Simple Summary
Interval-compressed chemotherapy was evaluated in Asian children and young adults with sarcomas to assess the feasibility of this treatment strategy. Ultimately, the goal is to improve survival in this group of individuals with high-risk malignancies without increasing short-term or long-term toxicities. The feasibility of this strategy in Asian patients with sarcomas needed to be established prior to evaluating the efficacy of this approach in a prospective trial.
Abstract
Twelve Asian patients with sarcoma received interval-compressed (ic-) chemotherapy scheduled every 14 days with a regimen of vincristine (2 mg/m2), doxorubicin (75 mg/m2), and cyclophosphamide (1200–2200 mg/m2) (VDC) alternating with a regimen of ifosfamide (9000 mg/m2) and etoposide (500 mg/m2) (IE), with filgrastim (5–10 mcg/kg/day) between cycles. Carboplatin (800 mg/m2) was added for CIC-rearranged sarcoma. The patients were treated with 129 cycles of ic-VDC/IE with a median interval of 19 days (interquartile range [IQR], 15–24 days. Median nadirs (IQR) were neutrophil count, 134 (30–396) × 106/L at day 11 (10–12), recovery by day 15 (14–17) and platelet count, 35 (23–83) × 109/L at day 11 (10–13), recovery by day 17 (14–21). Fever and bacteremia were observed in 36% and 8% of cycles, respectively. The diagnoses were Ewing sarcoma (6), rhabdomyosarcoma (3), myoepithelial carcinoma (1), malignant peripheral nerve sheath tumor (1), and CIC-DUX4 Sarcoma (1). Seven of the nine patients with measurable tumors responded (one CR and six PR). Interval-compressed chemotherapy is feasible in the treatment of Asian children and young adults with sarcomas.
1. Introduction
The outcome of children and young adults with sarcoma has improved dramatically with the use of systemic chemotherapy in the context of multimodality treatment [1]. In spite of these improvements, there is still a need for better therapy for the sarcomas. The modern chemotherapy regimens for high-risk sarcomas incorporate non-cross-resistant chemotherapy regimens that are largely derived from the development of chemotherapy in Ewing sarcoma (ES). In a randomized controlled trial, the addition of ifosfamide and etoposide (IE) to standard chemotherapy with doxorubicin, vincristine, cyclophosphamide (VDC), and dactinomycin significantly improved the survival of patients with localized ES [2].
Based on these results of alternating chemotherapy, a strategy of increasing the dose intensity has been evaluated in ES in two ways. Increasing the dose of the agents did not improve the outcome [3]; however, giving interval-compressed (ic-) chemotherapy with VDC/IE scheduled every 14 days with the use of granulocyte colony stimulating factor (G-CSF) was found to improve the event-free survival of localized ES [4] compared to VDC/IE scheduled every 21 days. The concept of ic-VDC/IE has also been successfully incorporated in the treatment of intermediate-risk and high-risk rhabdomyosarcoma [5,6]. The other prospective treatments for sarcomas in children and young adults that need to be evaluated in the future include immunotherapy [7] and targeted therapy [8].
Currently, there is a lack of data for the use of ic-chemotherapy in Asian children and young adults. This study demonstrates the feasibility and toxicity of interval-compressed chemotherapy in treating sarcomas in children and young adults in Asia.
2. Materials and Methods
2.1. Patient Eligibility
From December 2016 to December 2020, Asian patients younger than 30 years of age with newly diagnosed sarcomas treated with ic-VDC/IE at two university healthcare systems in Taiwan were included in this retrospective analysis of the feasibility, toxicity, and early efficacy of this regimen.
2.2. Diagnosis and Staging
The diagnosis of sarcoma was established morphologically and with the use of immunohistochemistry and the detection of gene rearrangements to determine the specific diagnosis when indicted. The molecular diagnosis of fusion genes was performed by fluorescent in situ hybridization (FISH) or ribonucleic acid sequencing (RNA-Seq) (see below).
Staging work-up included computerized tomography (CT) or magnetic resonance imaging (MRI) of the primary site, CT of the lungs, and whole-body bone scan. The TNM Staging System (American Joint Committee on Cancer, 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk) was used [9].
2.3. Fluorescence In Situ Hybridization (FISH)
To detect fusion genes and confirm their fusion candidates, we performed FISH for EWSR1, FLI1, FOXO1, CIC, and DUX4 break-apart as modified from previously described methods [10]. Commercial probes for EWSR1 and FLI1 were purchased from ZytoVision (Bremerhaven, Germany). Custom probes were made by bacterial artificial chromosomes (BAC) clones flanking the genes of interest according to the UCSC genome browser (http://genome.ucsc.edu, accessed on 20 July 2019) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA, USA; http://bacpac.chori.org, accessed on 20 July 209). DNA from each BAC was isolated according to the manufacturer’s instructions. The BAC clones were labeled with fluorochromes (fluorescent-labeled dUTPs, Enzo Life Sciences, Farmingdale, NY, USA) by nick translation and validated on normal metaphase chromosomes. The 4 μm-thick FFPE slides were deparaffinized, pretreated, and hybridized with denatured probes. After overnight incubation, the slides were washed, stained with 4′,6-diamidino-2-phenylindole (DAPI), mounted with an antifade solution, and then examined on an Olympus BX63 automated fluorescence microscope (Evident corporation, Tokyo, Japan) controlled by GenASIs software, Version 8.0.1 (Applied Spectral Imaging, Carlsband, CA, USA).
