A Systematic Review of Intermittent Theta Burst Stimulation for Neurocognitive Dysfunction in Older Adults with Schizophrenia
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment of Each Study
3. Results
3.1. Results of the Search
3.2. Sample Characteristics
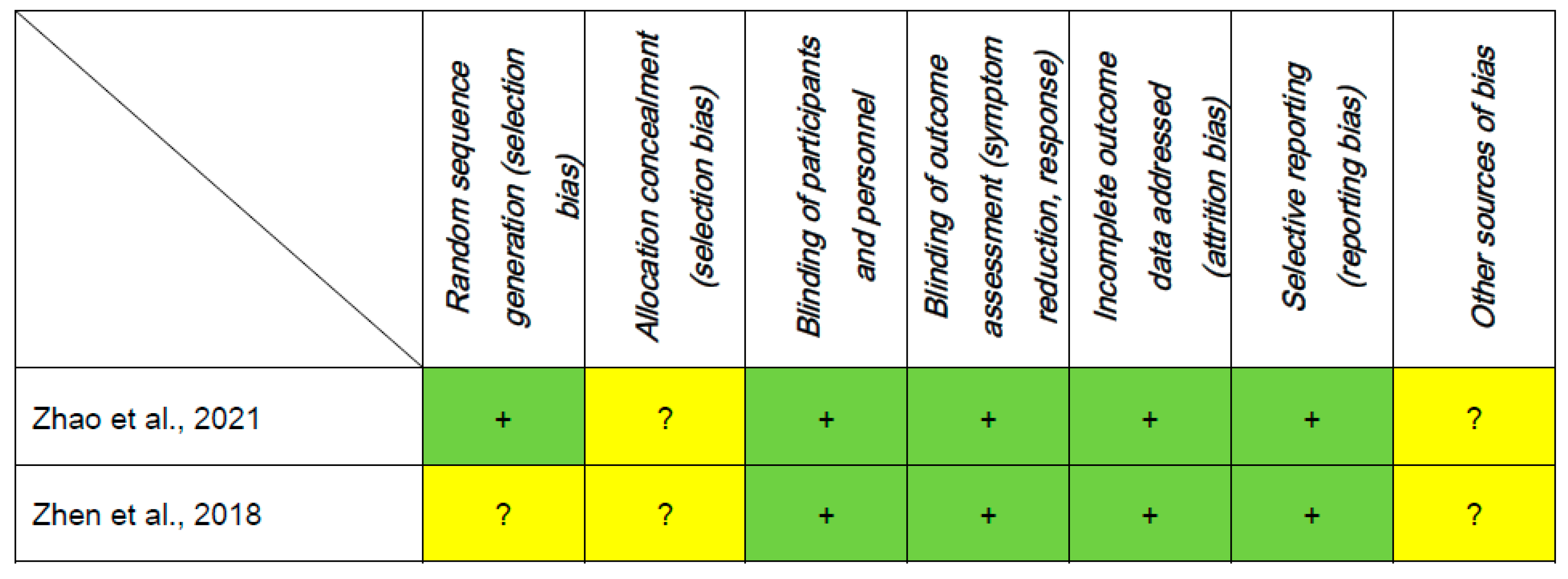
3.3. Assessment of Study Quality
3.4. Neurocognitive Function
3.5. Total Psychopathology
3.6. Dropout Rate and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, B.W.; Heaton, R.K.; Paulsen, J.S.; Kuck, J.; Braff, D.; Harris, M.J.; Zisook, S.; Jeste, D.V. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology 1997, 11, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.; Fenton, W.S. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr. Bull. 2007, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Murante, T.; Cohen, C.I. Cognitive functioning in older adults with schizophrenia. Focus 2017, 15, 26–34. [Google Scholar] [CrossRef] [PubMed]
- McCleery, A.; Nuechterlein, K.H. Cognitive impairment in psychotic illness: Prevalence, profile of impairment, developmental course, and treatment considerations. Dialogues Clin. Neurosci. 2019, 21, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic exercise improves cognitive functioning in people with schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017, 43, 546–556. [Google Scholar] [CrossRef]
- Golas, A.C.; Kalache, S.M.; Tsoutsoulas, C.; Mulsant, B.H.; Bowie, C.R.; Rajji, T.K. Cognitive remediation for older community-dwelling individuals with schizophrenia: A pilot and feasibility study. Int. J. Geriatr. Psychiatry 2015, 30, 1129–1134. [Google Scholar] [CrossRef]
- Mogg, A.; Purvis, R.; Eranti, S.; Contell, F.; Taylor, J.P.; Nicholson, T.; Brown, R.G.; McLoughlin, D.M. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: A randomized controlled pilot study. Schizophr. Res. 2007, 93, 221–228. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Sampaio-Junior, B.; Moffa, A.H.; Aparício, L.V.; Gordon, P.; Klein, I.; Rios, R.M.; Razza, L.B.; Loo, C.; Padberg, F.; et al. Noninvasive brain stimulation in psychiatric disorders: A primer. Braz. J. Psychiatry 2019, 41, 70–81. [Google Scholar] [CrossRef]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Goyal, N.; Nizamie, S.H.; Desarkar, P. Efficacy of adjuvant high frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: Preliminary results of a double-blind sham-controlled study. J. Neuropsychiatry Clin. Neurosci 2007, 19, 464–467. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, W.; Chung, Y.-C.; Jung, K.-H.; Bahk, W.-M.; Jun, T.-Y.; Kim, K.-S.; George, M.S.; Chae, J.-H. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci. Lett. 2005, 376, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-T.; Zeng, B.-S.; Hung, C.-M.; Liang, C.-S.; Stubbs, B.; Carvalho, A.F.; Brunoni, A.R.; Su, K.