Abstract
Orofacial pain (OFP) is a dental specialty that includes the diagnosis, management and treatment of disorders of the jaw, mouth, face, head and neck. Evidence-based understanding is critical in effectively treating OFPs as the pathophysiology of these conditions is multifactorial. Since OFP impacts the quality of life of the affected individuals, treating patients successfully is of the utmost significance. Despite the therapeutic choices available, treating OFP is still quite challenging, owing to inter-patient variations. The emerging trends in precision medicine could probably lead us to a paradigm shift in effectively managing the untreatable long-standing pain conditions. Precision medicine is designed based on the patient’s genetic profile to meet their needs. Several significant relationships have been discovered based on the genetics and genomics of pain in the past, and some of the notable targets are discussed in this review. The scope of this review is to discuss preclinical and clinical trials that include approaches used in targeted therapy for orofacial pain. Future developments in pain medicine should benefit from current trends in research into novel therapeutic approaches.
1. Introduction
Orofacial pain (OFP) is the term used to define pain affecting the hard and soft tissues of the face and/or oral cavity [1,2]. Pain is a stimulus modality, it is subjective and serves as a warning sign for response to damaged tissue in the body [3,4]. The prevalence of OFP is reported to be between 16.1 and 33.2%, out of which 10% is considered to contribute to chronic orofacial pain [5]. OFP can range from a straightforward intraoral pain affecting the teeth or periodontium to more extensive involvement of cranial nerves or lesions or sometimes OFP also resembles headaches [6]. The pathophysiology of these OFPs is multifactorial and is being extensively studied to understand the underlying mechanism. OFP lasting more than three months is defined as chronic OFP. Chronic pain is a disease state, that outlasts even the time of healing when associated with illness or injury. Chronic pain widely relies on a multidisciplinary approach [7]. Of paramount importance is the effective treatment of patients as OFP affects the quality of life of the affected individuals. Despite the availability of a multitude of therapeutic options, effective treatment of OFP remains quite challenging in a wide variety of cases. This could be owed to the inter-patient variations.
The emerging trends in “precision medicine” might lead us to a paradigm shift in effectively managing untreatable long-standing pain conditions. Precision medicine refers to the tailor-made optimization of therapeutics to individuals, or a particular group of patients based on their genetic or molecular profiling. In other words, it is the customization of medical management rather than “one drug fitting all”. It is occasionally also called personalized medicine or personalized care. Currently, precision medicine is rapidly evolving in the field of oncology and rare genetic diseases [8,9,10].
The heterogeneity of orofacial pain and the inter-patient variations in analgesic treatment are fundamental to focusing on individualized medicine. An example for such a scenario is that some patients may not respond to morphine therapy, however they may respond when changing to other opioids. There seems to be a linkage between the genetic makeup and analgesic response [11,12,13].
2. History of Precision Medicine
The idea of patient-centered care has existed for ages, dating back to Hippocrates (460–370 BCE), who accurately stated “It’s far more important to know what person the disease has than what disease the person has” [14]. The term personalized medicine was indeed already reported in an article by W.M. Gibson in 1971, who envisioned the family physician’s role as a scientist–physician. He precisely stated that the family physician “Within a few years will likely have available to him a computer programmed for medicine providing him with a great store of knowledge literally at his fingertips” [15].
Over the years, several terms have been used to describe individualized therapy, and in the past decade the preferred term has been “precision medicine”, shifted from personalized medicine. The National Research Council chose the word precision medicine due to concerns that that personalized medicine may be interpreted incorrectly as unique or individualized treatment for each person. Indeed, the term “precision medicine” has been used by Wasi in 1997 in the discussion of the future of genomic medicine [16]. He wrote that human genomics is fundamental for diagnosis, treatment, prognosis and prevention, it will give rise to “predictive-preventive medicine and precision medicine”. However, it was only in 2015, after Barack Obama mentioned about a “precision medicine initiative”, that it became a preferred term [17].