2.4. RNA Sequencing
RNA sequencing was performed with TruSeq RNA Exome (Illumina, San Diego, CA, USA) that converted total RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissues into template molecules of known strand origin, followed by sequence-specific capture of coding RNA. Paired-end RNA-seq at read lengths of 150 base pairs were performed with the HiSeq 2000 (Illumina). After being independently aligned by STAR (version 2.3) against the human reference genome (hg19), the reads were analyzed by STAR-Fusion algorithm for fusion discovery.
2.5. Treatment
Patients were treated with interval-compressed chemotherapy regimens with vincristine (2 mg/m2/dose; maximum, 2 mg/dose), doxorubicin (75 mg/m2/cycle), and cyclophosphamide (1200–2200 mg/m2/cycle) (VDC) alternating with Ifosfamide (9000 mg/m2/cycle) and Etoposide (500 mg/m2/cycle) (IE). Carboplatin (800 mg/m2/cycle) was added with IE for the CIC-rearranged sarcoma [11]. The interval-compressed chemotherapy cycles (ic-VDC/IE) were scheduled every 14 days; therapy was delivered when the neutrophil count had recovered to ≥750 × 106/L, and the platelet count had recovered to ≥75 × 109/L without transfusions.
To support the patients through neutrophil nadirs and to compress treatment intervals, granulocyte colony-stimulating factor (G-CSF; filgrastim 5–10 mcg/kg/day) was given subcutaneously daily, starting from 24 to 36 h after the last dose of chemotherapy to the recovery of neutrophil count to ≥750 × 106/L. Neutrophil and platelet counts were evaluated 3 times a week following each cycle of chemotherapy until recovery.
Treatment of the primary site with surgery was performed before chemotherapy in patients who required urgent decompression or if upfront resection was considered feasible. Otherwise, treatment of the primary site with surgery, radiation, or both was planned to begin at week 10–15 after initiation of systemic chemotherapy for patients with nonmetastatic disease or after maximal systemic control for patients with metastatic disease.
2.6. Evaluation of Treatment Response and Adverse Events
Treatment response was assessed by two-dimensional (2D) measurements according to the World Health Organization [12], which defined complete response (CR) as 100% reduction of tumor size, partial response (PR) as 50–99% reduction of tumor size, stable disease (SD) as 0–49% reduction or 0–24% enlargement of tumor size, and progressive disease (PD) as ≥25% enlargement of tumor size.
The adverse events were evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Acute adverse events of a cycle were defined as complications that occurred during the administration of chemotherapy or before the next chemotherapy cycle began. Significant adverse events included infections, shock, mucositis, cytopenias, impaired liver function, and abnormal renal function.
2.7. Statistical Analysis
Clinical characteristics of patients, including age at diagnosis, gender, tumor size, stage, treatments, and chemotherapy regimen were collected. Toxicities were collected for each chemotherapy cycle. The interval between chemotherapy cycles was defined by the number of days between the first day of two consecutive cycles; for the last cycle, recovery was defined by the number of days until neutrophil and platelet counts returned to ≥750 × 106/L and ≥75 × 109/L without transfusions, respectively. Continuous variables were presented by median and interquartile ranges (IQRs) and compared by the Kruskal-Wallis test. Progression-free survival (PFS) and overall survival (OS) were calculated by the Kaplan-Meier method and compared by the log-rank test.
3. Results
3.1. Patient Characteristics
From December 2016 to December 2020, twelve patients from Taiwan, Japan, or China with newly diagnosed sarcoma were treated at our institutions with ic-VDC/IE and were eligible for analysis. There were 6 males and 6 females with a median age of 14 years (range, 3–29 years) at diagnosis. Their diagnoses included six ES, three alveolar rhabdomyosarcoma, one myoepithelial carcinoma, one malignant peripheral nerve sheath tumor (MPNST), and one CIC-DUX4 sarcoma. The primary tumors originated in the soft tissue (n = 9) or bone (n = 3). The most common primary sites were trunk (n = 10), extremities (n = 1), and retroperitoneum (n = 1). Six of the twelve patients had metastatic diseases; the most common metastatic site was bone (n = 4). The clinical characteristics of the patients are shown in Table 1.

Table 1.
Clinical characteristics, histopathology, and molecular features of Asian patients with sarcomas.
3.2. Intensification of Chemotherapy Intervals
The ic-VDC/IE treatment cycles were planned at 14-day intervals. A total of 129 cycles of chemotherapy were given at a median interval of 19 days (interquartile range [IQR], 15–24 days). The intervals between neoadjuvant chemotherapy cycles (n = 64; median [IQR] = 16 [14–19] days) were significantly shorter than the intervals between concurrent chemoradiotherapy cycles (n = 15; median [IQR] = 21 [18–26] days) and the intervals after local control (n = 50, median [IQR] = 23 [20–32] days) (p = 0.0001).
3.3. Toxicity and Recovery
The toxicities during chemotherapy are shown in Table 2. Grade 3 and 4 hematological toxicities were common: neutropenia (92%), thrombocytopenia (62%), and anemia (33%). The most common grade 3 and 4 non-hematological toxicities were fever (36%), urinary tract infection (8%), bacteremia (6%), and mucositis (3%). No patient developed septic shock or infection-associated death during ic-VDC/IE.

Table 2.