-P.; Tu, Y.-K.; Wu, Y.-C.; et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: A systematic review and network meta-analysis. JAMA Psychiatry 2022, 79, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Hovington, C.L.; McGirr, A.; Lepage, M.; Berlim, M.T. Repetitive transcranial magnetic stimulation (rTMS) for treating major depression and schizophrenia: A systematic review of recent meta-analyses. Ann. Med. 2013, 45, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lan, X.-J.; Qin, Z.-J.; Yang, X.-H.; Shi, Z.-M. Low-frequency repetitive transcranial magnetic stimulation for children and adolescents with first-episode and drug-naïve major depressive disorder: A systematic review. Front. Psychiatry 2023, 14, 123. [Google Scholar]
- Whybird, M.; Coats, R.; Vuister, T.; Harrison, S.; Booth, S.; Burke, M. The role of the posterior parietal cortex on cognition: An exploratory study. Brain Res. 2021, 1764, 147452. [Google Scholar] [CrossRef]
- Hahn, B.; Robinson, B.M.; Leonard, C.J.; Luck, S.J.; Gold, J.M. Posterior parietal cortex dysfunction Is central to working memory storage and broad cognitive deficits in schizophrenia. J. Neurosci. 2018, 38, 8378–8387. [Google Scholar] [CrossRef]
- Zheng, L.N.; Guo, Q.; Li, H.; Li, C.B.; Wang, J.J. Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients. J. Peking Univ. Health Sci. 2012, 44, 732–736. [Google Scholar]
- Rachid, F. Safety and efficacy of theta-burst stimulation in the treatment of psychiatric disorders: A review of the literature. J. Nerv. Ment. Dis. 2017, 205, 823–839. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Mix, A.; Benali, A.; Eysel, U.T.; Funke, K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur. J. Neurosci. 2010, 32, 1575–1586. [Google Scholar] [CrossRef]
- Grossheinrich, N.; Rau, A.; Pogarell, O.; Hennig-Fast, K.; Reinl, M.; Karch, S.; Dieler, A.; Leicht, G.; Mulert, C.; Sterr, A.; et al. Theta burst stimulation of the prefrontal cortex: Safety and impact on cognition, mood, and resting electroencephalogram. Biol. Psychiatry 2009, 65, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Walther, S.; Kunz, M.; Müller, M.; Zürcher, C.; Vladimirova, I.; Bachofner, H.; Scherer, K.A.; Nadesalingam, N.; Stegmayer, K.; Bohlhalter, S.; et al. Single session transcranial magnetic stimulation ameliorates hand gesture deficits in schizophrenia. Schizophr. Bull. 2020, 46, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, Y.M.; Li, M.N.; Gao, J.; Fang, X.Y.; Zhou, C.; Chen, J.; Zhang, F.Q.; Zhang, X.R. Effects of theta burst stimulation mode repetitive transcranial magnetic stimulation on negative symptoms and cognitive function in elderly patients with chronic schizophrenia (in Chinese). Chin. J. Behav. Med. Brain Sci. 2021, 30, 577–583. [Google Scholar]
