How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test
Abstract
1. Introduction
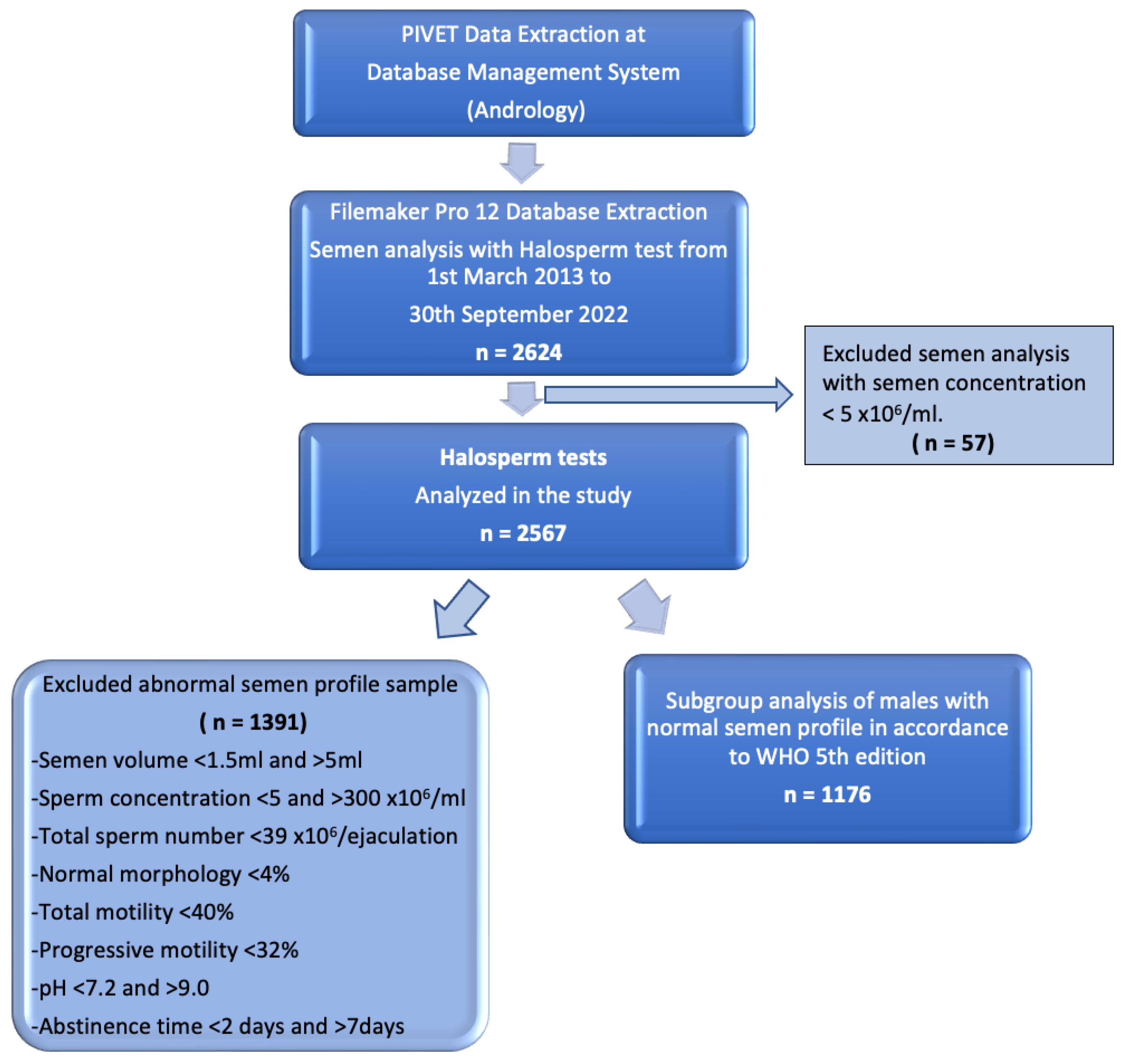
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Procedures
2.2.1. Semen Analysis
- Rapid progressive motility (A-grade sperm)—movement approximately equal to five head lengths per second.