Grade 3 and 4 toxicities observed during 129 cycles of ic-VDC/IE chemotherapy (number of cycles with the toxicities and their percentage of total cycles are presented).
Patients had their neutrophil count decreased to a median nadir (IQR) of 134 (30–396) × 106/L at median (IQR) day 11 (10–12) that had recovered by day 15 (14–17) with the use of G-CSF (Figure 1a). After the cessation of G-CSF use, the neutrophil count dropped back to the near-normal range. Patients had their platelet count decreased to a median nadir (IQR) of 35 (23–83) × 109/L at median (IQR) day 11 (10–13) that recovered by day 17 (14–21) (Figure 1b). The recovery of the platelet count was slower than the neutrophil count; no patients in this cohort used thrombopoietin.
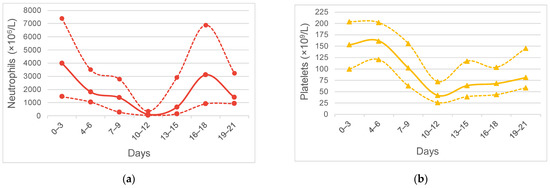
Figure 1.
Hematological toxicity of 129 cycles of ic-VDC/IE in young Asian patients: (a) Neutrophil count of cycles; (b) Platelet count of cycles. Solid lines are median counts, and dashed lines are 25th% and 75th% counts, respectively.
3.4. Treatment Response and Outcomes
Initial response to neoadjuvant chemotherapy alone was evaluable in the nine patients with measurable disease; responses included one CR, six PR, one SD, and one PD (overall response rate = 78%). Among the six patients with PR after ic-VDC/IE neoadjuvant chemotherapy, four achieved CR after local control with surgery and radiotherapy (n = 2) or radiotherapy alone (n = 2). The non-responders were the patient with CIC-rearranged sarcoma stage IV (No.9; SD) and the patient with MPNST (No.5; PD). The three other patients had primary tumor resection prior to chemotherapy.
All four patients with Ewing sarcoma without metastases at diagnosis achieved long-term systemic control and remain in CR. One patient (No.4) developed second malignancy with acute lymphoblastic leukemia at 17 months after an initial diagnosis of localized ES and remains in CR of both diseases. The other 2 patients with non-metastatic disease at diagnosis, MPNST (No.5) and ARMS (No.6), had disease progression at 11 and 31 months after diagnosis, respectively.
Five of six patients with metastatic disease had initial response to ic-VDC/IE, including 1 CR and 4 PR; however, all six patients with metastatic sarcoma progressed at 9–37 months after initial diagnosis, regardless of best response to treatment.
At a median follow-up of 43 months, all patients with localized sarcoma who received ic-VDC/IE remained in remission. The 2-year PFS in patients with localized disease at diagnosis was 83%; the 2-year PFS in patients with metastases at diagnosis was 33% (p = 0.05). The 2-year OS in patients with localized disease at diagnosis was 83%; the 2-year OS in patients with metastatic disease at diagnosis was 67% (p = 0.28).
The treatment and outcomes of patients were summarized in Table 3.

Table 3.
Treatment and outcomes of Asian patients with sarcomas.
4. Discussion
Sarcomas are a heterogenous group of malignant tumors arising in bone, soft tissue, and the peripheral nervous system. Most sarcomas in children and young adults are high-grade. Molecular diagnostics are now utilized in combination with histopathology and immunohistochemistry to characterize sarcomas. The most common round cell sarcomas in this age group, characterized by relatively small, round-to-oval, undifferentiated cells, include Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) and rhabdomyosarcoma [13]. In children and young adults, sarcomas have a slight male predilection, with overlapping but different epidemiological features and clinical presentations across histological and molecular types [14].
Race/ethnicity has an impact on the incidence and treatment outcomes of sarcomas in children and young adults [15,16]. In ES, the incidence in Caucasians is 2-fold higher than that in Asian/Pacific Islanders and 9-fold higher than that in African Americans [16]. Extraskeletal ES accounts for approximately 20% of all ES with a better prognosis in most studies [17,18]. Intriguingly, non-Caucasian patients had a higher incidence of Extraskeletal ES compared to Caucasians (36 vs. 19%) and an inferior survival [18,19]. In ARMS, the incidence rate in Caucasians is 4-fold higher than that in African Americans and 8-fold higher than that in other races [20]. These observations suggest the importance of careful study of the incidence and longitudinal follow-up of patients of different races and ethnic groups with sarcomas.
The standard chemotherapy regimens for treating sarcoma have evolved over the last five decades. The first multiagent chemotherapy regimen for ES was a combination of vincristine, dactinomycin, and cyclophosphamide (VAC) [21]. This was based on phase two trials of each drug that showed activity in the disease [22,23]. This combination improved the outcome for Ewing Sarcoma at the time but only modestly. When doxorubicin was approved for patients, phase two data showed activity in Ewing Sarcoma [24]. This agent was then added to the standard regimen at the time, VAC, with improvement in outcome for patients with both nonmetastatic [25] and metastatic disease [26]. In the 1980s, ifosfamide was shown to have activity in a phase two trial in recurrent patients [27]. The combination of etoposide with ifosfamide in the treatment of Ewing sarcoma showed significantly increased activity suggesting that this combination might be non-cross-resistant with the standard regimen of VAC plus doxorubicin [28]. This led to an intergroup study in the United States that demonstrated the benefit of adding this new regimen to the standard regimen [2].
