Modified SCOPE (mSCOPE) Score as a Tool to Predict Mortality in COVID-19 Critically Ill Patients
Abstract
1. Introduction
2. Materials and methods
2.1. Study Design
2.2. Calculation of the Severe COVID Prediction Estimate (SCOPE) Score
2.3. Study Participants
2.4. Charlson Comorbidity Index and APACHE Score Calculation
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
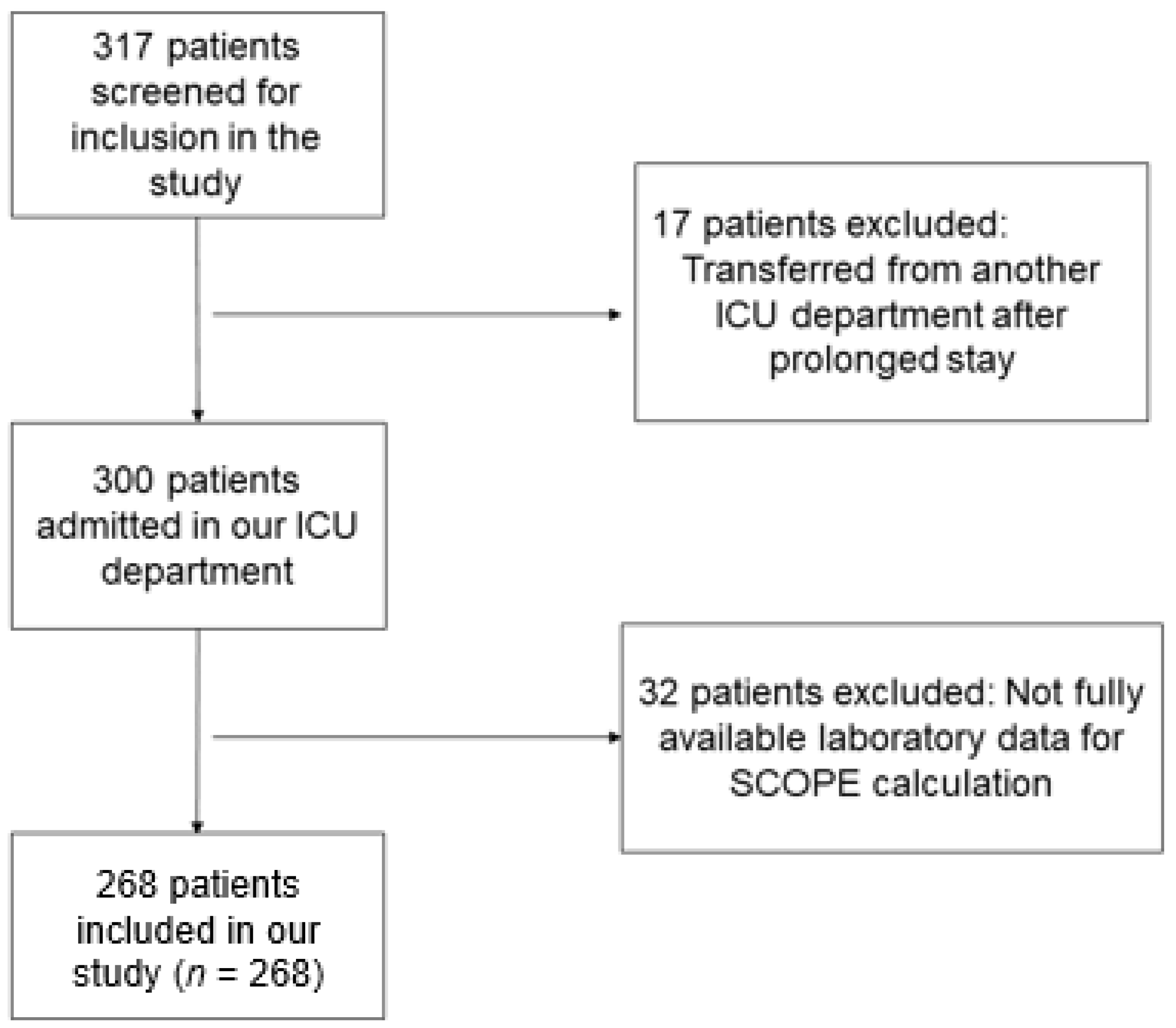
3.1. Study participants
3.2. Correlations of mSCOPE with Disease Severity and Outcomes
3.3. mSCOPE Score and the Presence of Comorbidities
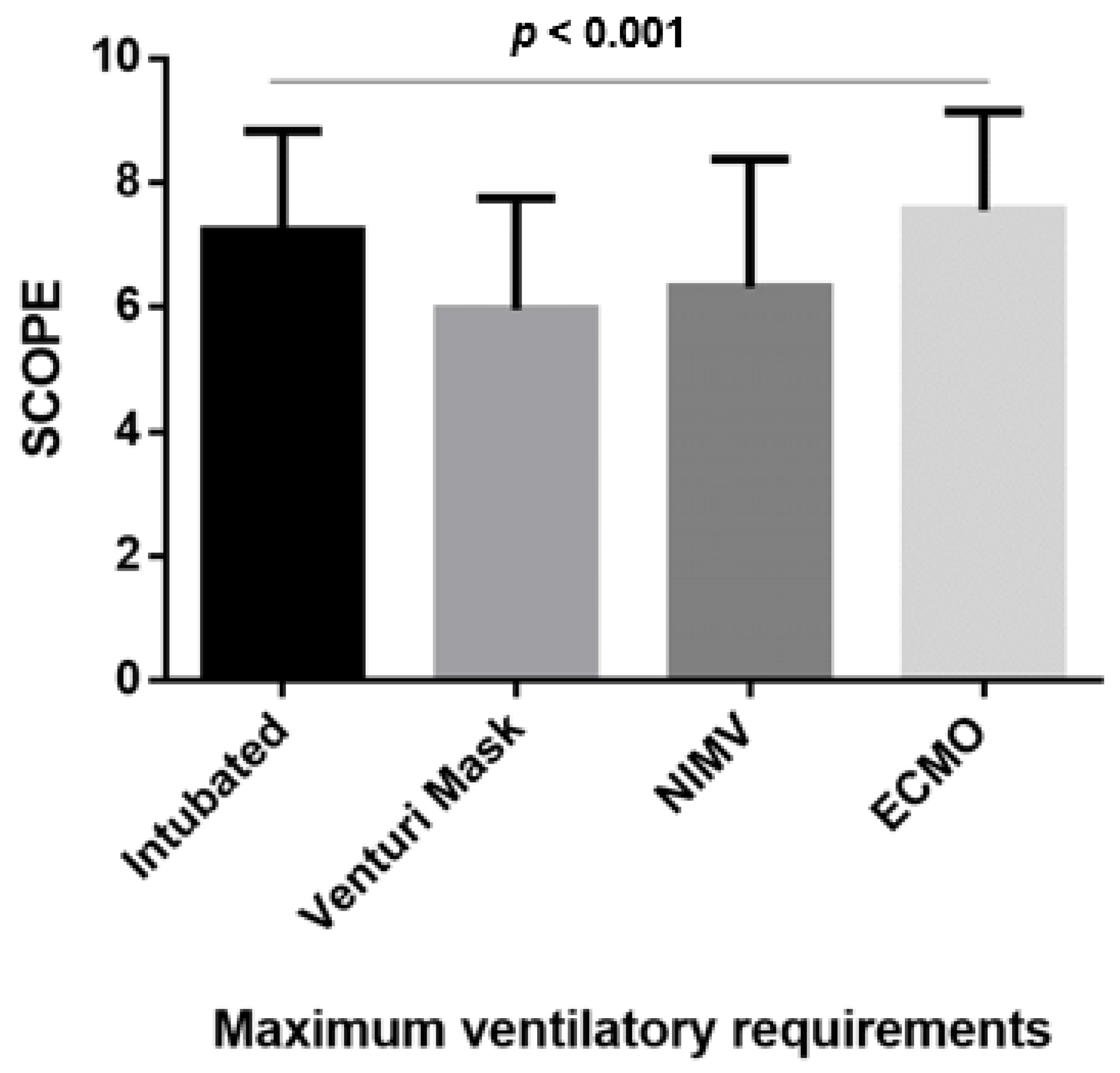
3.4. mSCOPE According to the Maximal Ventilation Requirements
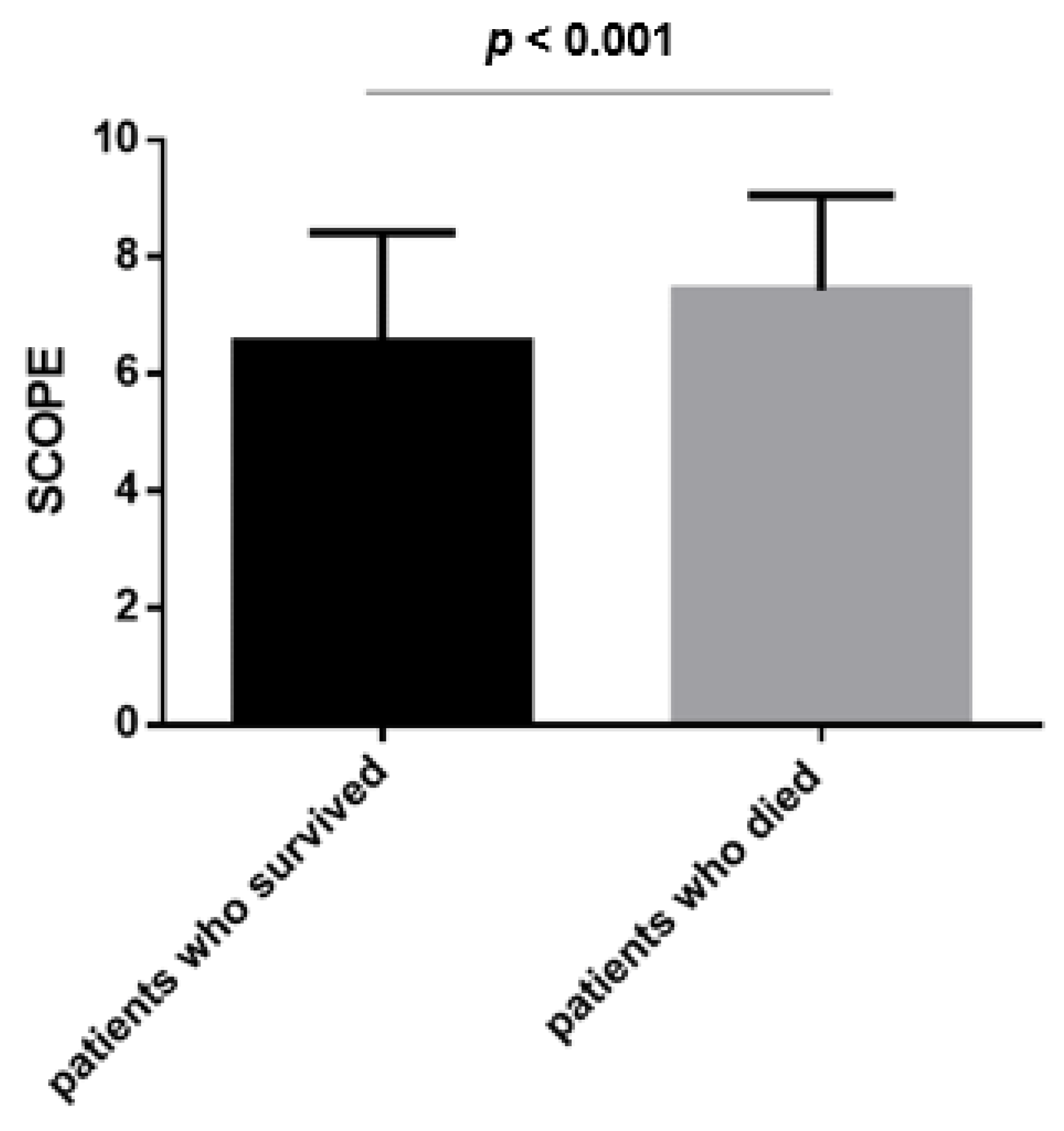
3.5. mSCOPE and Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [PubMed]
- Li, X.; Wang, W.; Zhao, X.; Zai, J.; Zhao, Q.; Li, Y.; Chaillon, A. Transmission dynamics and evolutionary history of 2019-nCoV. J. Med. Virol. 2020, 92, 501–511. [Google Scholar] [PubMed]
- Preventing and Managing COVID-19 Across Long-Term Care Services: Policy Brief. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy_Brief-Long-term_Care-2020 (accessed on 1 January 2020).
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [PubMed]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health Eur. 2021, 11, 100243. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Poulakou, G.; de Nooijer, A.; Milionis, H.; Metallidis, S.; Ploumidis, M.; Grigoropoulou, P.; Rapti, A.; Segala, F.; Balis, E.; et al. Development and validation of SCOPE score: A clinical score to predict COVID-19 pneumonia progression to severe respiratory failure. Cell Rep. Med. 2022, 3, 100560. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar]
- Salbach, C.; Mueller-Hennessen, M.; Biener, M.; Stoyanov, K.M.; Vafaie, M.; Preusch, M.R.; Kihm, L.P.; Merle, U.; Schnitzler, P.; Katus, H.A.; et al. Validation of two severity scores as predictors for outcome in Coronavirus Disease 2019 (COVID-19). PLoS ONE 2021, 16, e0247488. [Google Scholar]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Rovina, N.; Akinosoglou, K.; Eugen-Olsen, J.; Hayek, S.; Reiser, J.; Giamarellos-Bourboulis, E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Recommends Approval for Use of Kineret in Adults with COVID-19. 2021. Available online: https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19 (accessed on 1 January 2020).
- Kyriazopoulou, E.; Panagopoulos, P.; Metallidis, S.; Dalekos, G.; Poulakou, G.; Gatselis, N.; Karakike, E.; Saridaki, M.; Loli, G.; Stefos, A.; et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife 2021, 10, e66125. [Google Scholar]