3. The Need for Advancement of Precision Medicine in Pain Management
Chronic pain is a global burden, the current treatments available are only selectively effective or have side effects. Orofacial pain is one of the severe pains affecting approximately 25% of the population [5,18]. The type of orofacial pain is classified based on the pathophysiology and symptoms. The classification has been published by the International Classification of Orofacial Pain (Table 1) [6]. Non-odontogenic variants of orofacial pain are difficult to diagnose and usually misdiagnosed. Hence, for appropriate diagnosis and effective management, the underlying mechanism has been researched widely in recent decades. Despite abundant treatment options prevailing, the management of orofacial pain still remains unmet in some cases.

Table 1.
Classification describing the diagnostic criteria.
Over the years, there has been tremendous progress in the field of genetics and understanding the genomics of pain. Significant links of pain have been discovered and researched in the past, however these are still under a “trial and error” state, and need further validation through animal and human studies. The current treatment is based on pharmacotherapy, and advancements in genetics have paved way for precision medicine. Precision medicine aims to make better diagnoses and improve patient management by understanding more about the human condition. The potential for developing better medications based on a greater understanding of the pathophysiology of illnesses is a topic of considerable discussion. In precision medicine, pain management uses techniques to evaluate every patient individually, determine their risk profile for experiencing exaggerated pain or the emergence of chronic pain, and then refine therapeutic approaches to target particular pathological processes underlying chronic pain. Current trends in exploring the new therapeutic avenues in pain management should aid in improving the precision medicine for pain in the future.
4. Precision Medicine Targeting Orofacial Pain
Conventional treatment involves the selection of drugs based on the cause of pain, the pain characteristics, patient history, age, gender, etc. Further, the dose is modified based on the patient response of pain relief and side effects. The existing lacunae in this kind of treatment is the failure to consider genomic criteria in the treatment strategy. Hence, there is limited efficacy in these routine pharmacotherapies, further leading to the need for precision medicine.
Precision medicine is thoroughly based on the genetic makeup of the patient, specially tailored based on the patient needs. The development of precision medicine for pain is relatively slow when compared to that of other fields. Genetic analysis together with pharmacological analysis can predict the efficacy of drugs in a patient-specific manner. Based on the genetics and genomics of orofacial pain, several important links have been identified in the past and some of the notable targets are shown in Figure 1.
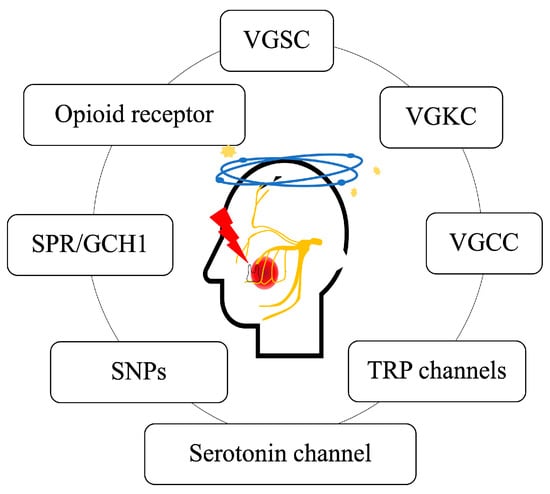
Figure 1.
Potential targets of orofacial pain. VGSC: voltage-gated sodium channel; VGKC: voltage-gated potassium channel; VGCC: voltage-gated calcium channel; TRP: transient receptor potential channels; SNP: single-nucleotide polymorphism; SPR: sepiapterin reductase; GCH1: GTP cyclohydroxylase 1.
Ion channels are classified based on what closes and opens the channel and the nature of ions passing through the pores (gates). We have discussed below the role of the various gated channels.
4.1. Voltage-Gated Sodium Channel
Voltage-gated sodium channels (VGSCs) function as hetero-multimeric proteins that consist of an α subunit and an auxiliary β subunit. There are nine distinct VGSCs subtypes: Nav1.1 to Nav1.9. The Nav1.3, Nav1.7, Nav1.8 and Nav1.9 channels have been discovered to have contributed to neuropathic pain [19]. In the ongoing research trend, sodium channels are the key targets for treating neuropathic pain. However, currently available drugs are of limited efficacy and poor tolerability. For further promising results, screening strategies to identify molecules binding to distinct subtypes would resolve the concerns.
