Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
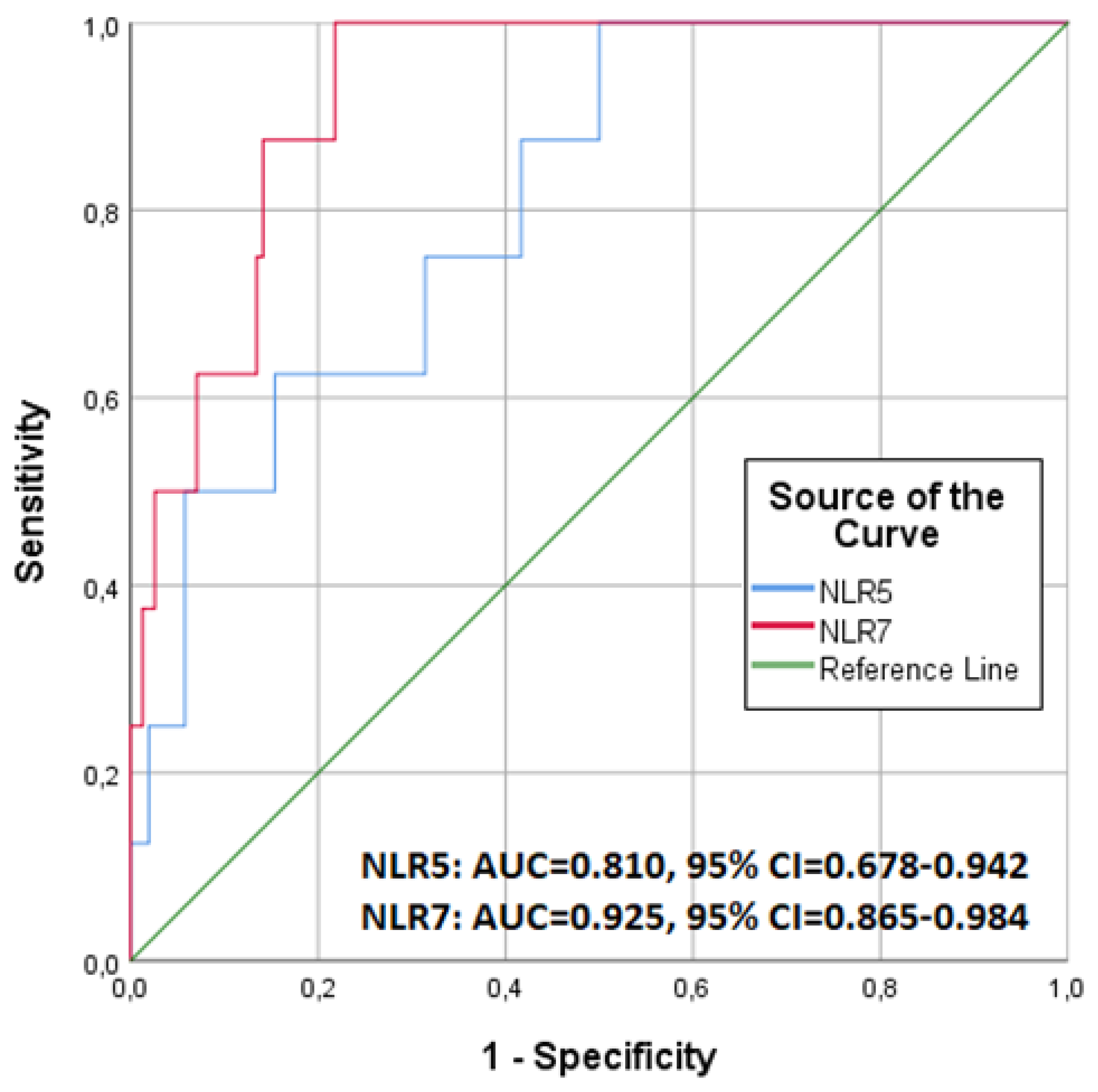
3.1. NLR and 90-Day Mortality
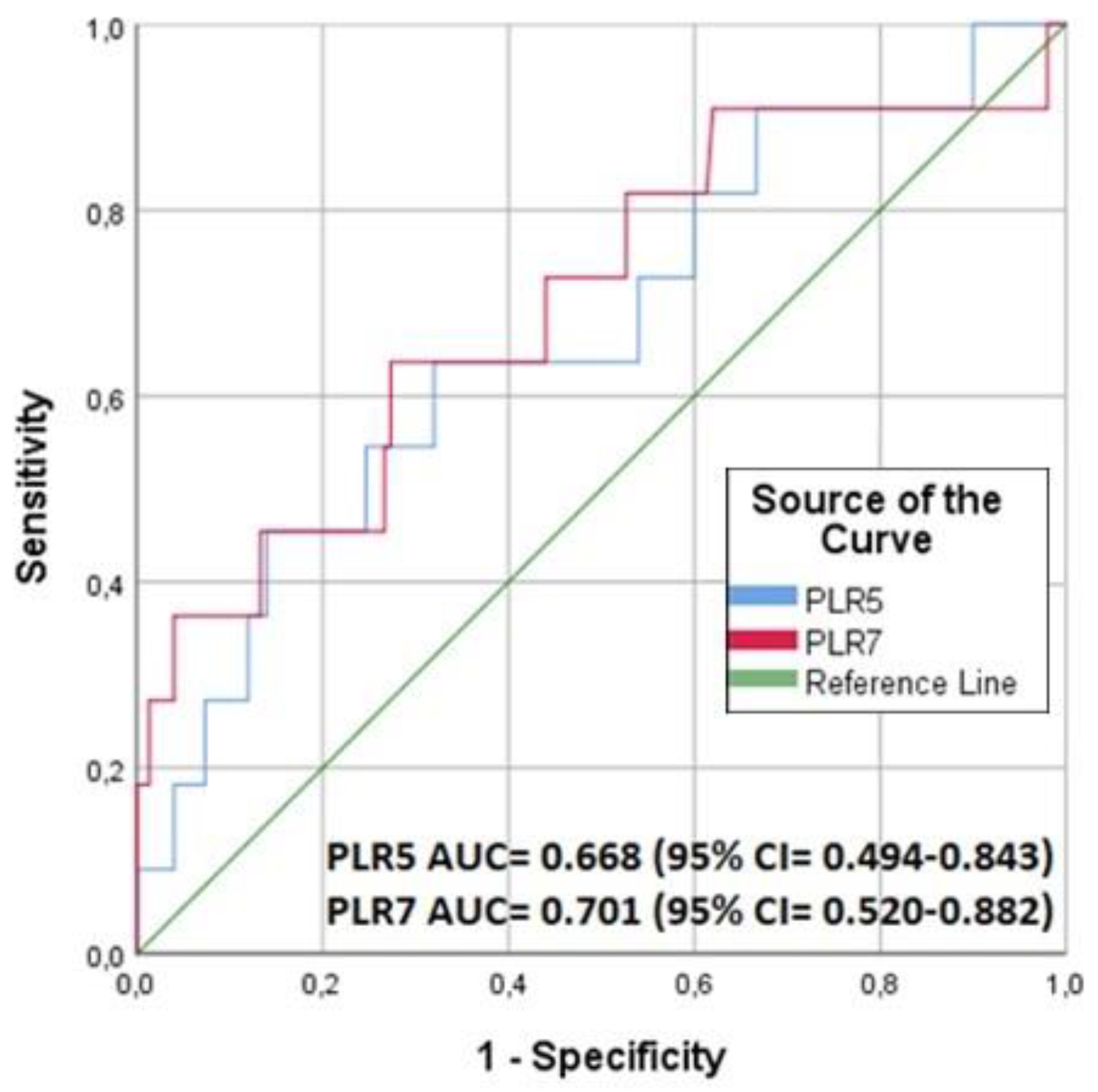
3.2. PLR and 90 Day Mortality
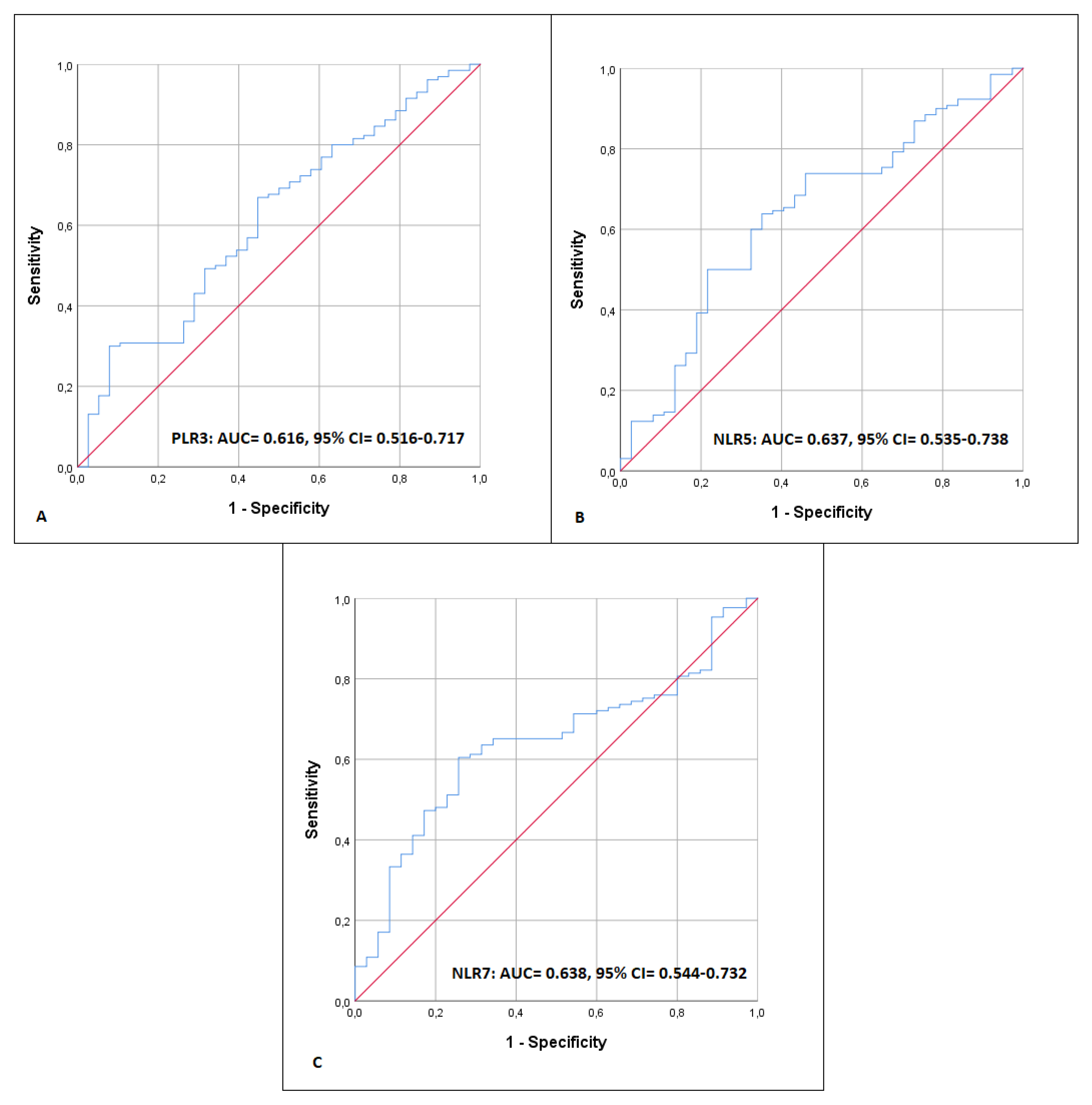
3.3. NLR, PLR, and 90-Day Mortality
3.4. NLR, PLR, and Length of Hospital Stay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moisa, E.; Corneci, D.; Negoita, S.; Filimon, C.R.; Serbu, A.; Negutu, M.I.; Grintescu, I.M. Dynamic Changes of the Neutrophil-to-Lymphocyte Ratio, Systemic Inflammation Index, and Derived Neutrophil-to-Lymphocyte Ratio Independently Predict Invasive Mechanical Ventilation Need and Death in Critically Ill COVID-19 Patients. Biomedicines 2021, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Fernandes, P.C., Jr.; Silva, M.J.B.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Shao, Y.; Zhu, D.; Zheng, X.; Zhou, Q.; Zhou, W.; Ni, X.; Wu, C.; Jiang, J. Systemic Immune-Inflammation Index Predicts Prognosis of Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-matched Analysis. Sci. Rep. 2016, 6, 39482. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Geng, Y.; Gu, W.; Ning, Z.; Huang, J.; Pei, H.; Jiang, J. Assessment of Lymph Node Ratio to Replace the pN Categories System of Classification of the TNM System in Esophageal Squamous Cell Carcinoma. J. Thorac. Oncol. 2016, 11, 1774–1784. [Google Scholar] [CrossRef]