- Slow or sluggish progressive motility (B-grade sperm).
- Non-progressive motility (C-grade sperm).
- Non-motile (D-grade sperm).
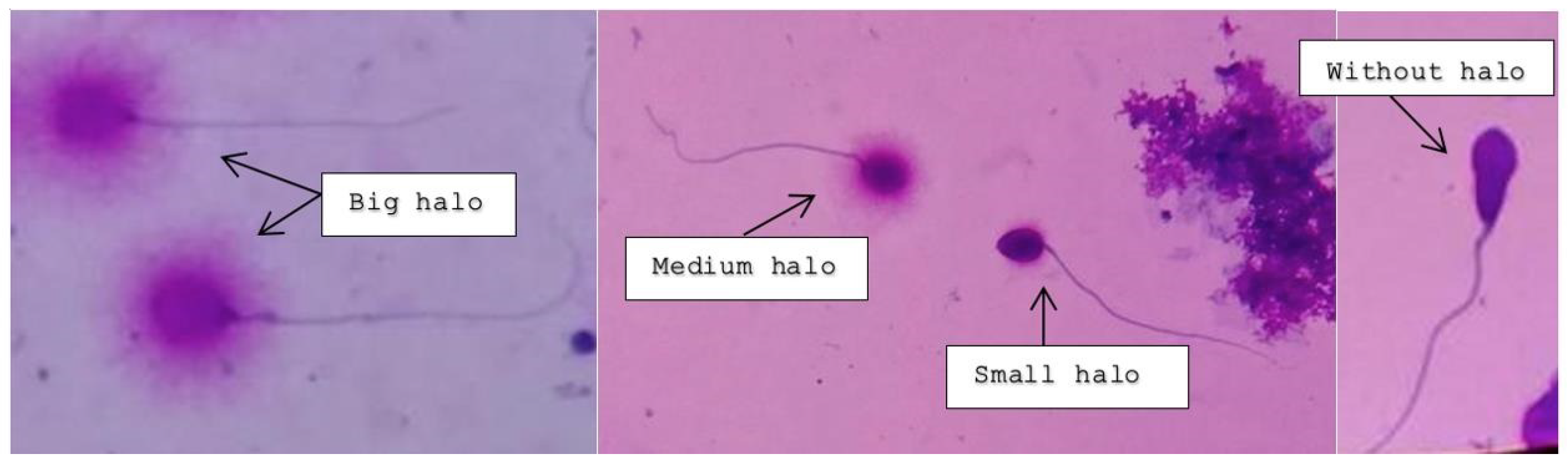
2.2.2. Sperm Chromatin Dispersion Test—Halosperm® G2
Principle of the Halosperm Test
Procedure
2.2.3. Assessment under Light Microscopy
2.3. DNA Fragmentation Index (DFI) Calculation
- 0–4.9%: Excellent sperm DNA Integrity
- 5–14.9%: Adequate sperm DNA Integrity
- 15–29.9%: Elevated levels of DNA fragmentation. This may impact upon fertility potential; ICSI recommended.
- ≥30%: Severely elevated levels of DNA fragmentation. This is very likely to impact upon fertility potential. ICSI required.