4.2. Voltage-Gated Potassium Channel
The family of voltage-gated potassium channels (VGKC), often known as Kv channels, has 12 different members. Kv channels play a crucial role in regulating neuronal excitability by participating in action potential repolarization and damping membrane depolarization. Compared to sodium channel blockers, drugs that target potassium channels have received significantly less research [20]. A more detailed understanding is necessary on medications that alter potassium channels since they may be a potent addition to the current options for alleviating neuropathic pain. Identification of specific sensory defects and genetic profiling of patients may predict the therapeutic benefit of Kv channels [20,21].
4.3. Voltage-Gated Calcium Channel
The voltage-gated calcium channels (VGCC) are a group of ion channels found in the membrane of excitable cells, such as muscle, neurons, glial cells, etc. They are broadly classified into high-voltage-activated and low-voltage-activated channels. There are several different subunits (α1, α2δ, β1–4, and γ) [22,23]. VGCCs are well-known pain signal mediators in primary afferent neurons [24]. Even though calcium channels have been studied as a possible therapeutic target for more than 20 years, these agents are not reported yet to be effective in clinical management of pain [25].
4.4. Transient Receptor Potential Channels
Transient Receptor Potential (TRP) channels are a type of cationic channels that function as signal transducers by modifying intracellular calcium or membrane potential. The TRP channel superfamily is classified into six subfamilies: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPA (Ankyrin), TRPML (Mucolipin) and TRPP (Polycystic) [26]. These channels have been identified to have a role in a range of pain modalities, including inflammatory pain, neuropathic pain, visceral pain and pain related to specific pathological disorders, such as cancer or migraine [27]. TRPV1 and TRPA1 have been explored due to their antinociceptive properties and were found to be beneficial in relieving cancer-derived pain [28]. TRP channels are improving their importance in different areas, becoming suitable as promising candidates for precision medicine.
4.5. Serotonin-Gated Ion Channels
Serotonin-gated ion channels are opened directly by the neurotransmitter acetylcholine, glycine or glutamate. Serotonin directly activates 5-hydroxytryptamine (5-HT3) channels, which are nonselective cationic ion channels [29]. The role of 5-HT in neuropathic pain has been reported in several studies but the specific receptors have not been studied [30].
4.6. Single Nucleotide Polymorphisms
Single nucleotide polymorphisms (SNP) are the most common type of genetic variation among people. They aid in predicting a person’s susceptibility to specific medications, tolerance to environmental factors such as toxins, and risk of contracting diseases. SNPs can also be used to monitor how disease-related genetic variations are passed down across families. Techniques based on gene therapy and the regulation of epigenetic alterations broaden future options to enhance the understanding of pain mechanisms and their treatment by novel medicines, opening doors to precision medicine [31]. Some recent promising SNPs ideal for targeted therapy have been discussed below.
- Solute carrier family 17 member 9 (SLC17A9) and purinergic receptor P2Y12 (P2RY12) have been reported to be associated with neuropathic pain. Further studies are needed for understanding the detailed mechanisms of pain signal transduction in humans [32].
- Catechol-O-methyltransferase [COMT] is a metabolic enzyme found primarily in postsynaptic neurons and glial cells. Previous reports have shown the participation of COMT in the regulation of neurotransmitters such as dopamine, noradrenaline and adrenaline related to pain [33,34,35,36]. The literature shows evidence of the association of COMT with pain modulation in temporomandibular disorders, which would aid in progressing toward precision medicine.
4.7. Sepiapterin Reductase/GTP Cyclohydrolase 1
Sepiapterin reductase (SPR) is a prime enzyme in the synthesis of tetrahydrobiopterin (BH4). GTP cyclohydroxylase 1 catalyzes the initial and rate-limiting steps in the synthetic pathway of BH4. BH4 plays a major role in cardiovascular function, mood, inflammation and neurotransmission. An association between BH4 increase and axonal injury has been reported previously [37]. Further, the association between GCH1 and orofacial pain is yet to be explored. SPR is emerging as a novel therapeutic target for treating neuropathic pain [38].
4.8. Opioid Receptors
Opioid receptors are a group of inhibitory G-protein-coupled receptors with opioids as ligands. There are three types of opioid receptors, designated as mu, delta and kappa. Opioid analgesics are well established for their use in analgesia, including their abusive risks [39]. Hopefully, the recent knowledge of opioid analgesic drugs may aid in new drug development for newer therapeutic approaches in precision medicine [40].