Similar to the experience with Ewing Sarcoma, the standard regimen of VAC was developed based on phase two data for each agent alone and was shown to have benefit in newly diagnosed rhabdomyosarcoma [29]. Subsequent studies demonstrated that doxorubicin had activity in rhabdomyosarcoma and significantly benefitted patients with alveolar rhabdomyosarcoma [30]. The new combination of ifosfamide plus etoposide combined with vincristine was evaluated in patients with stage III rhabdomyosarcoma and was demonstrated to have activity that was equivalent to the standard regimen of VAC [31,32]. The use of ic-VDC/IE in rhabdomyosarcoma has been evaluated in initial studies and requires further investigations in prospective, randomized trials [5,6]. The treatment of Ewing-like sarcomas is less well established because of their rarity; however, the current treatment regimens for these entities are based on the treatment of Ewing Sarcoma.
The use of a non-cross-resistant regimen in our patients is based on the Ewing sarcoma trial in the United States which added the combination of ifosfamide and etoposide to the standard regimen of VAC plus doxorubicin with 5-year overall survival (5y-OS) of 72% [2]. The addition of IE to VDC in a non-cross-resistant chemotherapy regimen also improved survival in non-metastatic ES in Japan (5-year overall survival, 80.1%) [17] and in Taiwan (5-year overall survival, 61.6%) [33].
The concept of dose intensity related to outcome was initially evaluated in breast cancer [34,35]. It was also evaluated in sarcomas demonstrating the importance of dose intensity in this population [36]. In these studies, increasing dose intensity was related to an improved outcome.
Following the development of G-CSF, interval compression of chemotherapy was developed to intensify the delivered dosing [37]. The feasibility of ic-VDC/IE was first demonstrated in a pilot study in the United States of children with ES/PNET, rhabdomyosarcoma, and other advanced soft tissue sarcomas. The median interval of chemotherapy cycles was 16 days, representing a 1.27-fold increase of intensity comparing with the traditional schedule of 21-day intervals [37].
Further trials in ES and rhabdomyosarcoma have confirmed the usefulness of ic-VDC/IE by increasing the dose intensity without increasing the total dose [4,5]. A large cooperative group trial evaluating interval compression of this chemotherapy regimen demonstrated a significant improvement in the outcome for patients with nonmetastatic ES. The efficacy of ic-VDC/IE has been validated in adult ES studies [38,39] and is being evaluated in a large European trial with promising early data [40,41]. By contrast, the use of dose escalation of VDC/IE has not improved the event-free survival and overall survival of localized ES [3,42]. Furthermore, a cohort study of 81 adult Chinese patients with ES receiving every-3-week VDC/IE found that chemotherapy delay of more than 3 days was associated with worse survival by multivariate Cox regression analysis, highlighting the importance of interval intensity [43].
Toxicities associated with ic-VDC/IE chemotherapy include febrile neutropenia, non-neutropenia related infection, anemia, thrombocytopenia, and mucositis. In our study, we did not observe grade 3 and 4 renal toxicity or encephalopathy. According to the report of Weigel et al., the most common toxicity was infection with and without neutropenia in 50 and 60% of patients during ic-VDC/IE, rates similar to those in our cohort [5]. As bone marrow toxicity and infections were the major toxicities observed in ic-VDC/IE, dose reductions instead of dose delays should be considered when these side effects occur [44].
The major clinical benefits of interval-compressed chemotherapy are: (1) to enable rapid shrinkage of the primary tumor that can enhance the success rate of surgery at the primary site after maximal response has been achieved; and (2) to prevent the development of metastatic disease. Further interval-intensive systemic control can be continued during the period of local therapy [45]. Thus, the main pro of interval-compressed chemotherapy is the improvement of survival in nonmetastatic sarcoma as confirmed in the two large trials in ES in the U.S. and Europe [4,41]. In addition, the ability to complete chemotherapy in a shorter period of time is appealing to many patients. Further, to give more cycles of interval-compressed chemotherapy prior to surgery may enhance local control. The major con of interval-compressed chemotherapy is the loss of rest time between chemotherapy cycles; however, the toxicity for the courses was not more than conventional chemotherapy [39].
Metastatic disease [20,46] is the major challenge in treating children and young adults with sarcomas despite the good results of ic-VDC/IE in localized sarcomas. Patients with metastatic sarcomas often have a good initial response to chemotherapy; however, this is usually followed by progression within 2 years. In metastatic ES/PNET, the addition of IE [47], dose escalation of VDC/IE [48], or the use of ic-VDC/IE [4] have not been associated with improved EFS or OS. In high-risk rhabdomyosarcomas, ic-VDC/IE was associated with better EFS only in embryonal rhabdomyosarcoma but not in ARMS [5]. In our study, three of the three patients with ARMS had disease progression in contrast to two progressions out of the six patients with ES. More aggressive treatment is required to improve the treatment outcome of metastatic sarcomas. To further intensify the systemic therapy in sarcomas, European studies utilized HDC/ASCR with busulfan/melphalan that improved the EFS and OS in localized ES [49] and achieved a similar outcome of whole-lung irradiation in ES with pulmonary metastases [50]. In a single institutional study in the U.S., adding topotecan to busulfan/melphalan as the HDC/ASCR regimen achieved a 10-year OS of 78% in patients at 1st CR and of 66% in patients at 2nd CR or 1st PR of metastatic or relapsed ES [51]. In rhabdomyosarcoma, the use of thiotepa and melphalan in Japan has resulted in a 3-year PFS > 50% for ARMS [20,52].