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlations between Routine Semen Parameters and Sperm DFI
3.3. Multivariate Logistic Regression Analysis of Semen Parameters Associated with Sperm DFI
3.4. Subgroup Analysis of Normal Semen Parameters Associated with Sperm DFI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Wiweko, B.; Utami, P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin. Androl. 2017, 27, 1. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef]
- Hammen, R. Studies on impaired fertility in man with special reference to the male. Acta Obstet. Gynecol. Scand. 1944, 24, 1–206. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Yang, H.; Li, G.; Jin, H.; Guo, Y.; Sun, Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl. Androl. Urol. 2019, 8, 356–365. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Horta, F.; Catt, S.; Ramachandran, P.; Vollenhoven, B.; Temple-Smith, P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum. Reprod. 2020, 35, 529–544. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, T.A.T.; Nguyen, H.T.T.; Nguyen, T.T.T.; Nguyen, V.T.; Le, D.D.; Nguyen, V.Q.H.; Cao, N.T. Does sperm DNA fragmentation correlate with semen parameters? Reprod. Med. Biol. 2019, 18, 390–396. [Google Scholar] [CrossRef]
- Lewis, S.E. Should sperm DNA fragmentation testing be included in the male infertility work-up? Reprod. Biomed. Online 2015, 31, 134–137. [Google Scholar] [CrossRef]
- Lopez, G.; Lafuente, R.; Checa, M.A.; Carreras, R.; Brassesco, M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J. Androl. 2013, 15, 790–794. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Zini, A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med. 2011, 57, 78–85. [Google Scholar] [CrossRef]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef]