The preclinical and clinical trials reported in recent years on each potential target, specifically on the orofacial region, have been summarized in Table 2 and Table 3.

Table 2.
Potential targets for orofacial pain—preclinical trials.

Table 3.
Potential targets for orofacial pain—clinical trials.
5. Challenges
Translational issues in precision medicine, particularly in neuropathic pain, are one of the main challenges. New drugs fail to be implemented in clinical practice due to the translational gap from traditional animal models to clinical application. Although there has been a persistent desire to focus on precision medicine as a method of treating the condition’s underlying cause, it is exceptionally rare for a disorder to originate from just a single gene mutation [63]. In pain, more frequently, mutations are characterized in sodium channels. Despite several novel blockers targeting these sodium channels, the translation of these laboratory results to practical day-to-day use in humans is not generally available [64]. The major reason for the translational gap is failure in clinical studies due to inappropriate endpoint selection for validation [63]. For example, 5HT3-antagonist when used for neuropathy patients showed positive results when ongoing pain [65] was evaluated, whereas it showed negative results when dynamic pain was evaluated [66]. Hence, it is necessary to pick an ideal endpoint for validation of the efficacy in patients.
6. Multifaced Perspectives
There are various aspects to be considered for effective precision medicine. Figure 2 shows a schematic representation of the multifaced perspectives.
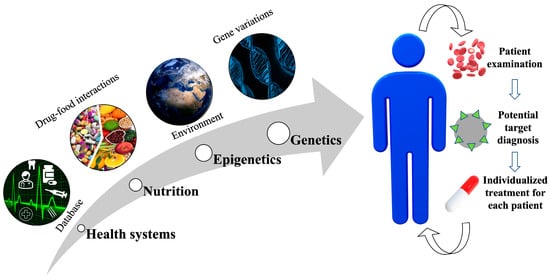
Figure 2.
Scheme for various perspectives for effective precision medicine. Various factors include databases by health systems, nutrition, epigenetics and genetics. A multifaced approach would aid in the diagnosis of the potential target, leading to an effective individualized treatment for each patient.
6.1. Health Systems
The database systems are essential for clinical evaluation, decisions, monitoring and to improve efficiency. Large-scale data access is essential for determining the suitable precision medicine for an individual [67].
6.2. Nutrition
The drug–food interactions play a vital factor as they may often result in malabsorption of the medicine. Hence, the diet of the individual is to be considered in precision medicine [68].
6.3. Epigenetics
Depression, stress, nerve damage and alcohol intake all cause epigenetic alterations that both directly and indirectly affect pain and the effects of drugs [69,70,71,72].
6.4. Genetics
The application of genetics in clinical decision making has existed for many years. For instance, keeping track of a patient’s family history provides insights into heritable patterns that indicate disease susceptibility or perhaps help choose the appropriate treatment regimen. We must investigate how common and rare genetic variations prevalent across many regions of the world affect health and treatment response if personalized medicine is to be credible and applicable to the global communities [73].
7. Future Directions
Precision medicine is of great ongoing interest. Immense research has been unraveling to tailor therapies to benefit the individual patient with maximum efficacy and reduced adverse effects. However, we must understand how common and rare genetic variations present in various geographic locations affect health and drug response if personalized medicine is to be meaningful and applicable to the population of the entire planet. For the implementation of precision medicine in clinical practice, there is a need for a better understanding of these potential targets’ (ion channels and TRP channels) role in pain pathogenesis [45,48]. The association of pain-related single-nucleotide polymorphism with the clinical phenotypes is essential for targeted therapy implementation [32,50]. The importance of GTP cyclohydrolase 1, sepiapterin reductase and catechol-O-methyltransferase has been extensively established in the context of pain mechanisms, although clinical trials in orofacial research are still required [37,38,74]. Despite an array of pharmacological drugs, sodium channel modulators are not yet pharmacotherapeutic for neuropathic pain; future research should consider the heterogeneity in pain phenotypes [55,56,75]. Furthermore, genetic testing needs to be reasonably priced for precision medicine to be effectively implemented. Large-scale studies on inherited pain would provide better perspectives in a broader population. Treatment strategies to target specific channel by selective blocking would aid in enhanced therapeutics in pain with reduced side effects. The exploration of drugs with new mechanisms based on genomic analysis would aid in more effective treatment. Enhanced research in pain genetics can widen the scope for individualized treatment for some rather than all.
