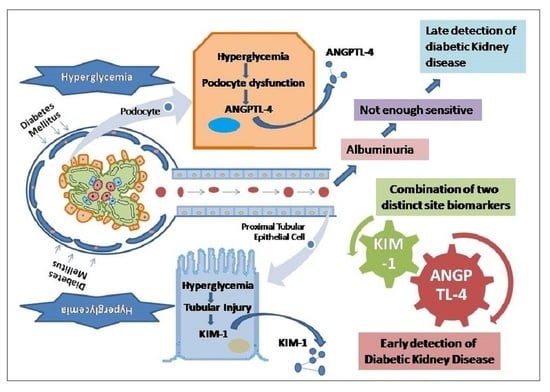
Expression of Angiopoetin-Like Protein-4 and Kidney Injury Molecule-1 as Preliminary Diagnostic Markers for Diabetes-Related Kidney Disease: A Single Center-Based Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size
2.3. Data Collection
2.4. Sample Collection and Biochemical Parameters Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Population Characteristics and Biochemical Parameter Assessment
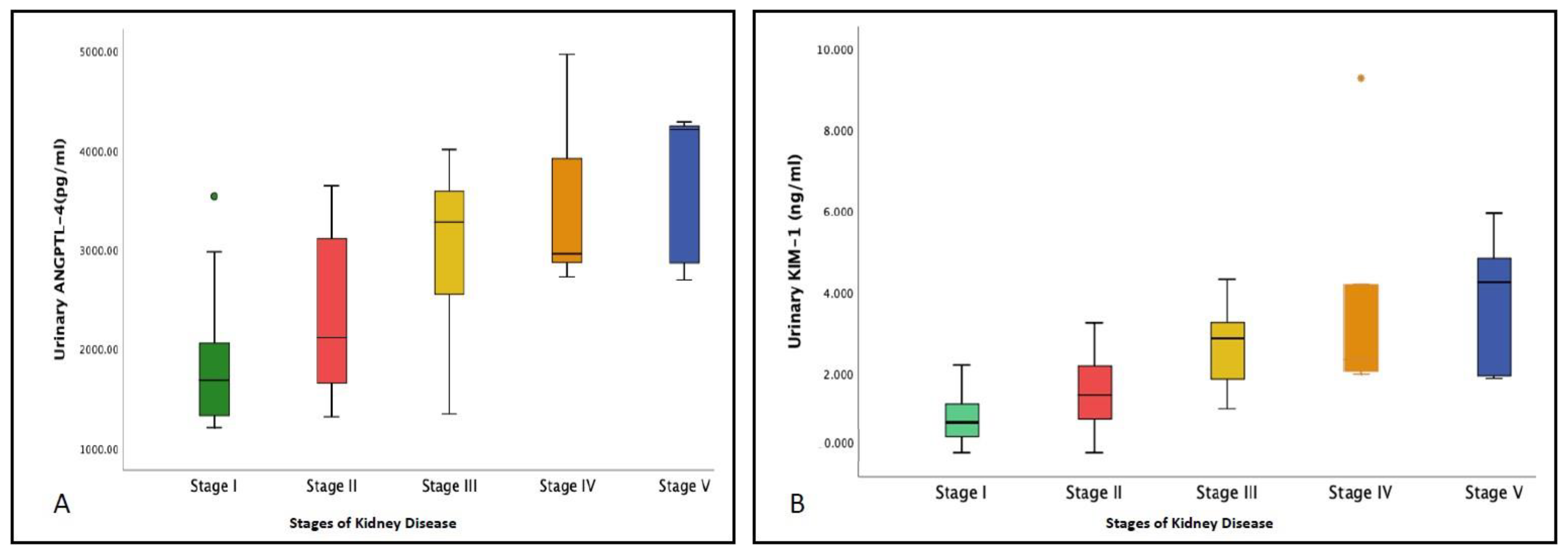
3.2. Comparison of Urinary Levels of ANGPTL-4 and KIM-1 among the Different Study Groups
3.3. Correlation of KIM-1 and ANGPTL-4 with Clinical Parameters
3.4. Associations between Urinary ANGPTL-4 and KIM-1 and Diabetic Kidney Disease
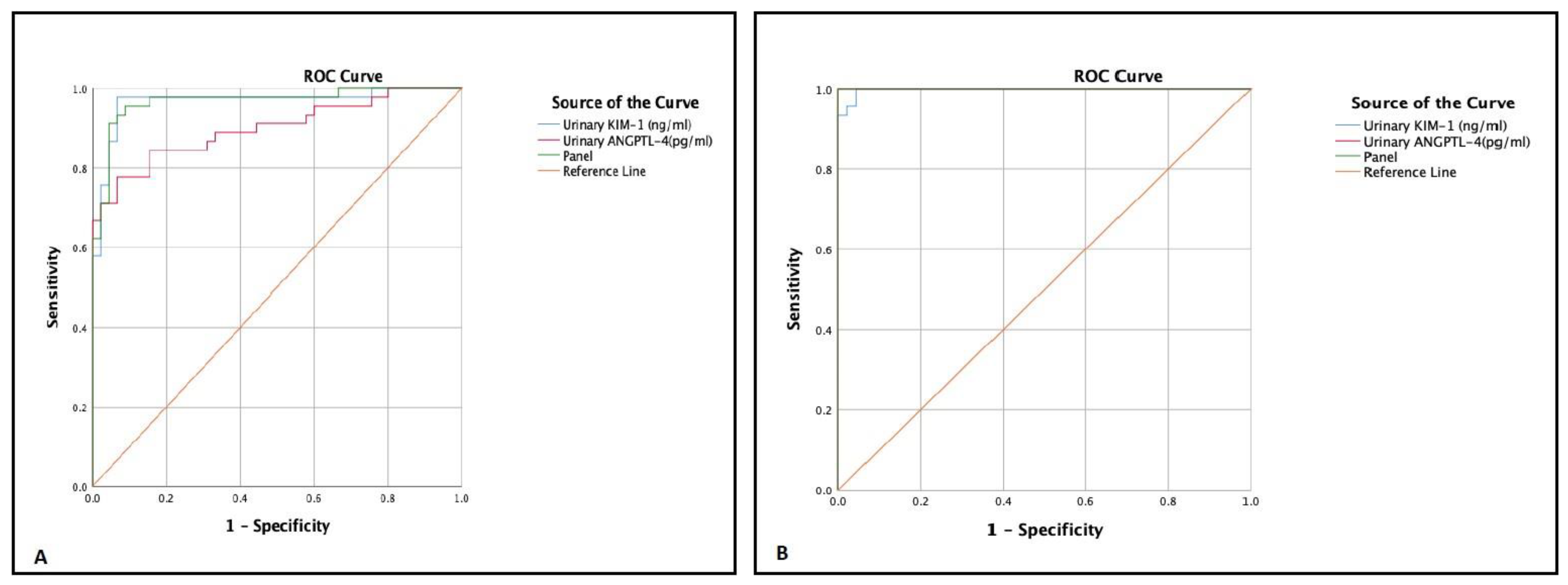
3.5. Sensitivity and Specificity of ANGPTL-4 and KIM-1 in the Diagnosis of DKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donate-Correa, J.; Martin-Nunez, E.; Muros-de-Fuentes, M.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Inflammatory cytokines in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 948417. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Persson, F.; Frimodt-Moller, M. Prognosis and treatment of diabetic nephropathy: Recent advances and perspectives. Nephrol. Ther. 2018, 14 (Suppl. S1), S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Lim, A. Diabetic nephropathy-complications and treatment. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Almas, S.; Prabhakar, S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J. Investig. Med. 2017, 65, 1093–1101. [Google Scholar] [CrossRef]