- Zhen, L.L.; Zou, X.J.; Peng, G.H.; Zou, K. Effects of theta burst stimulation mode repetitive transcranial magnetic stimulation on executive function in elderly patients with chronic schizophrenia (in Chinese). Chin. J. Gerontol. 2018, 38, 2947–2950. [Google Scholar]
- Wu, Y.; Wang, L.; Yu, F.; Ji, G.J.; Xiao, G.; Feifei, X.; Chunyan, Z.; Xingui, C.; Wang, K. Intermittent theta burst stimulation (iTBS) as an optimal treatment for schizophrenia risk decision: An ERSP study. Front. Psychiatry 2021, 12, 594102. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zheng, W.; Luo, X.N.; Li, H.Y.; Ke, X.Y.; Dai, Q.; Zhang, C.J.; Ng, C.H.; Ungvari, G.S.; Xiang, Y.T.; Ning, Y.P. Prevalence of insomnia symptoms and their associated factors in patients treated in outpatient clinics of four general hospitals in Guangzhou, China. BMC Psychiatry 2018, 18, 232. [Google Scholar] [CrossRef]
- Overall, J.E.; Beller, S.A. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research: I. Factor structure on an inpatient unit. J. Gerontol. 1984, 39, 187–193. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Li, M.Z.; Chen, L.C.; Rong, H.; Xu, S.X.; Li, Y.; Yang, Q.F.; Deng, W.F.; Yang, H.Z.; Kong, X.M.; Xiao, L.; et al. Low-charge electrotherapy for patients with schizophrenia: A double-blind, randomised controlled pilot clinical trial. Psychiatry Res. 2019, 272, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, M.L.; He, H.B.; Li, R.P.; Li, Q.L.; Zhang, C.P.; Zhou, S.M.; Yan, S.; Ning, Y.P.; Huang, X. A preliminary study of adjunctive nonconvulsive electrotherapy for treatment-refractory depression. Psychiatr. Q. 2021, 92, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.B.; Zhou, H.R.; Liang, W.N.; Gu, L.M.; He, M.; Huang, X.; Shi, Z.M.; Hou, H.C.; Zheng, W. Adjunctive nonconvulsive electrotherapy for patients with depression: A systematic review. Psychiatr. Q. 2021, 92, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Regenold, W.T.; Noorani, R.J.; Piez, D.; Patel, P. Nonconvulsive electrotherapy for treatment resistant unipolar and bipolar major depressive disorder: A proof-of-concept trial. Brain Stimul. 2015, 8, 855–861. [Google Scholar] [CrossRef]
- Matteau, E.; Dupré, N.; Langlois, M.; Jean, L.; Thivierge, S.; Provencher, P.; Simard, M. Mattis Dementia Rating Scale 2: Screening for MCI and dementia. Am. J. Alzheimers Dis. Other Demen 2011, 26, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, H.D.; Liang, W.N.; Yang, X.H.; Cai, D.B.; Huang, X.; Huang, X.B.; Liu, C.Y.; Zheng, W. Adjunctive magnetic seizure therapy for schizophrenia: A systematic review. Front. Psychiatry 2021, 12, 813590. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Xu, Y.; Zhang, B.; Sheng, J.; Liu, D.; Wang, W.; Yang, F.; Guo, X.; Li, Q.; et al. Magnetic seizure therapy compared to electroconvulsive therapy for schizophrenia: A randomized controlled Trial. Front. Psychiatry 2021, 12, 770647. [Google Scholar] [CrossRef]