- Koukaki, E.; Rovina, N.; Tzannis, K.; Sotiropoulou, Z.; Loverdos, K.; Koutsoukou, A.; Dimopoulos, G. Fungal Infections in the ICU during the COVID-19 Era: Descriptive and Comparative Analysis of 178 Patients. J. Fungi 2022, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host. Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Rovina, N.; Galani, I.E.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021, 22, 32–40. [Google Scholar]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Du, C.; Zhang, Y.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.P.; Draper, E.A. Acute physiology and chronic health evaluation (APACHE II) and Medicare reimbursement. Health Care Financ. Rev. 1984, 1984, 91–105. [Google Scholar]
- Beigmohammadi, M.T.; Amoozadeh, L.; Motlagh, F.R.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K. Mortality Predictive Value of APACHE II and SOFA Scores in COVID-19 Patients in the Intensive Care Unit. Can. Respir. J. 2022, 2022, 5129314. [Google Scholar] [CrossRef] [PubMed]
- Tuty Kuswardhani, R.A.; Henrina, J.; Pranata, R.; Lim, M.A.; Lawrensia, S.; Suastika, K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 2103–2109. [Google Scholar] [CrossRef]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar]
- Wang, Q.; Cheng, J.; Shang, J.; Wang, Y.; Wan, J.; Yan, Y.Q.; Liu, W.B.; Zhang, H.P.; Wang, J.P.; Wang, X.Y.; et al. Clinical value of laboratory indicators for predicting disease progression and death in patients with COVID-19: A retrospective cohort study. BMJ Open 2021, 11, e043790. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R.; Lim, M.A.; Oehadian, A.; Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: A meta-analysis. Ther. Adv. Respir Dis. 2020, 14, 1753466620937175. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Al Mejedea, H.; Nazer, R.; Alhusaini, Y.; Alhamdan, A.; Al Jawad, A. Occurrence, Risk Factors, and Outcomes of Pulmonary Barotrauma in Critically Ill COVID-19 Patients: A Retrospective Cohort Study. Crit. Care Res. Pract. 2023, 2023, 4675910. [Google Scholar] [CrossRef]
- Büyükkarabacak, Y.; Pirzirenli, M.G.; Gurz, S.; Abacı, H.; Şengül, A.T.; Çelik, B.; Basoğlu, A. COVID-19 and pneumothorax, pneumomediastinum, subcutaneous emphysema: Analysis of risk factors. Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 69–77. [Google Scholar]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Udi, J.; Lang, C.N.; Zotzmann, V.; Krueger, K.; Fluegler, A.; Bamberg, F.; Bode, C.; Duerschmied, D.; Wengenmayer, T.; Staudacher, D.L. Incidence of Barotrauma in Patients With COVID-19 Pneumonia During Prolonged Invasive Mechanical Ventilation—A Case-Control Study. J. Intensive Care Med. 2021, 36, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.; Al Mutair, A.; Alghazal, H.A.; Alhaddad, A.J.; Al-Helal, H.; Al Salman, S.A.; Alali, J.; Almahmoud, S.; Alhejy, Z.M.; Albagshi, A.A.; et al. Extracorporeal membrane oxygenation support for SARS-CoV-2: A multi-centered, prospective, observational study in critically ill 92 patients in Saudi Arabia. Eur. J. Med. Res. 2021, 26, 141. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.N.; Sivanushanthan, S.; Etling, E.; Hockstein, M.; Yohannes, S.; Clark, P. Incidence of barotrauma in patients with COVID-19 (alpha- and beta-predominant period) requiring mechanical ventilation: Single-center retrospective study. SAGE Open Med. 2023, 11, 20503121231159479. [Google Scholar] [CrossRef] [PubMed]

| D-Dimers (mg/mL) | CRP (mg/L) | Ferritin (ng/mL) | Points |
|---|---|---|---|
| 0.10–0.40 | 0.3–25.0 | 10.0–225.0 | 0 |
| 0.41–0.57 | 25.1–45.0 | 225.1–450.0 | 1 |
| 0.58–0.90 | 45.1–85.0 | 450.1–750.0 | 2 |
| >0.91 | >85.1 | >750.1 | 3 |
| Variable | |
|---|---|
| Age (years) | 61.0 (51.0, 69.7) |
| Sex (M) N (%) | 189 (70.5) |
| BMI kg/m2 | 29.3 (26.0, 33.0) |
| Smoking (never/current/ex) N (%) | 157/33/78 (58.6/12.3/29.1) |
| APACHE Score | 11.0 (8.0, 14.0) |
| Comorbidities N (%) Respiratory disease Diabetes mellitus Arterial hypertension Thyroid disease Coronary disease | 39 (14.6) 56 (20.9) 121 (45.1) 43 (16) 27 (10.1) |
| Ventilatory requirements N (%) Mechanical ventilation Venturi Mask NIMV ECMO | 146 (54.4) 52 (19.4) 61 (22.8) 9 (3.4) |
| Length of stay in ICU (days) | 10.0 (6.0,23.0) |
| Length of stay in the Hospital (days) | 26.0 (17.0, 41.0) |