- Remizid, G.; Ruggenenti, P.; Benigni, A. Understanding the nature of renal disease progression. Kidney Int. 1997, 51, 2–15. [Google Scholar] [CrossRef]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- Papadopoulou-Marketou, N.; Kanaka-Gantenbein, C.; Marketos, N.; Chrousos, G.P.; Papassotiriou, I. Biomarkers of diabetic nephropathy: A 2017 update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 326–342. [Google Scholar] [CrossRef]
- Thipsawat, S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. DiabetesVasc. Dis. Res. 2021, 18, 14791641211058856. [Google Scholar] [CrossRef]
- Cara-Fuentes, G.; Segarra, A.; Silva-Sanchez, C.; Wang, H.; Lanaspa, M.A.; Johnson, R.J.; Garin, E.H. Angiopoietin-like-4 and minimal change disease. PLoS ONE 2017, 12, e0176198. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Tjeerdema, N.; Georgiadi, A.; Jonker, J.T.; van Glabbeek, M.; Alizadeh Dehnavi, R.; Tamsma, J.T.; Smit, J.W.; Kersten, S.; Rensen, P.C. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res. Care 2014, 2, e000034. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, X.; Li, J.S.; Peng, L.; Wei, S.Y.; Zhao, S.L.; Li, T.; Zhu, D.; He, Y.X.; Wei, Q.J.; et al. Upregulation of podocyte-secreted angiopoietin-like-4 in diabetic nephropathy. Endocrine 2015, 49, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Mace, C.; Clement, L.C.; Del Nogal Avila, M.; Marshall, C.B. Angiopoietin-like 4 based therapeutics for proteinuria and kidney disease. Front. Pharmacol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Clement, L.C.; Avila-Casado, C.; Mace, C.; Soria, E.; Bakker, W.W.; Kersten, S.; Chugh, S.S. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 2011, 17, 117–122. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Niewczas, M.A.; Ficociello, L.H.; Johnson, A.C.; Collings, F.B.; Warram, J.H.; Krolewski, A.S.; Bonventre, J.V. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011, 79, 464–470. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S151–S167. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 12 October 2020).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 10 September 2022).