The survival of patients with CIC-rearranged sarcoma is significantly worse than patients with conventional ES [53]. The 5-year OS of less than 50% in both localized and metastatic disease demonstrates the need for better therapy for this histology. VDC/IE can induce durable complete responses, however [54,55].
New approaches to treat metastatic sarcomas include targeted agents and immunotherapy. A novel targeted agent, ganitumab, a monoclonal antibody against IGF-1R, is being tested in a phase III clinical trial (NCT02306161). Further, tyrosine kinase inhibitors and anti-angiogenic agents are being evaluated in clinical trials [8]. Novel immunotherapy with HER2 chimeric antigen receptor T cells has been tested in an early-phase clinical trial. The efficacy of immune checkpoint inhibition has been limited in ES and other sarcomas [56,57,58]. Further, most sarcomas are immune “cold” tumors; only alveolar soft part sarcoma and undifferentiated pleomorphic sarcoma have responded to immune checkpoint inhibitors [8]. Recently, down-regulation or deletion of major histocompatibility complex class I antigens was found to be a common phenomenon in sarcomas that restricts tumor-specific CD8+ T cell response [7]. This may explain the failure of immune checkpoint inhibition in the treatment of many sarcomas.
Our study has several limitations. First, it is a retrospective analysis of a small number of cases. Given the rarity of sarcomas, however, our data demonstrates the feasibility of ic-VDC/IE in Asian patients. The clinical benefit of interval-compressed chemotherapy should be thoroughly studied in histology-driven treatment protocols and validated in future prospective studies. Second, the median interval between cycles in our study was 19 days, which was longer than the 15–16 days reported in Western literature, which might, in part, be the result of delayed platelet recovery. Whether the use of thrombopoietin receptor agonists can further compress the interval between chemotherapy cycles by maintaining platelet counts may be worth exploring [59]. Third, we did not observe long-term disease control in all patients with metastatic sarcomas although four of them achieved transient CR or PR. This observation highlights the need for more improved therapy, such as high-dose chemotherapy with autologous stem cell rescue, for patients with metastatic sarcomas.
5. Conclusions
In this study, we demonstrate that interval-compressed chemotherapy is feasible in treating Asian children and young adults with sarcoma with tolerable toxicity. Neoadjuvant ic-VDC/IE may enhance local control with rapid shrinkage of primary tumors and addresses the systemic disease early in the treatment. New approaches to the treatment of metastatic disease are needed and may include more aggressive regimens, targeted therapy, immunotherapy, or high-dose consolidation chemotherapy. Our experience needs to be validated in prospective, histology-driven studies, especially in rhabdomyosarcoma, in a larger population of Asian patients by incorporating ic-VDC/IE into multidisciplinary approaches that integrate surgery, radiotherapy, and chemotherapy.
Author Contributions
Conceptualization, Y.-L.L. and J.S.M.; methodology, Y.-C.K., T.-H.H. and Y.-R.L.; validation, Y.-C.K. and J.S.M.; formal analysis, J.-H.H., S.-H.C., Y.-M.L. and Y.-L.L.; investigation, Y.-M.L., Y.-C.K., W.-L.H., H.C., M.-L.T., H.-L.L., C.-C.K., S.-H.T., C.-Y.C., K.L.-C.H., L.-S.L., Y.-J.C., J.-F.C., W.H., W.-T.L., Y.-C.W., W.-C.W., J.-L.W., J.-J.T., K.T., C.K. and T.-T.W.; writing—original draft preparation, J.-H.H., S.-H.C., J.S.M. and Y.-L.L.; writing—review and editing, Y.-M.L., Y.-C.K., W.-L.H., H.C., M.-L.T., H.-L.L., C.-C.K., S.-H.T., C.-Y.C., K.L.-C.H., L.-S.L., Y.-J.C., J.-F.C., T.-H.H., Y.-R.L., W.H., W.-T.L., Y.-C.W., W.-C.W., J.-L.W., J.-J.T., K.T., C.K. and T.-T.W.; visualization, J.-H.H. and Y.-L.L.; supervision, T.-T.W. and J.S.M.; funding acquisition, Y.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the National Science and Technology Council, Taiwan, Grant Number NSC-109-2314-B-038-072.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB) No.N202002077.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, except for patients whose consent was waived by the TMU-JIRB if they had died or transferred to another institution at study enrollment.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy considerations.