- Yovich, S.; Ottolini, C.; Stanger, J.D.; Yovich, J.L. Sperm Chromatin Structure Assay: Population profile and Correlation with Semen parameters and Sperm Function Test. ANZJOG 2007, 47, A34. [Google Scholar]
- Layali, I.; Tahmasbpour, E.; Joulaei, M.; Jorsaraei, S.G.; Farzanegi, P. Total antioxidant capacity and lipid peroxidation in semen of patient with hyperviscosity. Cell J. 2015, 16, 554–559. [Google Scholar] [CrossRef]
- Ohl, D.A.; Quallich, S.A.; Sonksen, J.; Brackett, N.L.; Lynne, C.M. Anejaculation and retrograde ejaculation. Urol. Clin. N. Am. 2008, 35, 211–220. [Google Scholar] [CrossRef]
- Ferrigno, A.; Ruvolo, G.; Capra, G.; Serra, N.; Bosco, L. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J. Assist. Reprod. Genet. 2021, 38, 979–986. [Google Scholar] [CrossRef]
- Yovich, J.M.; Edirisinghe, W.R.; Yovich, J.L. Use of the acrosome reaction to ionophore challenge test in managing patients in an assisted reproduction program: A prospective, double-blind, randomized controlled study. Fertil. Steril. 1994, 61, 902–910. [Google Scholar] [CrossRef]
- Cummins, J.M.; Pember, S.M.; Jequier, A.M.; Yovich, J.L.; Hartmann, P.E. A test of the human sperm acrosome reaction following ionophore challenge. Relationship to fertility and other seminal parameters. J. Androl. 1991, 12, 98–103. [Google Scholar]
- Li, M.W.; Lloyd, K.C.K. DNA fragmentation index (DFI) as a measure of sperm quality and fertility in mice. Sci. Rep. 2020, 10, 3833. [Google Scholar] [CrossRef]