- Thomas, B. The Global Burden of Diabetic Kidney Disease: Time Trends and Gender Gaps. Curr. Diabetes Rep. 2019, 19, 18. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Tamhane, A.R.; Westfall, A.O.; Burkholder, G.A.; Cutter, G.R. Prevalence odds ratio versus prevalence ratio: Choice comes with consequences. Stat. Med. 2016, 35, 5730–5735. [Google Scholar] [CrossRef]
- Hardin, J.W. The sandwich estimate of variance. AdvEconom 2003, 17, 45–73. [Google Scholar]
- Tabaei, B.P.; Al-Kassab, A.S.; Ilag, L.L.; Zawacki, C.M.; Herman, W.H. Does microalbuminuria predict diabetic nephropathy? Diabetes Care 2001, 24, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001, 44, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Dalla Vestra, M.; Masiero, A.; Roiter, A.M.; Saller, A.; Crepaldi, G.; Fioretto, P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 2003, 52, 1031–1035. [Google Scholar] [CrossRef]
- Vanarsa, K.; Soomro, S.; Zhang, T.; Strachan, B.; Pedroza, C.; Nidhi, M.; Cicalese, P.; Gidley, C.; Dasari, S.; Mohan, S.; et al. Quantitative planar array screen of 1000 proteins uncovers novel urinary protein biomarkers of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1349–1361. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Renal function in diabetic disease models: The tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Tatsch, E.; Hausen, B.S.; Bollick, Y.S.; Moretto, M.B.; Duarte, T.; Duarte, M.M.; Londero, S.W.; Premaor, M.O.; Comim, F.V.; et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin. Biochem. 2016, 49, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Katz, R.; Bonventre, J.V.; Sabbisetti, V.; Siscovick, D.; Sarnak, M.; Shlipak, M.G. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 2012, 60, 904–911. [Google Scholar] [CrossRef]