- Tang, V.M.; Blumberger, D.M.; McClintock, S.M.; Kaster, T.S.; Rajji, T.K.; Downar, J.; Fitzgerald, P.B.; Daskalakis, Z.J. Magnetic seizure therapy in treatment-resistant schizophrenia: A pilot study. Front. Psychiatry 2017, 8, 310. [Google Scholar] [CrossRef]
- Wischnewski, M.; Schutter, D.J.L.G. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 2015, 8, 685–692. [Google Scholar] [CrossRef]
- Marder, S.R.; Fenton, W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr. Res. 2004, 72, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, G.J.; Zhu, C.; Bai, X.; Wang, L.; He, K.; Gao, Y.; Tao, L.; Yu, F.; Tian, Y.; et al. Neural correlates of auditory verbal hallucinations in schizophrenia and the therapeutic response to theta-burst transcranial magnetic stimulation. Schizophr. Bull. 2019, 45, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Wu, Y.; He, K.; Xu, F.; Xiao, G.; Hu, P.; Qiu, B.; Ji, G.J.; Wang, K. Intermittent theta burst stimulation (iTBS) adjustment effects of schizophrenia: Results from an exploratory outcome of a randomized double-blind controlled study. Schizophr. Res. 2020, 216, 550–553. [Google Scholar] [CrossRef]
- Jin, H.; Zisook, S.; Palmer, B.W.; Patterson, T.L.; Heaton, R.K.; Jeste, D.V. Association of depressive symptoms with worse functioning in schizophrenia: A study in older outpatients. J. Clin. Psychiatry 2001, 62, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Rado, J.; Janicak, P.G. Pharmacological and clinical profile of recently approved second-generation antipsychotics: Implications for treatment of schizophrenia in older patients. Drugs Aging 2012, 29, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kasckow, J.; Fellows, I.; Golshan, S.; Solorzano, E.; Meeks, T.; Zisook, S. Treatment of subsyndromal depressive symptoms in middle-age and older patients with schizophrenia: Effect of age on response. Am. J. Geriatr. Psychiatry 2010, 18, 853–857. [Google Scholar] [CrossRef]
- Oberman, L.; Edwards, D.; Eldaief, M.; Pascual-Leone, A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J. Clin. Neurophysiol. 2011, 28, 67–74. [Google Scholar] [CrossRef]
- Elmaghraby, R.; Sun, Q.; Ozger, C.; Shekunov, J.; Romanowicz, M.; Croarkin, P.E. A systematic review of the safety and tolerability of theta burst stimulation in children and adolescents. Neuromodulation 2022, 25, 494–503. [Google Scholar] [CrossRef]
- Valiengo, L.d.C.L.; Goerigk, S.; Gordon, P.C.; Padberg, F.; Serpa, M.H.; Koebe, S.; Santos, L.A.D.; Lovera, R.A.M.; Carvalho, J.B.d.; van de Bilt, M.; et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: A randomized clinical trial. JAMA Psychiatry 2020, 77, 121–129. [Google Scholar] [CrossRef]