- Stanger, J.D.; Vo, L.; Yovich, J.L.; Almahbobi, G. Hypo-osmotic swelling test identifies individual spermatozoa with minimal DNA fragmentation. Reprod. Biomed. Online 2010, 21, 474–484. [Google Scholar] [CrossRef]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosalvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef]
- Oleszczuk, K.; Augustinsson, L.; Bayat, N.; Giwercman, A.; Bungum, M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013, 1, 357–360. [Google Scholar] [CrossRef]
- Simon, L.; Proutski, I.; Stevenson, M.; Jennings, D.; McManus, J.; Lutton, D.; Lewis, S.E. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod. Biomed. Online 2013, 26, 68–78. [Google Scholar] [CrossRef]
- Cohen-Bacrie, P.; Belloc, S.; Menezo, Y.J.; Clement, P.; Hamidi, J.; Benkhalifa, M. Correlation between DNA damage and sperm parameters: A prospective study of 1633 patients. Fertil. Steril. 2009, 91, 1801–1805. [Google Scholar] [CrossRef]
- Lu, R.; Chen, X.; Yu, W.; Jiang, F.; Zhou, X.; Xu, Y.; Wang, F. Analysis of age-associated alternation of SCSA sperm DNA fragmentation index and semen characteristics of 1790 subfertile males in China. J. Clin. Lab. Anal. 2020, 34, e23548. [Google Scholar] [CrossRef]
- Hasanzadeh Keshteli, S.; Farsi, M.M.; Khafri, S. Should We Perform Semen Analysis, DNA Fragmentation, and Hypo-osmotic Swelling Tests together? Int. J. Mol. Cell. Med. 2016, 5, 246–254. [Google Scholar]
- Abad, C.; Amengual, M.J.; Gosalvez, J.; Coward, K.; Hannaoui, N.; Benet, J.; Garcia-Peiro, A.; Prats, J. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 2013, 45, 211–216. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef]