- Allgaier, R.; Strack, C.; Wallner, S.; Hubauer, U.; Uecer, E.; Lehn, P.; Keyser, A.; Luchner, A.; Maier, L.; Jungbauer, C. N-acetyl-b-D-glucosaminidase: A potential cardiorenal biomarker with a relevant impact on ICD shock therapies and mortality. Nephrology 2020, 25, 888–896. [Google Scholar] [CrossRef]
- Schrauben, S.J.; Shou, H.; Zhang, X.; Anderson, A.H.; Bonventre, J.V.; Chen, J.; Coca, S.; Furth, S.L.; Greenberg, J.H.; Gutierrez, O.M.; et al. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2021, 32, 115–126. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, L.M.; Pang, Y.; Wu, W.J.; Tan, Y.; Yu, F.; Zhao, M.H. Composite urinary biomarkers to predict pathological tubulointerstitial lesions in lupus nephritis. Lupus 2018, 27, 1778–1789. [Google Scholar] [CrossRef] [PubMed]

| Background Parameters | Normoalbuminuria (Control Group) (n = 45) | Microalbuminuria (n = 45) | Macroalbuminuria (n = 45) | p-Value |
|---|---|---|---|---|
| Demographic variables | ||||
| Age(year) | 56.9 ± 8.7 | 61.9 ± 12.0 | 61.4 ± 10.9 | 0.0527 |
| Female gender (%) | 23 (51.1) | 21 (46.7) | 22 (48.9) | 0.915 |
| Education (%) | ||||
| Illiterate | 10 (22.2) | 13 (28.9) | 11 (24.4) | 0.318 |
| Upto Senior Secondary | 21 (46.7) | 17 (37.8) | 24 (53.3) | |
| Graduation and Above | 14 (31.1) | 15 (33.3) | 7 (15.6) | |
| Life style and clinical variables | ||||
| Smokers (%) | 11 (24.4) | 13 (28.9) | 8 (17.8) | 0.459 |
| Family History of T2DM (%) | 17 (37.8%) | 18 (40%) | 17 (37.8%) | |
| Duration of T2DM (year) | 6.3 ± 5.1 | 10.1 ± 8.6 | 11.9 ± 6.8 | 0.0008 |
| BMI (Kg/m2) | 27.7 ± 3.5 | 30.1 ± 5.3 | 30.0 ± 5.5 | 0.0276 |