Acknowledgments
This work was supported in part by the “TMU Research Center of Cancer Translational Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The authors thank Yu-Han Chung at the Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan for her assistance with study coordination and data management.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| ARMS | alveolar rhabdomyosarcoma; |
| G-CSF | Granulocyte colony-stimulating factor; |
| ic- | Interval compressed; |
| IE | Ifosfamide, etoposide; |
| IQR | interquartile range; |
| MEC | myoepithelial carcinoma; |
| MPNST | malignant peripheral nerve sheath tumor; |
| PNET | primitive neuroectodermal tumor; |
| UTI | urinary tract infection; |
| VDC | Vincristine, doxorubicin, cyclophosphamide |
References
- Lessnick, S.L.; Dei Tos, A.P.; Sorensen, P.H.; Dileo, P.; Baker, L.H.; Ferrari, S.; Hall, K.S. Small round cell sarcomas. Semin. Oncol. 2009, 36, 338–346. [Google Scholar] [CrossRef]
- Grier, H.E.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N. Engl. J. Med. 2003, 348, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Granowetter, L.; Womer, R.; Devidas, M.; Krailo, M.; Wang, C.; Bernstein, M.; Marina, N.; Leavey, P.; Gebhardt, M.; Healey, J.; et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children’s Oncology Group Study. J. Clin. Oncol. 2009, 27, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Weigel, B.J.; Lyden, E.; Anderson, J.R.; Meyer, W.H.; Parham, D.M.; Rodeberg, D.A.; Michalski, J.M.; Hawkins, D.S.; Arndt, C.A. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2016, 34, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.A.; Hawkins, D.S.; Meyer, W.H.; Sencer, S.F.; Neglia, J.P.; Anderson, J.R. Comparison of results of a pilot study of alternating vincristine/doxorubicin/cyclophosphamide and etoposide/ifosfamide with IRS-IV in intermediate risk rhabdomyosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 50, 33–36. [Google Scholar] [CrossRef]
- Mosca, L.; de Angelis, A.; Ronchi, A.; De Chiara, A.; Fazioli, F.; Ruosi, C.; Altucci, L.; Conte, M.; de Nigris, F. Sarcoma Common MHC-I Haplotype Restricts Tumor-Specific CD8+ T Cell Response. Cancers 2022, 14, 3414. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tie, Y.; Wei, Y.Q.; Tu, C.Q.; Wei, X.W. Targeted and immuno-based therapies in sarcoma: Mechanisms and advances in clinical trials. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188606. [Google Scholar] [CrossRef]
- Cates, J.M.M. The AJCC 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database. J. Natl. Compr. Cancer Netw. 2018, 16, 144–152. [Google Scholar] [CrossRef]
- Kao, Y.C.; Flucke, U.; Eijkelenboom, A.; Zhang, L.; Sung, Y.S.; Suurmeijer, A.J.H.; Antonescu, C.R. Novel EWSR1-SMAD3 Gene Fusions in a Group of Acral Fibroblastic Spindle Cell Neoplasms. Am. J. Surg. Pathol. 2018, 42, 522–528. [Google Scholar] [CrossRef]
- Cairo, M.S.; Shen, V.; Krailo, M.D.; Bauer, M.; Miser, J.S.; Sato, J.K.; Blatt, J.; Blazar, B.R.; Frierdich, S.; Liu-Mares, W.; et al. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: A children’s cancer group report. J. Pediatr. Hematol. Oncol. 2001, 23, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Aghighi, M.; Boe, J.; Rosenberg, J.; Von Eyben, R.; Gawande, R.S.; Petit, P.; Sethi, T.K.; Sharib, J.; Marina, N.M.; DuBois, S.G.; et al. Three-dimensional Radiologic Assessment of Chemotherapy Response in Ewing Sarcoma Can Be Used to Predict Clinical Outcome. Radiology 2016, 280, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Domanski, H.A. The Small Round Cell Sarcomas Complexities and Desmoplastic Presentation. Acta Cytol. 2022, 66, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.M.; Gronchi, A. WHO Pathology: Highlights of the 2020 Sarcoma Update. Surg. Oncol. Clin. N. Am. 2022, 31, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.Y.; Scharschmidt, T.J.; Mayerson, J.L.; Fisher, J.L. Incidence and survival in sarcoma in the United States: A focus on musculoskeletal lesions. Anticancer Res. 2013, 33, 2597–2604. [Google Scholar]
- Jawad, M.U.; Cheung, M.C.; Min, E.S.; Schneiderbauer, M.M.; Koniaris, L.G.; Scully, S.P. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: An analysis of 1631 cases from the SEER database, 1973–2005. Cancer 2009, 115, 3526–3536. [Google Scholar] [CrossRef]
- Takenaka, S.; Naka, N.; Obata, H.; Joyama, S.; Hamada, K.; Imura, Y.; Kakunaga, S.; Aoki, Y.; Ueda, T.; Araki, N.; et al. Treatment outcomes of Japanese patients with Ewing sarcoma: Differences between skeletal and extraskeletal Ewing sarcoma. Jpn. J. Clin. Oncol. 2016, 46, 522–528. [Google Scholar] [CrossRef]
- Cash, T.; McIlvaine, E.; Krailo, M.D.; Lessnick, S.L.; Lawlor, E.R.; Laack, N.; Sorger, J.; Marina, N.; Grier, H.E.; Granowetter, L.; et al. Comparison of clinical features and outcomes in patients with extraskeletal versus skeletal localized Ewing sarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2016, 63, 1771–1779. [Google Scholar] [CrossRef]