- Noegroho, B.S.; Siregar, S.; Tampubolon, K.A.G. Antioxidant Supplementation on Sperm DNA Fragmentation and Sperm Parameters: A Systematic Review and Meta-Analysis. Turk. J. Urol. 2022, 48, 375–384. [Google Scholar] [CrossRef]
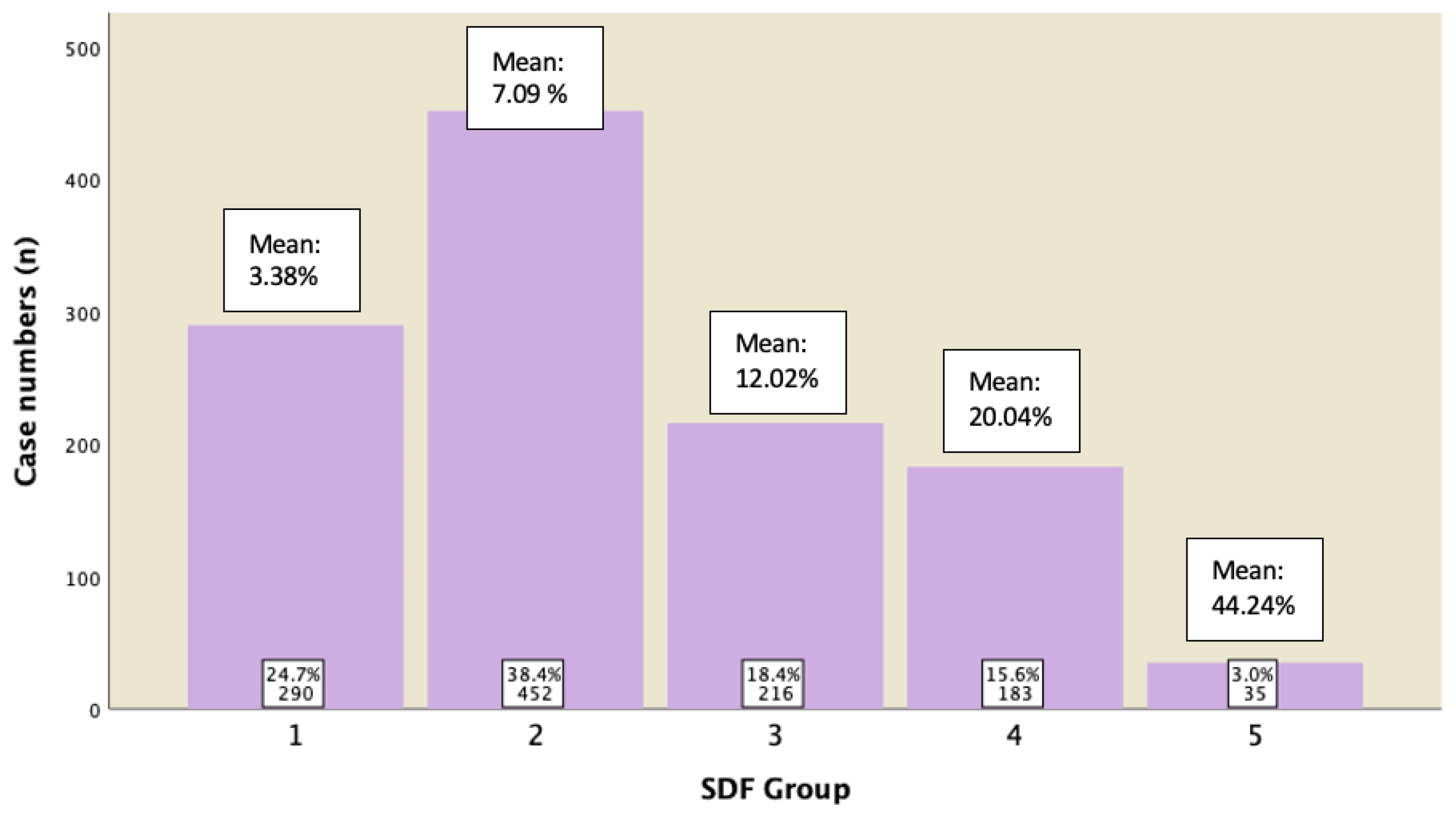
| Variable | 1 (n = 548) Mean ± SD (95% CI) | 2 (n = 933) Mean ± SD (95% CI) | SDF Group 3 (n = 477) Mean ± SD (95% CI) | 4 (n = 470) Mean ± SD (95% CI) | 5 (n = 139) Mean ± SD (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Sperm concentration (106/mL) | 66.31 ± 44.80 (62.55 − 70.07) | 68.35 ± 52.65 (64.97 − 71.73) | 69.45 ± 55.08 (64.50 − 74.41) | 64.21 ± 50.59 (59.62 − 68.79) | 66.98 ± 60.53 (56.82 − 77.13) | 0.540 |
| Normal morphology (%) | 5.47 ± 3.00 (5.22 − 5.72) | 5.40 ± 3.26 (5.19 − 5.61) | 5.50 ± 3.11 (5.22 − 5.78) | 5.01 ± 3.26 (4.72 − 5.31) | 4.36 ± 3.26 (3.81 − 4.91) | <0.001 |
| Head defects (%) | 81.22 ± 9.18 (80.45 − 81.99) | 81.02 ±10.69% (80.33 − 81.70) | 82.65 ± 35.05 (79.49 − 85.80) | 81.00 ± 13.01 (79.82 − 82.18) | 82.51 ± 8.31 (81.12 − 83.90) | 0.473 |
| Midpiece defects (%) | 9.60 ± 4.70 (9.21 − 10.00) | 9.40 ± 4.92% (9.08 − 9.72) | 9.06 ± 5.91 (8.52 − 9.59) | 8.77 ± 5.40 (8.28 − 9.26) | 8.74 ± 5.55 (7.81 − 9.67) | 0.053 |
| Tail defects (%) | 3.15 ± 4.60 (2.77 − 3.54) | 3.13 ± 3.01% (2.94 − 3.33) | 3.43 ± 3.81 (3.09 − 3.78) | 3.35 ± 3.41 (3.04 − 3.66) | 4.38 ± 6.63 (3.27 − 5.49) | 0.007 |
| Total motility (%) | 66.37 ± 12.33 (65.34 − 67.41) | 65.43 ± 12.23% (64.64 − 66.21) | 64.60 ± 12.45 (63.48 − 65.72) | 60.97 ± 13.92 (59.71 − 62.23) | 52.32 ± 17.54 (49.38 − 55.26) | <0.0001 |
| Progressive motility (%) | 61.54 ± 12.93 (60.46 − 62.63) | 60.32 ± 12.81% (59.49 − 61.14) | 58.94 ± 12.91 (57.78 − 60.10) | 55.38 ± 14.11 (54.10 − 56.65) | 46.42 ± 17.36 (43.51 − 49.33) | <0.0001 |