| SBP (mmHg) | 139.9 ± 13.4 | 141.6 ± 11.7 | 137.0 ± 10.8 | 0.1810 |
| DBP (mmHg) | 85.0 ± 6.1 | 87.1 ± 6.8 | 84.2 ± 6.0 | 0.0791 |
| Laboratory variables | ||||
| FPG (mg/dL) | 152.4 ± 44.6 | 182.4 ± 53.3 | 190.1 ± 42.3 | 0.0005 |
| HbA1c (%) | 7.2 ± 0.8 | 9.4 ± 1.8 | 9.8 ± 1.1 | <0.001 |
| Albumin (g/dL) | 4.4 ± 0.3 | 4.1 ± 0.5 | 4.0 ± 0.5 | 0.0001 |
| Globulin (g/dL) | 2.9 ± 0.4 | 2.9 ± 0.3 | 3.1 ± 0.4 | 0.0021 |
| Albumin Globulin Ratio | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.3 | 0.0001 |
| Protein Total (g/dL) | 7.3 ± 0.5 | 7.0 ± 0.6 | 7.1 ± 0.4 | 0.0382 |
| Urea (mg/dL) | 27.1 ± 12.4 | 34.3 ± 13.4 | 37.1 ± 10.2 | 0.0005 |
| BUN (mg/dL) | 13.0 ± 6.4 | 16.1 ± 7.0 | 17.6 ± 4.7 | 0.0016 |
| Uric Acid (mg/dL) | 4.0 ± 0.9 | 5.5 ± 0.7 | 6.4 ± 0.7 | <0.001 |
| Serum Creatinine (mg/dL) | 0.9 ± 0.1 | 1.2 ± 0.6 | 1.3 ± 0.7 | 0.0002 |
| eGFR (mL/min/1.73 m2) | 88.7 ± 11.7 | 68.1 ± 24.8 | 62.4 ± 22.6 | <0.001 |
| Total Cholesterol (mg/dL) | 178.5 ± 42.1 | 198.8 ± 46.4 | 196.6 ± 46.2 | 0.0661 |
| Na (mmol/L) | 140.3 ± 3.2 | 140.4 ± 3.1 | 140.6 ± 3.1 | 0.8805 |
| K (mmol/L) | 4.7 ± 0.6 | 4.8 ± 0.6 | 4.9 ± 0.6 | 0.0428 |
| Cl (mmol/L) | 103.3 ± 3.0 | 103.4 ± 3.3 | 104.1 ± 2.9 | 0.3581 |
| Ca (mg/dL) | 9.1 ± 0.7 | 8.6 ± 0.8 | 8.7 ± 0.8 | 0.0105 |
| P (mg/dL) | 3.5 ± 0.7 | 3.9 ± 0.7 | 4.0 ± 0.8 | 0.0043 |
| Alkaline Phosphatase (U/L) | 94.9 ± 13.0 | 96.3 ± 16.5 | 104.6 ± 17.8 | 0.0092 |
| Urine Albumin (mg/L) | 10.4 ± 4.9 | 170.4 ± 81.5 | 442.9 ± 84.1 | <0.001 |
| Urine Creatinine (mg/dL) | 167.9 ± 30.5 | 135.1 ± 29.4 | 110.5 ± 11.2 | <0.001 |
| Urine Albumin/Creatinine (mg/g) | 6.7 ± 3.9 | 131.4 ± 72.0 | 403.2 ± 81.0 | <0.001 |
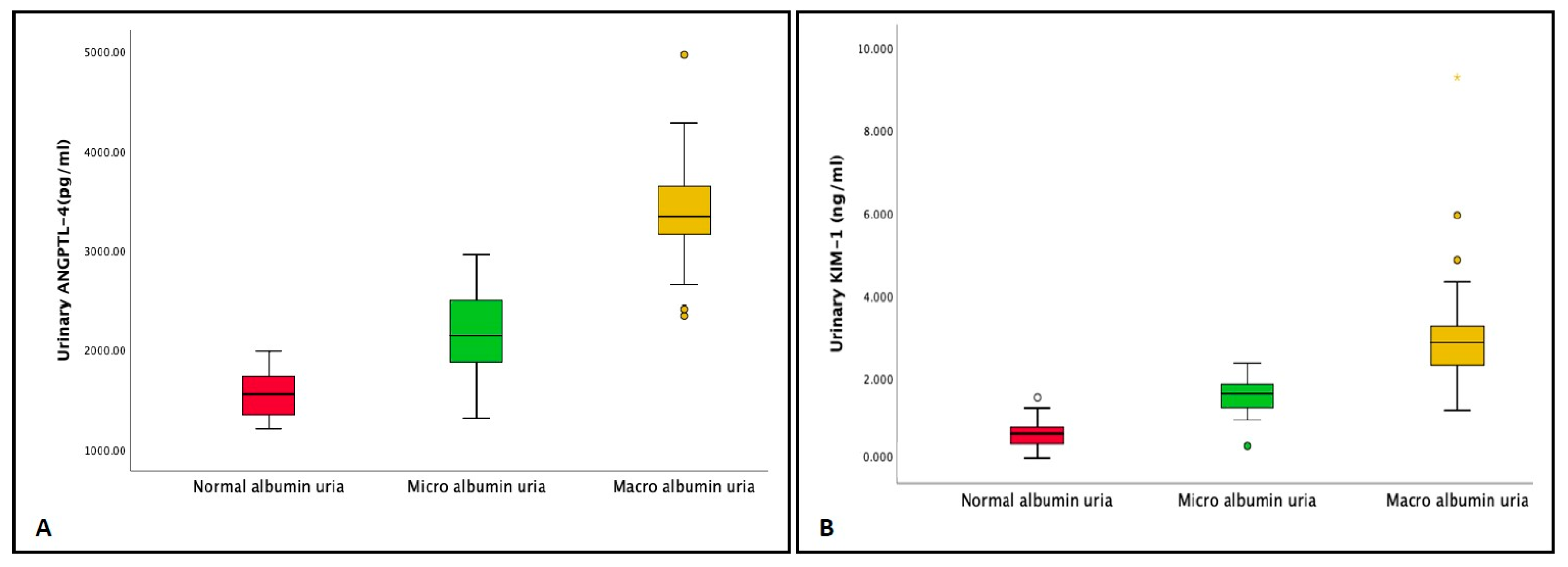
| Urinary ANGPTL-4 (pg/mL) | 1549.6 ± 229.5 | 2180.5 ± 424.5 | 3404.7 ± 482.0 | <0.001 |