8. Conclusions
Precision medicine tendencies might create a turning point in treating chronic pain illnesses, currently incurable in some patients. Precision medicine for orofacial pain is still in the preliminary stage despite several novel and potential targets being reported in the past. Overcoming the translational gaps and additionally considering standard protocols for screening would be promising for the success in precision medicine of the orofacial region in the near future.
Author Contributions
Conceptualization, S.R. and Y.M.; writing original draft preparation, S.R. and Y.M.; writing review and editing, S.R., D.I., K.O. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 18H02992, 22H03281).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zakrzewska, J.M.; Hamlyn, P.J. Facial pain. In Epidemiology of Pain Seattle; IASP Press: Washington, DC, USA, 1999; pp. 171–202. [Google Scholar]
- Sessle, B. Chronic Orofacial Pain: Models, Mechanisms, and Genetic and Related Environmental Influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef] [PubMed]
- Pak, D.J.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Chronification of pain: Mechanisms, current understanding, and clinical implications. Curr. Pain Headache Rep. 2018, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Rotpenpian, N.; Yakkaphan, P. Review of literatures: Physiology of orofacial pain in dentistry. Eneuro 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Ananthan, S.; Benoliel, R. Chronic orofacial pain. J. Neural Transm. 2020, 127, 575–588. [Google Scholar] [CrossRef]
- Benoliel, R.; May, A.; Svensson, P.; Pigg, M.; Alstergren, P.; Baad-Hansen, L. International Classification of Orofacial Pain, (ICOP). Cephalalgia 2020, 40, 129–221. [Google Scholar]
- Ghurye, S.; McMillan, R. Orofacial pain–an update on diagnosis and management. Br. Dent. J. 2017, 223, 639–647. [Google Scholar] [CrossRef]
- Li, C.C.; Shen, Z.; Bavarian, R.; Yang, F.; Bhattacharya, A. Oral cancer: Genetics and the role of precision medicine. Dent. Clin. N. Am. 2018, 62, 29–46. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Gil, Z.; Billan, S. Precision medicine in head and neck cancer. Drug Resist. Updates 2018, 40, 13–16. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; El Achkar, C.M.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- Branford, R.; Droney, J.; Ross, J.R. Opioid genetics: The key to personalized pain control? Clin. Genet. 2012, 82, 301–310. [Google Scholar] [CrossRef]
- Klepstad, P.; Rakvåg, T.T.; Kaasa, S.; Holthe, M.; Dale, O.; Borchgrevink, P.C.; Baar, C.; Vikan, T.; Krokan, H.E.; Skorpen, F. The 118 A> G polymorphism in the human µ-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol. Scand. 2004, 48, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sia, A.T.; Lim, Y.; Lim, E.C.; Goh, R.W.; Law, H.Y.; Landau, R.; Teo, Y.Y.; Tan, E.C. A118G single nucleotide polymorphism of human μ-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 2008, 109, 520–526. [Google Scholar] [CrossRef]
- Gibson, W.M. Can personalized medicine survive? Can. Fam. Physician 1971, 17, 29–88. [Google Scholar] [PubMed]
- Litman, T. Personalized medicine—Concepts, technologies, and applications in inflammatory skin diseases. Apmis 2019, 127, 386–424. [Google Scholar] [CrossRef] [PubMed]
- Wasi, P. Human genomics: Implications for health. Southeast Asian J. Trop. Med. Public Health 1997, 28, 19–24. [Google Scholar] [PubMed]
- Peck, R.W. Precision medicine is not just genomics: The right dose for every patient. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 105–122. [Google Scholar] [CrossRef]