| Grade A motility (%) | 39.62 ± 14.55 (38.40 − 40.84) | 36.11 ± 15.65% (35.10 − 37.12) | 34.39 ± 14.77 (33.06 − 35.72) | 29.43 ± 14.68 (28.10 − 30.76) | 23.84 ± 14.39 (21.43 − 26.25) | <0.0001 |
| Grade B motility (%) | 21.92 ± 10.72 (21.02 − 22.82) | 24.21 ± 13.01% (23.37 − 25.04) | 24.55 ± 12.24 (23.44 − 25.65) | 25.95 ± 13.36 (24.74 − 27.16) | 22.58 ± 13.12 (20.37 − 24.78) | <0.0001 |
| Grade C motility (%) | 4.83 ± 4.63 (4.44 − 5.22) | 5.11 ± 4.04% (4.85 − 5.37) | 5.66 ± 4.61 (5.24 − 6.07) | 5.59 ± 4.17 (5.21 − 5.97) | 5.90 ± 5.48 (4.98 − 6.82) | 0.003 |
| Grade D motility (%) | 33.62 ± 12.35 (32.58 − 34.65) | 34.22 ± 11.83% (33.46 − 4.98) | 34.97 ± 11.94 (33.90 − 36.04) | 38.60 ± 13.58 (37.37 − 39.83) | 47.65 ± 17.56 (44.71 − 50.60) | <0.0001 |
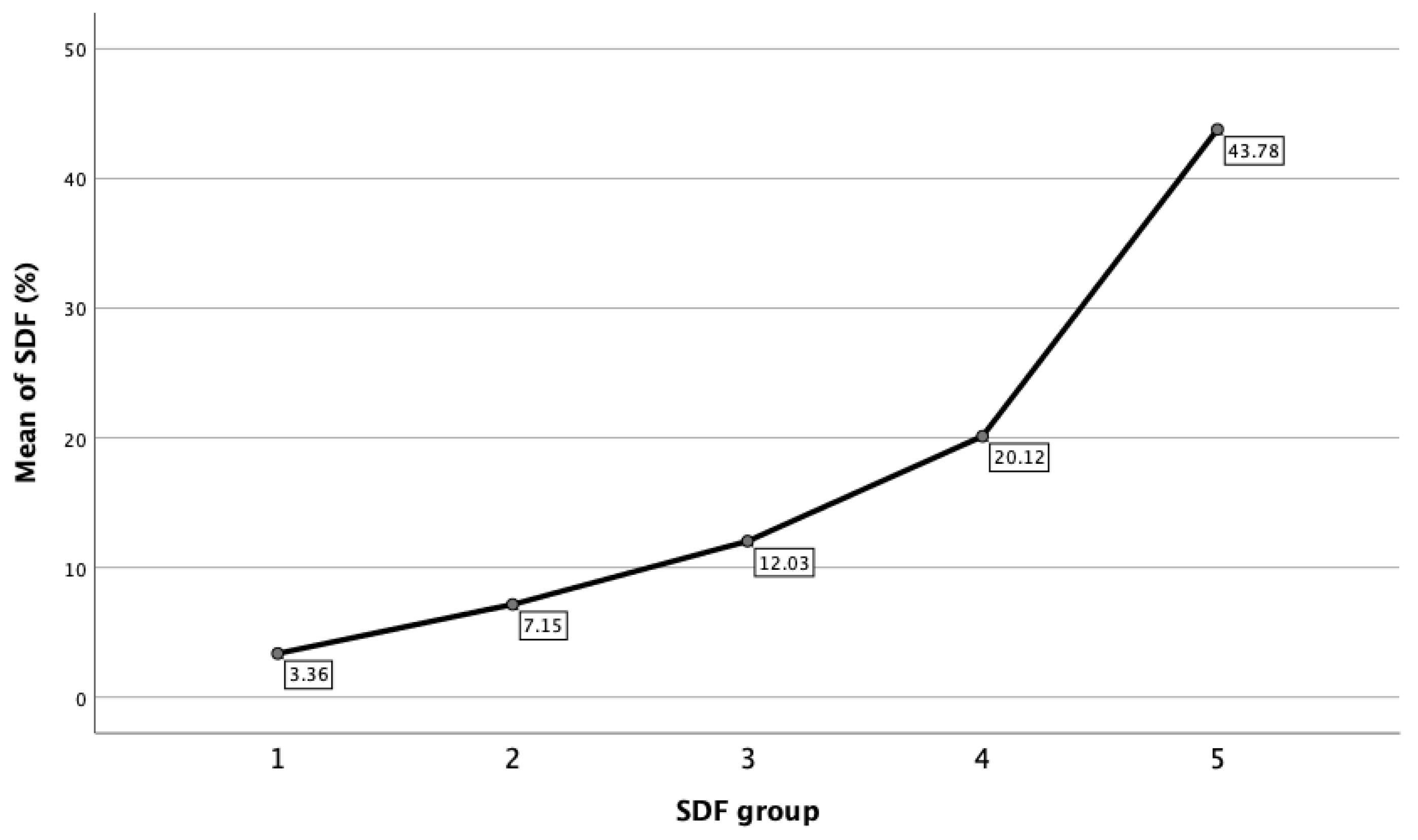
| DFI (%) | 3.36 ± 1.16 (3.26 − 3.46) | 7.15 ± 1.38 (7.06 − 7.24) | 12.03 ± 1.37 (11.91 − 12.15) | 20.12 ± 4.04 (19.76 − 20.49) | 43.78 ± 14.36 (41.38 − 46.19) | <0.0001 |
| Age (years) | 35.48 ± 6.06 (34.97 − 35.99) | 36.30 ± 6.38 (35.89 − 36.71) | 36.25 ± 6.06 (35.71 − 6.80) | 36.95 ± 6.78 (36.33 − 37.56) | 38.12 ± 7.30 (36.89 − 39.34) | <0.0001 |
| Abstinence time (days) | 3.70 ± 3.64 (3.40 − 4.01) | 4.34 ± 4.93 (4.02 − 4.65) | 4.96 ± 5.61 (4.45 − 5.46) | 4.79 ± 4.62 (4.38 − 5.21) | 5.02 ± 5.47 (4.11 − 5.94) | 0.0001 |
| Semen volume (mL) | 3.35 ± 1.58 (3.22 − 3.49) | 3.54 ± 1.56 (3.44 − 3.64) | 3.70 ± 1.61 (3.55 − 3.84) | 3.81 ± 1.72 (3.66 − 3.97) | 3.64 ± 1.82 (3.33 − 3.95) | <0.0001 |
| Semen pH | 8.08 ± 0.25 (8.06 − 8.10) | 8.07 ± 0.27 (8.05 − 8.09) | 8.06 ± 0.28 (8.03 − 8.08) | 8.04 ± 0.28 (8.01 − 8.06) | 8.00 ± 0.31 (7.95 − 8.06) | 0.009 |