| Urinary KIM-1 (ng/mL) | 0.6 ± 0.4 | 1.6 ± 0.4 | 3.0 ± 1.3 | <0.001 |
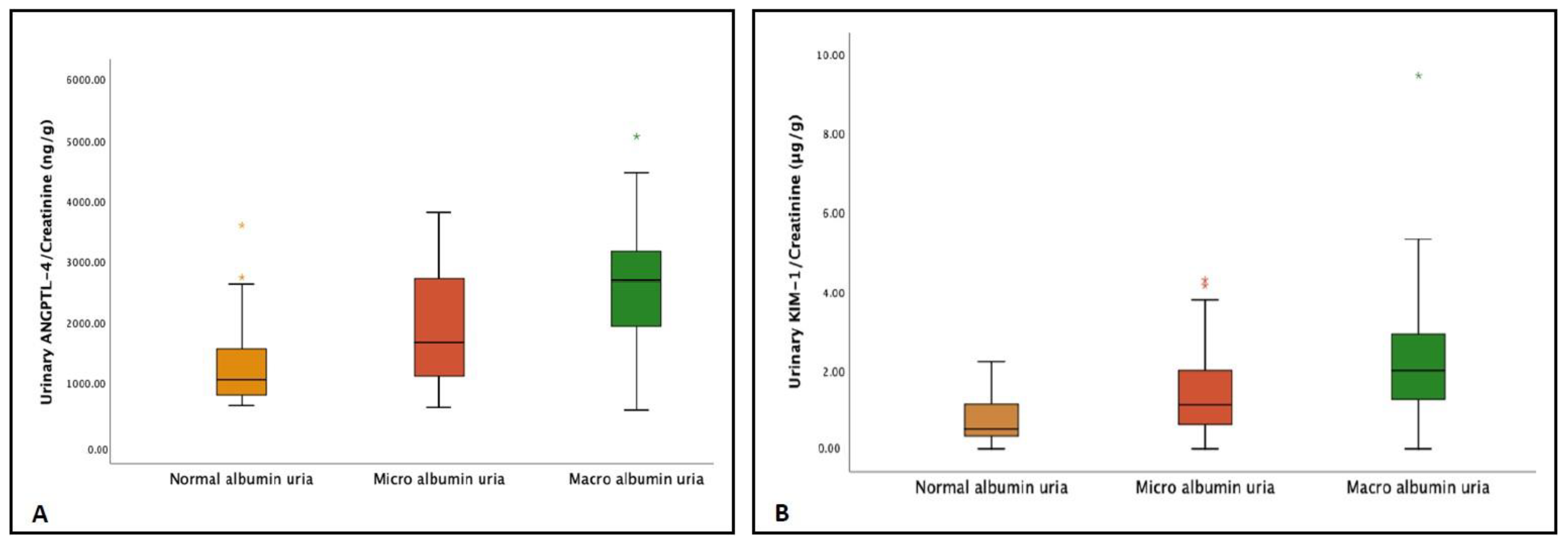
| Urinary ANGPTL-4/Creatinine (ng/g) | 965.2 ± 282.6 | 1691.2 ± 511.2 | 3119.0 ± 600.5 | <0.001 |
| Urinary KIM-1/Creatinine (µg/g) | 0.4 ± 0.3 | 1.2 ± 0.4 | 2.8 ± 1.4 | <0.001 |
| Parameters | Urinary ANGPTL-4 | Urinary KIM-1 | ||||
|---|---|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | Normoalbuminuria | Microalbuminuria | Macroalbuminuria | |
| Age (Year) | 0.39 * | 0.39 * | 0.39 * | 0.14 | 0.57 *** | 0.45 * |
| Duration of T2DM (year) | 0.36 * | 0.48 ** | 0.36 * | 0.02 | 0.66 *** | 0.40 * |
| BMI (Kg/m2) | 0.28 | 0.31 * | 0.28 | 0.19 | 0.04 | 0.31 * |
| SBP (mmHg) | 0.21 | 0.30 * | 0.21 | 0.30 * | 0.47 * | 0.36 * |
| DBP (mmHg) | 0.26 | 0.26 | 0.26 | 0.36 * | 0.32 * | 0.27 |
| FPG (mg/dL) | 0.42 * | 0.33 * | 0.42 * | 0.18 | 0.44 * | 0.33 * |
| HbA1c (%) | 0.43 * | 0.32 * | 0.43 * | 0.40 * | 0.34 * | 0.36 * |
| Albumin (g/dL) | −0.01 | 0.23 | −0.01 | 0.09 | 0.07 | −0.23 |
| Globulin (g/dL) | 0.14 | −0.03 | 0.14 | 0.64 *** | −0.12 | 0.36 * |
| Albumin Globulin Ratio | −0.07 | 0.14 | −0.07 | −0.45 * | 0.09 | −0.30 * |
| Protein Total (g/dL) | 0.16 | 0.16 | 0.16 | 0.55 ** | −0.04 | 0.11 |
| Urea (mg/dL) | 0.30 * | 0.43 * | 0.30 * | 0.38 * | 0.64 *** | 0.39 * |
| BUN (mg/dL) | 0.29 | 0.31 * | 0.29 | 0.39 * | 0.52 ** | 0.39 * |
| Uric Acid (mg/dL) | −0.02 | 0.15 | −0.02 | −0.18 | 0.14 | −0.02 |
| Serum Creatinine (mg/dL) | 0.70 *** | 0.53 *** | 0.70 *** | −0.03 | 0.73 *** | 0.68 *** |
| eGFR (mL/min/1.73 m2) | −0.20 *** | −0.52 *** | −0.81 *** | −0.33 * | −0.68 *** | −0.85 *** |
| Total Cholesterol (mg/dL) | 0.42 * | 0.28 | 0.42 * | −0.16 | 0.12 | 0.60 *** |
| Urine Albumin (mg/L) | 0.80 *** | 0.71 *** | 0.80 *** | 0.32 * | 0.82 *** | 0.69 *** |