- Farcas, A.; Iftene, F. Findings, limitations and new directions in tACS studies in schizophrenia research: A scoping review. J. Psychiatr. Res. 2022, 151, 291–298. [Google Scholar] [CrossRef]

| Study (Country) | N a | Sex b: Male (%) | Diagnosis (%) | Diagnostic Criteria | Age b: yrs (Range) | Duration b of Illness (yrs) | -Design -Analysis -Setting | AP: Dose (mg/d) | Intervention: -Device -Brain region -Stimulation Intensity | Treatment Duration (n/wks) | Jadad Scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al., 2021 (China) | total: 52 iTBS: 26 sham: 26 | 66.7 | SCZ (100%) | ICD-10 | 63.3 (59–71) | 33.2 | -DB -OC -inpatients | iTBS: CPZ-equ = 495 sham: CPZ-equ = 527 | -NR -L-DLPFC -iTBS (100% MT) versus sham (100% MT, tilt the coil 90 degrees from the original stimulation position relative to the scalp) | 4 (5/wks) | 5 |
| Zhen et al., 2018 (China) | total: 80 iTBS: 40 sham: 40 | 45.5 | SCZ (100%) | DSM-IV | 63.4 (NR) | 7.5 | -DB -OC -inpatients | iTBS: CPZ-equ = 554 c sham: CPZ-equ = 516 c | -Magpro R100 (Tonica Elektronik A/S) -L-DLPFC -iTBS (80% MT) versus sham (80% MT, tilt the coil 180 degrees from the original stimulation position relative to the scalp) | 4 (5/wks) | 4 |
| Study | Cognitive Function | Pre-iTBS (Mean ± SD) | Post-iTBS (Mean ± SD) | Pre-Sham (Mean ± SD) | Post-Sham (Mean ± SD) | Findings a |
|---|---|---|---|---|---|---|
| Zhao et al., 2021 | MDRS-2: | |||||
| Total scores | 109.7 ± 6.8 | 116.5 ± 6.7 | 107.9 ± 7.5 | 108.7 ± 7.3 | p < 0.05 | |
| Attention | 30.3 ± 2.0 | 32.1 ± 2.0 | 30.3 ± 2.2 | 30.4 ± 2.2 | p < 0.05 | |
| Initiation/perseveration | 31.1 ± 1.8 | 33.3 ± 1.8 | 30.3 ± 2.2 | 30.5 ± 2.2 | p < 0.05 | |
| Conceptualization | 30.0 ± 1.5 | 31.4 ± 2.0 | 30.0 ± 1.9 | 30.3 ± 1.8 | p < 0.05 | |
| Construction | 4.9 ± 0.7 | 5.3 ± 0.5 | 4.6 ± 0.6 | 4.7 ± 0.6 | p < 0.05 | |
| Memory | 13.3 ± 2.1 | 14.4 ± 2.5 | 12.7 ± 1.6 | 12.9 ± 1.6 | p < 0.05 | |
| Zhen et al., 2018 | Digit span test: | |||||
| Total scores | 15.6 ± 4.1 | 16.1 ± 4.2 | 16.2 ± 4.4 | 15.8 ± 3.9 | NS | |
| Digit span forward | 10.6 ± 2.5 | 11.3 ± 1.9 | 10.8 ± 2.1 | 10.6 ± 2.5 | p < 0.05 | |
| Digit span backward | 5.2 ± 2.5 | 5.4 ± 2.1 | 5.1 ± 1.9 | 4.9 ± 1.6 | NS | |
| Spatial span test: | ||||||
| Total scores | 12.0 ± 4.4 | 16.3 ± 4.2 | 11.9 ± 4.1 | 10.2 ± 3.9 | p < 0.05 | |
| Spatial span forward | 5.8 ± 2.1 | 7.5 ± 2.2 | 5.6 ± 1.9 | 6.2 ± 2.1 | p < 0.05 | |
| Spatial span backward | 6.2 ± 2.4 | 6.9 ± 3.1 | 6.5 ± 1.7 | 5.9 ± 2.4 | NS | |
| WCST: | ||||||
| Response administered | 118.0 ± 14.0 | 120.0 ± 17.0 | 121.0 ± 15.0 | 120.0 ± 11.0 | NS | |
| Categories completed | 4.7 ± 1.3 | 5.2 ± 1.1 | 4.7 ± 1.1 | 4.6 ± 1.3 | NS | |
| Total correct responses | 50.0 ± 10.0 | 54.0 ± 9.0 | 51.0 ± 9.0 | 49.0 ± 10.0 | NS | |
| Total errors | 70.0 ± 18.0 | 63.0 ± 22.0 | 69.0 ± 21.0 | 72.0 ± 19.0 | NS | |