| Outcome (death) N (%) | 70 (26.1) |
| mSCOPE | 7.0 (6.0, 8.0) |
| Comorbidity | mSCOPE | p-Value | |
|---|---|---|---|
| Patients w/o the Comorbidity | Patients with the Comorbidity | ||
| Respiratory disease | 7.0 (6.0, 8.0) | 6.0 (5.0, 8.0) | 0.176 |
| Diabetes mellitus | 7.0 (5.0, 8.0) | 7.0 (6.0, 8.7) | 0.202 |
| Hypertension | 7.0 (5.0, 8.0) | 7.0 (6.0, 9.0) | 0.306 |
| Thyroid disease | 7.0 (6.0, 8.0) | 6.0 (5.0, 8.0) | 0.101 |
| Coronary disease | 7.0 (5.5, 8.0) | 7.0 (6.0, 9.0) | 0.764 |
| Optimal Cut-Off Point | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | AUC (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
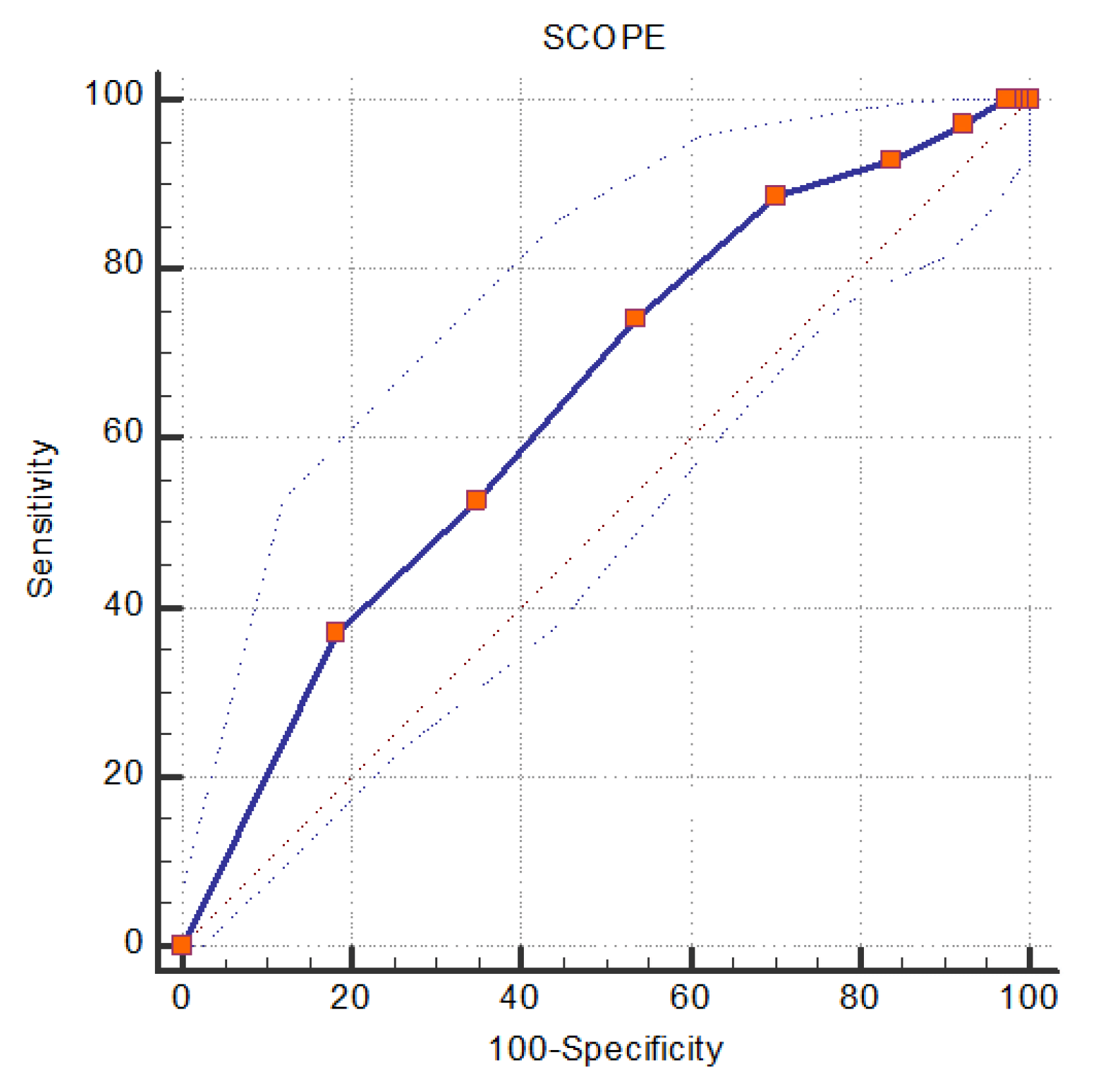
| SCOPE score | ≥6 | 88.57 (78.7, 94.9) | 29.69(23.3, 36.7) | 31.5 (25.1, 38.5) | 87.7 (77.2, 94.5) | 0.643 (0.582, 0.701) | <0.001 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.075 | 1.051–1.099 | <0.001 | 0.984 | 0.952–1.017 | 0.332 |
| Sex | 0.882 | 0.521–1.494 | 0.640 | |||
| APACHE score | 1.135 | 1.091–1.181 | <0.001 | 1.090 | 1.038–1.145 | 0.001 |
| CCI | 1.672 | 1.501–1.864 | <0.001 | 1.693 | 1.417–2.023 | <0.001 |
| mSCOPE score | 1.297 | 1.121–1.500 | <0.001 | 1.219 | 1.010–1.471 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelli, S.; Bakakos, A.; Sotiropoulou, Z.; Papaioannou, A.I.; Koukaki, E.; Potamianou, E.; Kyriakoudi, A.; Kaniaris, E.; Bakakos, P.; Giamarellos-Bourboulis, E.J.; et