primary early-stage sinonasal adenocarcinoma: A retrospective bi-institutional case-control study. J. Surg. Oncol. 2015, 112, 561–567. [Google Scholar] [CrossRef]
- Mattavelli, D.; Lombardi, D.; Missale, F.; Calza, S.; Battocchio, S.; Paderno, A.; Bozzola, A.; Bossi, P.; Vermi, W.; Piazza, C.; et al. Prognostic nomograms in oral squamous cell carcinoma: The negative impact of low neutrophil to lymphocyte ratio. Front. Oncol. 2019, 9, 339. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Galon, J.; Fridman, W.H.; Smyth, M.J. From mice to humans: Developments in cancer immunoediting. J. Clin. Investig. 2015, 125, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1354. [Google Scholar] [CrossRef]

| Variable | CD3+ Lymphocyte Density (cell/mm2) | p-Value * | CD8+ Lymphocyte Density (cell/mm2) | p-Value * |
|---|---|---|---|---|
| Age | rs = −0.127 | 0.375 | rs = −0.101 | 0.480 |
| Sex | Male: 234.3 Female: 139.7 | 0.691 |
Male: 169.1 Female: 91.7 | 0.460 |
| Exposure to wood and/or leather dust | No: 204.8 Yes: 225.4 | 0.455 | No: 184.1 Yes: 152.3 | 0.840 |
| Primary or persistent/recurrent tumor | Primary: 217.3 Persistent/recurrent: 269.6 | 0.362 | Primary: 160.7 Persistent/recurrent: 132.9 | 0.700 |
| Pathologic T stage | T1: 198.0 T2: 217.6 T3: 163.0 T4a: 188.3 T4b: 176.6 | 0.737 | T1: 199.9 T2: 229.4 T3: 183.3 T4a: 205.5 T4b: 172.5 | 0.544 |
| Nodal status | N0: 222.5 N+: 167.1 | 0.734 | N0: 159.7 N+: 101.3 | 1.000 |
| Necrosis | Not present: 253.4 Present: 202.3 | 0.110 | Not present: 224.5 Present: 119.3 | 0.514 |
| Lymphovascular invasion | V0: 193.5 V1: 396.4 | 0.274 | V0: 140.4 V1: 272.1 | 0.292 |
| Perineural invasion | Pn0: 223.9 Pn1: 94.5 | 0.497 | Pn0: 160.5 Pn1: 58.1 | 0.541 |
| Histological subtype according to Barnes | Papillary: 523.3 Colonic: 176.1 Solid: 335.5 Mucinous: 145.8 Mixed: 159.5 | 0.516 |
Papillary: 401.1 Colonic: 125.7 Solid: 197.1 Mucinous: 128.0 Mixed: 72.8 | 0.518 |
| Histological subtype according to Kleinsasser and Schroeder | Papillary-tubular cylinder cell: 248:8 Alveolar goblet cell: 145.3 Signet ring-cell:_186.8 Transitional: 86.4 | 0.465 | Papillary-tubular cylinder cell: 174.5 Alveolar goblet cell: 139.6 Signet-ring cell: 86.5 Transitional: 81.7 | 0.804 |
| Mucinous type | No: 248.8 Yes: 148.7 | 0.151 | No: 174.5 Yes: 116.2 | 0.375 |
| Covariate | CD3+ TIL Density-Including Model | CD8+ TIL Density-Including Model | ||
|---|---|---|---|---|
| HR (95%-CI) | p-Value | HR (95%-CI) | p-Value | |
| Categorized CD3+ TIL density | <45 cell/mm2: REF 45–368 cell/mm2: 0.23 (0.06–0.98) >368 cell/mm2: 0.50 (0.08–3.28) | REF 0.046 0.468 | Not included | |
| Categorized CD8+ TIL density | Not included | <55 cell/mm2: REF 55–101 cell/mm2: 0.98 (0.17–5.52) >101 cell/mm2: 0.38 (0.10–1.50) | REF 0.982 0.167 | |
| Age | 1.03 (0.96–1.09) | 0.398 | 1.05 (0.98–1.12) | 0.212 |
| ITAC subtype according to Barnes | Papillary, colonic, mixed: REF Solid, mucinous: 0.29 (0.05–1.74) | REF 0.174 | Papillary, colonic, mixed: REF Solid, mucinous: 0.65 (0.10–4.20) | REF 0.655 |
| Pathological T category | T1-3: REF T4: 3.20 (0.83–12.34) | 0.091 | T1-3: REF T4: 1.63 (0.34–7.76) | REF 0.538 |
| Margin status | Uninvolved: REF Involved: 1.58 (0.47–5.35) | 0.464 | Uninvolved: REF Involved: 2.06 (0.51–8.33) | REF 0.310 |
| Adjuvant RT | No: REF Yes: 0.17 (0.05–0.56) | REF 0.004 | No: REF Yes: 0.15 (0.04–0.51) | REF 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, M.; Alessandrini, L.; Savietto, E.; Cazzador, D.; Schiavo, G.; Taboni, S.; Carobbio, A.L.C.; Calvanese, L.; Contro, G.; Gaudioso, P.; et al. The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes. J. Pers. Med. 2023, 13, 726. https://doi.org/10.3390/jpm13050726
Ferrari M, Alessandrini L, Savietto E, Cazzador D, Schiavo G, Taboni S, Carobbio ALC, Calvanese L, Contro G, Gaudioso P, et al. The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes. Journal of Personalized Medicine. 2023; 13(5):726. https://doi.org/10.3390/jpm13050726
Chicago/Turabian StyleFerrari, Marco, Lara Alessandrini, Enrico Savietto, Diego Cazzador, Gloria Schiavo, Stefano Taboni, Andrea L. C. Carobbio, Leonardo Calvanese, Giacomo Contro, Piergiorgio Gaudioso, and et al. 2023. "The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes" Journal of Personalized Medicine 13, no. 5: 726. https://doi.org/10.3390/jpm13050726
APA StyleFerrari, M., Alessandrini, L., Savietto, E., Cazzador, D., Schiavo, G., Taboni, S., Carobbio, A. L. C., Calvanese, L., Contro, G., Gaudioso, P., Emanuelli, E., Sbaraglia, M., Zanoletti, E., Marioni, G., Dei Tos, A. P., & Nicolai, P. (2023). The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes. Journal of Personalized Medicine, 13(5), 726. https://doi.org/10.3390/jpm13050726