- Worch, J.; Matthay, K.K.; Neuhaus, J.; Goldsby, R.; DuBois, S.G. Ethnic and racial differences in patients with Ewing sarcoma. Cancer 2010, 116, 983–988. [Google Scholar] [CrossRef]
- Amer, K.M.; Thomson, J.E.; Congiusta, D.; Dobitsch, A.; Chaudhry, A.; Li, M.; Chaudhry, A.; Bozzo, A.; Siracuse, B.; Aytekin, M.N.; et al. Epidemiology, Incidence, and Survival of Rhabdomyosarcoma Subtypes: SEER and ICES Database Analysis. J. Orthop. Res. 2019, 37, 2226–2230. [Google Scholar] [CrossRef]
- Jaffe, N.; Paed, D.; Traggis, D.; Salian, S.; Cassady, J.R. Improved outlook for Ewing’s sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy. Cancer 1976, 38, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Hayes, F.A.; Thompson, E.I.; Hustu, H.O.; Kumar, M.; Coburn, T.; Webber, B. The response of Ewing’s sarcoma to sequential cyclophosphamide and adriamycin induction therapy. J. Clin. Oncol. 1983, 1, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, T.C.; Johnson, R.E. Combined modality therapy of Ewing’s sarcoma. Cancer 1975, 35, 36–47. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, R.M.; Luce, J.K.; Talley, R.W.; Gottlieb, J.A.; Baker, L.H.; Bonadonna, G. Phase II evaluation of adriamycin in human neoplasia. Cancer 1973, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.E., Jr.; Perez, C.A.; Tefft, M.; Burgert, E.O., Jr.; Vietti, T.J.; Kissane, J.; Pritchard, D.J.; Gehan, E.A. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: An Intergroup Study. Natl. Cancer Inst. Monogr. 1981, 255–262. [Google Scholar]
- Vietti, T.J.; Gehan, E.A.; Nesbit, M.E., Jr.; Burgert, E.O., Jr.; Pilepich, M.; Tefft, M.; Kissane, J.; Pritchard, D.J. Multimodal therapy in metastatic Ewing’s sarcoma: An Intergroup Study. Natl. Cancer Inst. Monogr. 1981, 279–284. [Google Scholar]
- Magrath, I.; Sandlund, J.; Raynor, A.; Rosenberg, S.; Arasi, V.; Miser, J. A phase II study of ifosfamide in the treatment of recurrent sarcomas in young people. Cancer Chemother. Pharmacol. 1986, 18 (Suppl. S2), S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Miser, J.S.; Kinsella, T.J.; Triche, T.J.; Tsokos, M.; Jarosinski, P.; Forquer, R.; Wesley, R.; Magrath, I. Ifosfamide with mesna uroprotection and etoposide: An effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J. Clin. Oncol. 1987, 5, 1191–1198. [Google Scholar] [CrossRef]
- Ruymann, F.B. The development of VAC chemotherapy in rhabdomyosarcoma: What does one do for an encore? Curr. Oncol. Rep. 2003, 5, 505–509. [Google Scholar] [CrossRef]
- Bergeron, C.; Thiesse, P.; Rey, A.; Orbach, D.; Boutard, P.; Thomas, C.; Schmitt, C.; Scopinaro, M.J.; Bernard, F.; Stevens, M.; et al. Revisiting the role of doxorubicin in the treatment of rhabdomyosarcoma: An up-front window study in newly diagnosed children with high-risk metastatic disease. Eur. J. Cancer 2008, 44, 427–431. [Google Scholar] [CrossRef]
- Crist, W.M.; Anderson, J.R.; Meza, J.L.; Fryer, C.; Raney, R.B.; Ruymann, F.B.; Breneman, J.; Qualman, S.J.; Wiener, E.; Wharam, M.; et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J. Clin. Oncol. 2001, 19, 3091–3102. [Google Scholar] [CrossRef]
- Arndt, C.A.; Nascimento, A.G.; Schroeder, G.; Schomberg, P.J.; Neglia, J.P.; Sencer, S.F.; Silberman, T.L.; Moertel, C.L.; Tillisch, J.K.; Miser, J.S. Treatment of intermediate risk rhabdomyosarcoma and undifferentiated sarcoma with alternating cycles of vincristine/doxorubicin/cyclophosphamide and etoposide/ifosfamide. Eur. J. Cancer 1998, 34, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Yen, C.C.; Yen, H.J.; Shiau, C.Y.; Chao, T.C.; Wu, P.K.; Chen, C.F.; Chen, P.C.; Wu, H.H.; Chiou, H.J.; et al. Outcomes of 50 Patients With Ewing Sarcoma Family of Tumors Treated at a Single Institution in Taiwan. Medicine 2016, 95, e3830. [Google Scholar] [CrossRef]
- Hryniuk, W.; Bush, H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J. Clin. Oncol. 1984, 2, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Bronchud, M.H.; Howell, A.; Crowther, D.; Hopwood, P.; Souza, L.; Dexter, T.M. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br. J. Cancer 1989, 60, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Ungerleider, R.S.; Horowitz, M.E.; Simon, R. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing’s sarcoma. J. Natl. Cancer Inst. 1991, 83, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; Daller, R.T.; Fenton, J.G.; Miser, J.S. Granulocyte colony stimulating factor permits dose intensification by interval compression in the treatment of Ewing’s sarcomas and soft tissue sarcomas in children. Eur. J. Cancer 2000, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Ryan, C.W.; Bassale, S.; Lim, J.Y.; Davis, L.E. Feasibility of Treating Adults with Ewing or Ewing-Like Sarcoma with Interval-Compressed Vincristine, Doxorubicin, and Cyclophosphamide Alternating with Ifosfamide and Etoposide. Oncologist 2020, 25, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Pretz, J.L.; Barysauskas, C.M.; George, S.; Hornick, J.L.; Raut, C.P.; Chen, Y.E.; Marcus, K.J.; Choy, E.; Hornicek, F.; Ready, J.E.; et al. Localized Adult Ewing Sarcoma: Favorable Outcomes with Alternating Vincristine, Doxorubicin, Cyclophosphamide, and Ifosfamide, Etoposide (VDC/IE)-Based Multimodality Therapy. Oncologist 2017, 22, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Anderton, J.; Moroz, V.; Marec-Berard, P.; Gaspar, N.; Laurence, V.; Martin-Broto, J.; Sastre, A.; Gelderblom, H.; Owens, C.; Kaiser, S.; et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours—EURO EWING 2012 Protocol. Trials 2020, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.; Kirton, L.; Marec-Berard, P.; Martin-Broto, J.; Gelderblom, H.; Gaspar, N.; Strauss, S.J.; Urgelles, A.S.; Anderton, J.; Laurence, V.; et al. Comparison of two chemotherapy regimens in Ewing sarcoma (ES): Overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012). J. Clin. Oncol. 2020, 38, 11500. [Google Scholar] [CrossRef]
- Leavey, P.J.; Laack, N.N.; Krailo, M.D.; Buxton, A.; Randall, R.L.; DuBois, S.G.; Reed, D.R.; Grier, H.E.; Hawkins, D.S.; Pawel, B.; et al. Phase III Trial Adding Vincristine-Topotecan-Cyclophosphamide to the Initial Treatment of Patients With Nonmetastatic Ewing Sarcoma: A Children’s Oncology Group Report. J. Clin. Oncol. 2021, 39, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Sun, Y.; He, A.; Zhou, Y.; Hu, H.; Yao, Y.; Shen, Z. Impact of chemotherapy cycles and intervals on outcomes of nonspinal Ewing sarcoma in adults: A real-world experience. BMC Cancer 2019, 19, 1168. [Google Scholar] [CrossRef]
- Minichsdorfer, C.; Steinbrecher, O.; Kölz, M.; Schmid, M.; Raderer, M.; Brodowicz, T.; Lamm, W. Adolescents and Young Adults (AYAs) With Initially Localized and Metastatic Bone Sarcomas: A Retrospective Single Center Analysis of Side Effect Management. In Vivo 2021, 35, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhi, X.; Xie, L.; Sun, X.; Liu, X.; Liu, K.; Guo, W. Long-term outcome and relapse patterns in Ewing sarcoma patients with extensive lung/pleural metastases after a complete response to systemic therapy. BMC Cancer 2022, 22, 500. [Google Scholar] [CrossRef]
- Miser, J.S.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide--a Children’s Cancer Group and Pediatric Oncology Group study. J. Clin. Oncol. 2004, 22, 2873–2876. [Google Scholar] [CrossRef]
- Miser, J.S.; Goldsby, R.E.; Chen, Z.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2007, 49, 894–900. [Google Scholar] [CrossRef]
- Whelan, J.; Le Deley, M.C.; Dirksen, U.; Le Teuff, G.; Brennan, B.; Gaspar, N.; Hawkins, D.S.; Amler, S.; Bauer, S.; Bielack, S.; et al. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J. Clin. Oncol. 2018, 36, 3110–3119. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, U.; Brennan, B.; Le Deley, M.C.; Cozic, N.; van den Berg, H.; Bhadri, V.; Brichard, B.; Claude, L.; Craft, A.; Amler, S.; et al. High-Dose Chemotherapy Compared With Standard Chemotherapy and Lung Radiation in Ewing Sarcoma With Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. J. Clin. Oncol. 2019, 37, 3192–3202. [Google Scholar] [CrossRef]
- Pawlowska, A.B.; Sun, V.; Calvert, G.T.; Karras, N.A.; Sato, J.K.; Anderson, C.P.; Cheng, J.C.; DiMundo, J.F.; Femino, J.D.; Lu, J.; et al. Long-Term Follow-up of High-Dose Chemotherapy with Autologous Stem Cell Transplantation in Children and Young Adults with Metastatic or Relapsed Ewing Sarcoma: A Single-Institution Experience. Transplant. Cell. Ther. 2021, 27, 72.e1–72.e7. [Google Scholar] [CrossRef]
- Hosoi, H. Current status of treatment for pediatric rhabdomyosarcoma in the USA and Japan. Pediatr. Int. 2016, 58, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Owosho, A.A.; Zhang, L.; Chen, S.; Deniz, K.; Huryn, J.M.; Kao, Y.C.; Huang, S.C.; Singer, S.; Tap, W.; et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. Am. J. Surg. Pathol. 2017, 41, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.A.; Bhadri, V.A.; Wake, J.; Ingley, K.M.; Lewin, J.; Bae, S.; Wong, D.D.; Long, A.P.; Pryor, D.; Thompson, S.R.; et al. Systemic treatments and outcomes in CIC-rearranged Sarcoma: A national multi-centre clinicopathological series and literature review. Cancer Med. 2022, 11, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, S.; Imamura, Y.; Kiyota, N.; Takakura, H.; Matsumoto, S.; Koyama, T.; Fujishima, Y.; Funakoshi, Y.; Toyoda, M.; Hirose, T.; et al. Secondary CIC-rearranged sarcoma responsive to chemotherapy regimens for Ewing sarcoma: A case report. Mol. Clin. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Lozano, M.L.; Rodeghiero, F. Thrombopoietin receptor agonist in chemotherapy-induced thrombocytopenia. Lancet Haematol. 2022, 9, e168–e169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