| Urine Creatinine (mg/dL) | −0.30 * | −0.23 | −0.30 * | −0.36 * | −0.24 | −0.44 ** |
| Urine Albumin/Creatinine (mg/g) | 0.88 *** | 0.73 *** | 0.97 *** | 0.38 * | 0.84 *** | 0.87 *** |
| Urinary KIM-1/Creatinine (µg/g) | 0.77 *** | 0.58 *** | 0.77 *** | 0.96 *** | 0.85 *** | 0.97 *** |
| Urinary ANGPTL-4/Creatinine (ng/g) | 0.84 *** | 0.76 *** | 0.84 *** | 0.62 *** | 0.60 *** | 0.77 *** |
| Biomarker | PR | 95% CI | p-Value |
|---|---|---|---|
| Log [UrinaryANGPTL-4] | |||
| Model 1: unadjusted | 4.36 | 3.04 to 6.24 | p < 0.001 |
| Model 2: adjusted for demographic and clinical history [age, gender, BMI, family history of T2DM, duration of T2DM] | 4.38 | 2.98 to 6.44 | p < 0.001 |
| Model 3: adjusted for laboratory parameters [SBP, DBP, HbA1C, urea, BUN, serum creatinine, eGFR, total cholesterol] | 3.08 | 2.00 to 4.75 | p < 0.001 |
| Model 4: adjusted for significant variables in model 2 and model 3 [HbA1C, serum creatinine] | 3.40 | 2.32 to 4.98 | p < 0.001 |
| Urinary KIM-1 | |||
| Model 1: unadjusted | 1.26 | 1.12 to 1.42 | p < 0.001 |
| Model 2: adjusted for demographic and clinical history [age, gender, BMI, family history of T2DM, duration of T2DM] | 1.30 | 1.18 to 1.43 | p < 0.001 |
| Model 3: adjusted for laboratory parameters [SBP, DBP, HbA1C, urea, BUN, serum creatinine, eGFR, total cholesterol] | 1.17 | 1.04 to 1.31 | 0.008 |
| Model 4: adjusted for significant variables in model 2 and model 3 [age, BMI, duration of T2DM, HbA1C, serum creatinine] | 1.25 | 1.14 to 1.38 | p < 0.001 |
| Test Result Variable(s) | Optimal Cutoff | SN | SP | Positive Likelihood Ratio | AUC | Asymptotic 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Urinary ANGPTL-4 (pg/mL) | ||||||||
| Microalbuminuria | 1868 | 77.8% | 93.3% | 11.61 | 0.90 | 0.83 | 0.97 | <0.0001 |