- De Rossi, S.S. Orofacial pain: A primer. Dent. Clin. N. Am. 2013, 57, 383–392. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 6, 663–678. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Jackson, M.; Gu, S.L.; Fiala, K.; Gu, J. Neuropathic pain and Kv7 voltage-gated potassium channels: The potential role of Kv7 activators in the treatment of neuropathic pain. Mol. Pain 2019, 15, 1744806919864256. [Google Scholar] [CrossRef]
- Smith, P.A. K+ channels in primary afferents and their role in nerve injury-induced pain. Front. Cell. Neurosci. 2020, 14, 566418. [Google Scholar] [CrossRef]
- Dolphin, A.C. Beta subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003, 35, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Tsubota, M.; Kawabata, A. Involvement of voltage-gated calcium channels in inflammation and inflammatory Pain. Biol. Pharm. Bull. 2018, 41, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Lewis, R.J.; Todorovic, S.M.; Arneric, S.P.; Snutch, T.P. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res. Rev. 2009, 60, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Alles, S.R.; Smith, P.A. Peripheral voltage-gated cation channels in neuropathic pain and their potential as therapeutic targets. Front. Pain Res. 2021, 2, 106. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar]
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in pain sensation. Front. Physiol. 2017, 8, 392. [Google Scholar] [CrossRef]
- Duitama, M.; Moreno, Y.; Santander, S.P.; Casas, Z.; Sutachan, J.J.; Torres, Y.P.; Albarracín, S.L. TRP Channels as Molecular Targets to Relieve Cancer Pain. Biomolecules 2021, 12, 1. [Google Scholar] [CrossRef]
- Panicker, S.; Cruz, H.; Arrabit, C.; Slesinger, P.A. Evidence for a centrally located gate in the pore of a serotonin-gated ion channel. J. Neurosci. 2002, 22, 1629–1639. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yao, X.X.; Gao, S.H.; Li, R.; Li, B.J.; Yang, W.; Cui, R.J. Role of 5-HT receptors in neuropathic pain: Potential therapeutic implications. Pharmacol. Res. 2020, 159, 104949. [Google Scholar] [CrossRef]
- Peiró, A.M. Pharmacogenetics in Pain Treatment. Adv. Pharmacol. 2018, 83, 247–273. [Google Scholar]
- Soeda, M.; Ohka, S.; Nishizawa, D.; Hasegawa, J.; Nakayama, K.; Ebata, Y.; Fukuda, K.I.; Ikeda, K. Single-nucleotide polymorphisms of the SLC17A9 and P2RY12 genes are significantly associated with phantom tooth pain. Mol. Pain 2022, 18, 17448069221089592. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Omar, H.A.; Hersi, F.; Nunes, A.C.F.; Noreddin, A.M. The impact of catechol-O-methyl transferase knockdown on the cell proliferation of hormone-responsive cancers. Mol. Cell. Endocrinol. 2019, 488, 79–88. [Google Scholar] [CrossRef] [PubMed]
- García-Carmona, J.A.; Georgiou, P.; Zanos, P.; Bailey, A.; Laorden, M.L. Methamphetamine withdrawal induces activation of CRF neurons in the brain stress system in parallel with an increased activity of cardiac sympathetic pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 423–434. [Google Scholar] [CrossRef]
- Slade, G.D.; Diatchenko, L.; Ohrbach, R.; Maixner, W. Orthodontic Treatment, Genetic Factors, and Risk of Temporomandibular Disorder. Semin. Orthod. 2008, 14, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, Z.; Chen, S.; Yao, F.; Liu, J.; Ouyang, Z.; Liao, Z. Mechanism of Catechol-O-methyltransferase Regulating Orofacial Pain Induced by Tooth Movement. BioMed Res. Int. 2021, 2021, 4229491. [Google Scholar] [CrossRef]
- Latremoliere, A.; Latini, A.; Andrews, N.; Cronin, S.J.; Fujita, M.; Gorska, K.; Hovius, R.; Romero, C.; Chuaiphichai, S.; Painter, M.; et al. Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron 2015, 86, 1393–1406. [Google Scholar] [CrossRef]
- Raman, S.; Waskitho, A.; Raju, R.; Iwasa, T.; Ikutame, D.; Okura, K.; Oshima, M.; Matsuka, Y. Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis. Int. J. Mol. Sci. 2022, 23, 5878. [Google Scholar] [CrossRef]