al. Modified SCOPE (mSCOPE) Score as a Tool to Predict Mortality in COVID-19 Critically Ill Patients. J. Pers. Med. 2023, 13, 628. https://doi.org/10.3390/jpm13040628
Zanelli S, Bakakos A, Sotiropoulou Z, Papaioannou AI, Koukaki E, Potamianou E, Kyriakoudi A, Kaniaris E, Bakakos P, Giamarellos-Bourboulis EJ, et al. Modified SCOPE (mSCOPE) Score as a Tool to Predict Mortality in COVID-19 Critically Ill Patients. Journal of Personalized Medicine. 2023; 13(4):628. https://doi.org/10.3390/jpm13040628
Chicago/Turabian StyleZanelli, Stavroula, Agamemnon Bakakos, Zoi Sotiropoulou, Andriana I. Papaioannou, Evangelia Koukaki, Efstathia Potamianou, Anna Kyriakoudi, Evangelos Kaniaris, Petros Bakakos, Evangelos J. Giamarellos-Bourboulis, and et al. 2023. "Modified SCOPE (mSCOPE) Score as a Tool to Predict Mortality in COVID-19 Critically Ill Patients" Journal of Personalized Medicine 13, no. 4: 628. https://doi.org/10.3390/jpm13040628
APA StyleZanelli, S., Bakakos, A., Sotiropoulou, Z., Papaioannou, A. I., Koukaki, E., Potamianou, E., Kyriakoudi, A., Kaniaris, E., Bakakos, P., Giamarellos-Bourboulis, E. J., Koutsoukou, A., & Rovina, N. (2023). Modified SCOPE (mSCOPE) Score as a Tool to Predict Mortality in COVID-19 Critically Ill Patients. Journal of Personalized Medicine, 13(4), 628. https://doi.org/10.3390/jpm13040628