| Macroalbuminuria | 2794 | 100% | 97.8% | 45.45 | 1.00 | 1.00 | 1.00 | <0.0001 |
| Urinary KIM-1 (ng/mL) | ||||||||
| Microalbuminuria | 0.96 | 97.8% | 93.3% | 14.59 | 0.97 | 0.93 | 1.00 | <0.0001 |
| Macroalbuminuria | 1.87 | 100% | 95.6% | 22.72 | 0.98 | 0.95 | 1.00 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bano, G.; Imam, M.T.; Bajpai, R.; Alem, G.; Kashyap, V.K.; Habib, A.; Najmi, A.K. Expression of Angiopoetin-Like Protein-4 and Kidney Injury Molecule-1 as Preliminary Diagnostic Markers for Diabetes-Related Kidney Disease: A Single Center-Based Cross-Sectional Study. J. Pers. Med. 2023, 13, 577. https://doi.org/10.3390/jpm13040577
Bano G, Imam MT, Bajpai R, Alem G, Kashyap VK, Habib A, Najmi AK. Expression of Angiopoetin-Like Protein-4 and Kidney Injury Molecule-1 as Preliminary Diagnostic Markers for Diabetes-Related Kidney Disease: A Single Center-Based Cross-Sectional Study. Journal of Personalized Medicine. 2023; 13(4):577. https://doi.org/10.3390/jpm13040577
Chicago/Turabian StyleBano, Gulnaz, Mohammad Tarique Imam, Ram Bajpai, Ghada Alem, Varun Kumar Kashyap, Anwar Habib, and Abul Kalam Najmi. 2023. "Expression of Angiopoetin-Like Protein-4 and Kidney Injury Molecule-1 as Preliminary Diagnostic Markers for Diabetes-Related Kidney Disease: A Single Center-Based Cross-Sectional Study" Journal of Personalized Medicine 13, no. 4: 577. https://doi.org/10.3390/jpm13040577
APA StyleBano, G., Imam, M. T., Bajpai, R., Alem, G., Kashyap, V. K., Habib, A., & Najmi, A. K. (2023). Expression of Angiopoetin-Like Protein-4 and Kidney Injury Molecule-1 as Preliminary Diagnostic Markers for Diabetes-Related Kidney Disease: A Single Center-Based Cross-Sectional Study. Journal of Personalized Medicine, 13(4), 577. https://doi.org/10.3390/jpm13040577