- Corbett, A.D.; Henderson, G.; McKnight, A.T.; Paterson, S.J. 75 years of opioid research: The exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006, 147, S153–S162. [Google Scholar] [CrossRef]
- Somogyi, A.A.; Musolino, S.T.; Barratt, D.T. New pharmacological perspectives and therapeutic options for opioids: Differences matter. Anaesth. Intensive Care 2022, 50, 127–140. [Google Scholar] [CrossRef]
- Eriksson, J.; Jablonski, A.; Persson, A.K.; Hao, J.X.; Kouya, P.F.; Wiesenfeld-Hallin, Z.; Xu, X.J.; Fried, K. Behavioral changes and trigeminal ganglion sodium channel regulation in an orofacial neuropathicpain model. Pain 2005, 119, 82–94. [Google Scholar] [CrossRef]
- Luiz, A.P.; Kopach, O.; Santana-Varela, S.; Wood, J.N. The role of Nav1.9 channel in the development of neuropathic orofacial pain associated with trigeminal neuralgia. Mol. Pain 2015, 25, 72. [Google Scholar]
- Madrid, R.; de la Peña, E.; Donovan-Rodriguez, T.; Belmonte, C.; Viana, F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J. Neurosci. 2009, 29, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Ling, J.; Chang, Y.-T.; Erol, F.; Viatchenko-Karpinski, V.; Yamada, A.; Noguchi, K.; Gu, J.G. Kv4.3 channel dysfunction contributes to trigeminal neuropathic pain manifested with orofacial cold hypersensitivity in rats. J. Neurosci. 2021, 41, 2091–2105. [Google Scholar] [CrossRef] [PubMed]
- Montera, M.; Goins, A.; Cmarko, L.; Weiss, N.; Westlund, K.N.; Alles, S.R.A. Trigeminal neuropathic pain is alleviated by inhibition of Cav3.3 T-type calcium channels in mice. Channels 2021, 15, 31–37. [Google Scholar] [CrossRef]
- Gambeta, E.; Gandini, M.A.; Souza, I.A.; Zamponi, G.W. Ca V 3.2 calcium channels contribute to trigeminal neuralgia. Pain 2022, 163, 2315–2325. [Google Scholar] [CrossRef]
- DeMartini, C.; Greco, R.; Zanaboni, A.M.; Francesconi, O.; Nativi, C.; Tassorelli, C.; Deseure, K. Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model. Int. J. Mol. Sci. 2018, 19, 3320. [Google Scholar] [CrossRef]
- Santos, S.A.A.R.; Damasceno, M.B.M.V.; Magalhães, F.E.A.; Sessle, B.J.; Oliveira, B.A.; Batista, F.L.A.; Vieira-Neto, A.E.; Campos, A.R. Transient receptor potential channel involvement in antinociceptive effect of citral in orofacial acute and chronic pain models. EXCLI J. 2022, 21, 869–887. [Google Scholar]
- Cornelison, L.E.; Woodman, S.E.; Durham, P.L. 5-HT3/7 and GABAB receptors mediate inhibition of trigeminal nociception by dietary supplementation of grape seed extract. Nutr. Neurosci. 2022, 25, 1565–1576. [Google Scholar] [CrossRef]
- Katagiri, A.; Shinoda, M.; Honda, K.; Toyofuku, A.; Sessle, B.J.; Iwata, K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol. Pain 2012, 8, 23–36. [Google Scholar] [CrossRef]
- Nũnéz, S.; Lee, J.S.; Zhang, Y.; Bai, G.; Ro, J.Y. Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain. J. Neurosci. 2007, 146, 1346–1354. [Google Scholar] [CrossRef]
- Erfanparast, A.; Tamaddonfard, E.; Seyedin, S. Involvement of central opiate receptors in modulation of centrally administered oxytocin-induced antinociception. Iran. J. Basic Med. Sci. 2018, 12, 1275. [Google Scholar]
- Saloman, J.L.; Niu, K.Y.; Ro, J.Y. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. J. Neurosci. 2011, 190, 379–385. [Google Scholar] [CrossRef]
- Siqueira, S.R.; Alves, B.; Malpartida, H.M.; Teixeira, M.J.; Siqueira, J.T. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience 2009, 164, 573–577. [Google Scholar] [CrossRef]
- Zakrzewska, J.M.; Palmer, J.; Morisset, V.; Giblin, G.M.; Obermann, M.; Ettlin, D.A.; Cruccu, G.; Bendtsen, L.; Estacion, M.; Derjean, D.; et al. Safety and efficacy of a nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: A double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 2017, 16, 291–300. [Google Scholar] [CrossRef]