| Percent errors | 59.8 ± 14.7 | 51.5 ± 12.2 | 56.4 ± 14.1 | 58.2 ± 12.1 | p < 0.05 | |
| Trials to complete first category | 25.2 ± 13.5 | 21.7 ± 14.1 | 23.5 ± 9.7 | 25.0 ± 11.4 | NS | |
| Perseverative responses | 37.0 ± 11.5 | 41.1 ± 12.3 | 35.9 ± 13.2 | 39.8 ± 14.3 | NS | |
| Perseverative errors | 55.3 ± 18.0 | 44.2 ± 17.6 | 51.5 ± 20.1 | 56.8 ± 22.3 | p < 0.05 | |
| Percent perseverative errors | 71.3 ± 12.6 | 62.7 ± 11.4 | 66.1 ± 11.7 | 70.5 ± 14.5 | p < 0.05 | |
| Nonperseverative errors | 17.4 ± 5.1 | 17.1 ± 7.5 | 18.6 ± 6.7 | 20.9 ± 5.8 | NS | |
| Percent nonperseverative errors | 28.7 ± 12.2 | 30.3 ± 13.1 | 29.7 ± 11.4 | 32.1 ± 13.6 | NS | |
| Failure to maintain set | 14.9 ± 6.8 | 13.3 ± 7.6 | 11.5 ± 6.7 | 13.5 ± 5.4 | NS | |
| Percent conceptual level responses | 53.3 ± 17.0 | 63.5 ± 15.9 | 51.8 ± 18.8 | 56.9 ± 17.6 | p < 0.05 | |
| Study | Clinical Effects | Pre-iTBS (Mean ± SD) | Post-iTBS (Mean ± SD) | Pre-Sham (Mean ± SD) | Post-Sham (Mean ± SD) | Findings a |
|---|---|---|---|---|---|---|
| Zhao et al., 2021 | BPRS | 32.1 ± 1.9 | 31.3 ±1.9 | 32.3 ± 2.4 | 32.2 ± 2.3 | NS |
| PANSS | 60.2 ± 3.7 | 56.7 ± 3.1 | 61.0 ± 2.8 | 60.7 ± 2.7 | p < 0.05 | |
| Zhen et al., 2018 | NR | |||||
| Study | Adverse Events | Dropout Rate | |||||
|---|---|---|---|---|---|---|---|
| Events | Total (%) | iTBS Group (%) | Sham Group (%) | Total (%) | iTBS Group (%) | Sham Group (%) | |
| Zhao et al., 2021 | Intolerance | 1 (1.9) | 1 (3.8) | 0 | 4 (7.7) | 2 (7.7) | 2 (7.7) |
| Zhen et al., 2018 | NR | 3 (3.8) | 2 (5.0) | 1 (2.5) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, X.; Shi, Z.; Xu, R.; Tan, J.; Yang, J.; Huang, X.; Huang, X.; Zheng, W. A Systematic Review of Intermittent Theta Burst Stimulation for Neurocognitive Dysfunction in Older Adults with Schizophrenia. J. Pers. Med. 2023, 13, 485. https://doi.org/10.3390/jpm13030485
Zhang X, Yang X, Shi Z, Xu R, Tan J, Yang J, Huang X, Huang X, Zheng W. A Systematic Review of Intermittent Theta Burst Stimulation for Neurocognitive Dysfunction in Older Adults with Schizophrenia. Journal of Personalized Medicine. 2023; 13(3):485. https://doi.org/10.3390/jpm13030485
Chicago/Turabian StyleZhang, Xinyang, Xinhu Yang, Zhanming Shi, Rui Xu, Jianqiang Tan, Jianwen Yang, Xiong Huang, Xingbing Huang, and Wei Zheng. 2023. "A Systematic Review of Intermittent Theta Burst Stimulation for Neurocognitive Dysfunction in Older Adults with Schizophrenia" Journal of Personalized Medicine 13, no. 3: 485. https://doi.org/10.3390/jpm13030485
APA StyleZhang, X., Yang, X., Shi, Z., Xu, R., Tan, J., Yang, J., Huang, X., Huang, X., & Zheng, W. (2023). A Systematic Review of Intermittent Theta Burst Stimulation for Neurocognitive Dysfunction in Older Adults with Schizophrenia. Journal of Personalized Medicine, 13(3), 485. https://doi.org/10.3390/jpm13030485