| Semen Parameters | ||
|---|---|---|
| Sperm DFI | ||
| p-Value | Correlation (r) | |
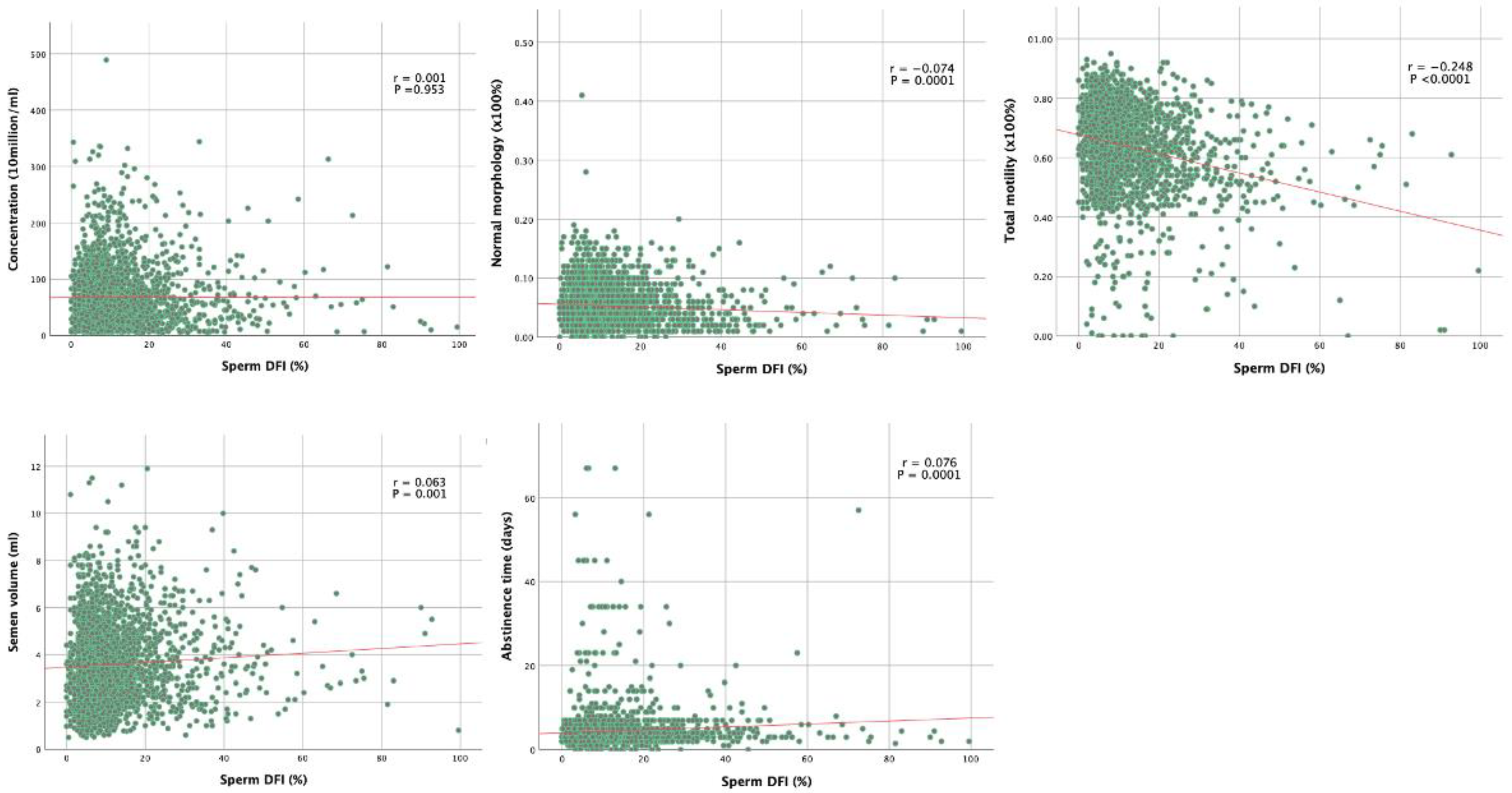
| Concentration (106/mL) | 0.001 | 0.953 |
| Total sperm number (106/Ejaculate) | 0.019 | 0.345 |
| Normal morphology (%) Head defects (%) Midpiece defects (%) Tail defects (%) | −0.074 0.009 −0.057 0.096 | 0.0001 0.645 0.004 <0.0001 |
| Total motility (%) Progressive motility (%) Motility Grade A (%) Motility Grade B (%) Motility Grade C (%) Motility Grade D (%) | −0.248 −0.257 −0.254 0.032 0.055 0.253 | <0.0001 <0.0001 <0.0001 0.106 0.005 <0.0001 |
| Age | 0.088 | <0.0001 |
| Abstinence time (Days) | 0.076 | 0.0001 |
| Volume (ml) | 0.063 | 0.001 |
| PH | −0.066 | 0.001 |
| Viscosity | −0.025 | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, S.C.; Yovich, S.J.; Hinchliffe, P.M.; Yovich, J.L. How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test. J. Pers. Med. 2023, 13, 518. https://doi.org/10.3390/jpm13030518
Chua SC, Yovich SJ, Hinchliffe PM, Yovich JL. How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test. Journal of Personalized Medicine. 2023; 13(3):518. https://doi.org/10.3390/jpm13030518
Chicago/Turabian StyleChua, Shiao Chuan, Steven John Yovich, Peter Michael Hinchliffe, and John Lui Yovich. 2023. "How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test" Journal of Personalized Medicine 13, no. 3: 518. https://doi.org/10.3390/jpm13030518
APA StyleChua, S. C., Yovich, S. J., Hinchliffe, P. M., & Yovich, J. L. (2023). How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test. Journal of Personalized Medicine, 13(3), 518. https://doi.org/10.3390/jpm13030518