- Kotecha, M.; Cheshire, W.P.; Finnigan, H.; Giblin, K.; Naik, H.; Palmer, J.; Tate, S.; Zakrzewska, J.M. Design of phase 3 studies evaluating vixotrigine for treatment of trigeminal neuralgia. J. Pain Res. 2020, 13, 1601–1609. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.M.; Hansen, J.M.; Guo, S.; Olesen, J.; Ashina, M. Opening of ATP-Sensitive Potassium Channels Causes Migraine Attacks: A New Target for the Treatment of Migraine. Brain 2019, 142, 2644–2654. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.M.; Ghanizada, H.; Hansen, J.M.; Aghazadeh, S.; Skovgaard, L.T.; Olesen, J.; Ashina, M. Extracranial activation of ATP-sensitive potassium channels induces vasodilation without nociceptive effects. Cephalalgia 2019, 39, 1789–1797. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.M.; Ghanizada, H.; Nielsen, C.A.W.; Skandarioon, C.; Snellman, J.; Lopez Lopez, C.; Hansen, J.M.; Ashina, M. Opening of BKCa channels alters cerebral hemodynamic and causes headache in healthy volunteers. Cephalalgia 2020, 40, 1145–1154. [Google Scholar] [CrossRef]
- Christidis, N.; Kang, I.; Cairns, B.E.; Kumar, U.; Dong, X.; Rosén, A.; Kopp, S.; Ernberg, M. Expression of 5-HT3 receptors and TTX resistant sodium channels (Na(V)1.8) on muscle nerve fibers in pain-free humans and patients with chronic myofascial temporomandibular disorders. J. Headache Pain 2014, 15, 63. [Google Scholar] [CrossRef]
- Slade, G.D.; Fillingim, R.B.; Ohrbach, R.; Hadgraft, H.; Willis, J.; Arbes Jr, S.J.; Tchivileva, I.E. COMT genotype and efficacy of propranolol for TMD pain: A randomized trial. J. Dent. Res. 2021, 100, 163–170. [Google Scholar] [CrossRef]
- Kleinert, R.; Lange, C.; Steup, A.; Black, P.; Goldberg, J.; Desjardins, P. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: The results of a randomized, double-blind, placebo-controlled study. Anesth. Analg. 2008, 107, 2048–2055. [Google Scholar] [CrossRef]
- Dickenson, A.H.; Patel, R. Translational issues in precision medicine in neuropathic pain. Can. J. Pain 2020, 4, 30–38. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- McCleane, G.J.; Suzuki, R.; Dickenson, A.H. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesth. Analg. 2003, 97, 1474–1478. [Google Scholar] [CrossRef]
- Tuveson, B.; Leffler, A.S.; Hansson, P. Ondansetron, a 5HT3-antagonist, does not alter dynamic mechanical allodynia or spontaneous ongoing pain in peripheral neuropathy. Clin. J. Pain 2011, 27, 323–329. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Phillips, K.A. Precision medicine: From science to value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]
- Franklin, T.B.; Mansuy, I.M. Epigenetic inheritance in mammals: Evidence for the impact of adverse environmental effects. Neurobiol. Dis. 2010, 39, 61–65. [Google Scholar] [CrossRef]
- Darnaudéry, M.; Maccari, S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 2008, 57, 571–585. [Google Scholar] [CrossRef]
- Ponomarev, I.; Wang, S.; Zhang, L.; Harris, R.A.; Mayfield, R.D. Gene Coexpression Networks in Human Brain Identify Epigenetic Modifications in Alcohol Dependence. J. Neurosci. 2012, 32, 1884–1897. [Google Scholar] [CrossRef]
- Ungerer, M.; Knezovich, J.; Ramsay, M. In utero alcohol exposure, epigenetic changes, and their consequences. Alcohol Res. Curr. Rev. 2013, 35, 37–46. [Google Scholar]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, A. Why are sodium channel modulators not yet pharmacotherapeutic trailblazers for neuropathic pain? Expert Opin. Pharmacother. 2021, 22, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).