- Passardi, A.; Scarpi, E.; Cavanna, L.; Dall’Agata, M.; Tassinari, D.; Leo, S.; Bernardini, I.; Gelsomino, F.; Tamberi, S.; Brandes, A.A.; et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016, 7, 33210–33219. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic Immune-Inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Mennitto, A.; Prisciandaro, M.; Huber, V.; Milano, M.; Rinaldi, L.; Cona, M.S.; Maggi, C.; Ferrari, B.; Manoukian, S.; et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict efficacy of platinum-based chemotherapy in patients with metastatic triple negative breast cancer. Sci. Rep. 2018, 8, 8703. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, P.; Huang, Z.; Huang, G.; Tang, J.; Guo, Y.; Huang, P.; Lai, Z.; Lin, F. The value of red cell distribution width in patients with ovarian cancer. Medicine 2017, 96, e6752. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Sun, Y.; Zhang, J.; Yao, Y.; Shen, Z.; Xiang, D.; He, A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci. Rep. 2016, 6, 39862. [Google Scholar] [CrossRef]
- Lu, A.; Li, H.; Zheng, Y.; Tang, M.; Li, J.; Wu, H.; Zhong, W.; Gao, J.; Ou, N.; Cai, Y. Prognostic Significance of Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio in Patients with Nasopharyngeal Carcinoma. BioMed Res. Int. 2017, 2017, 3047802. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Kolios, G.; Manousou, P.; Kazamias, P.; Paramythiotis, D.; Papavramidis, T.S.; Heliadis, S.; Kouroumalis, E.; Eleftheriadis, E. Oxidative stress due to anesthesia and surgical trauma: Importance of early enteral nutrition. Mol. Nutr. Food Res. 2009, 53, 770–779. [Google Scholar] [CrossRef] [PubMed]
- DiMaria-Ghalili, R.A.; Sullivan-Marx, E.M.; Compher, C. Inflammation, functional status, and weight loss during recovery from cardiac surgery in older adults: A pilot study. Biol. Res. Nurs. 2014, 16, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Aydınlı, B.; Demir, A.; Güçlü, Ç.Y.; Bölükbaşı, D.; Ünal, E.U.; Koçulu, R.; Selçuk, G. Hematological predictors and clinical outcomes in cardiac surgery. J. Anesth. 2016, 30, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Romeo, F.J.; Pizarro, R.; Vulcano, N.O.; Posatini, R.A.; Marenchino, R.G.; Berrocal, D.H.; Belziti, C.A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of survival after heart transplantation. ESC Heart Fail. 2018, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, G.; Panagidi, M.; Papakostas, P.; Grosomanidis, V.; Stavrou, G.; Kotzampassi, K. Phase Angle and Handgrip Strength as Complements to Body Composition Analysis for Refining Prognostic Accuracy in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Levine, M.; Ensom, M.H. Post hoc power analysis: An idea whose time has passed? Pharmacotherapy 2001, 21, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.A.; Liu, Z.; Loth, J.; Penny-Dimri, J.C.; Plummer, M.; Segal, R.; Smith, J. Perioperative Neutrophil-Lymphocyte Ratio Predicts Mortality after Cardiac Surgery: Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1296–1303. [Google Scholar] [CrossRef]
- Bashour, C.A.; Yared, J.P.; Ryan, T.A.; Rady, M.Y.; Mascha, E.; Leventhal, M.J.; Starr, N.J. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit. Care Med. 2000, 28, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio. Sixteen-year-long history since publication of our article in Bratislava Medical Journal. Bratisl. Lek. Listy 2017, 118, 321–323. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.M.; Rueda, F.R. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J. Pers. Med. 2023, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, G.; Açıkgoz, S.K.; Bozbay, M.; Altay, S.; Uğur, M.; Uluganyan, M.; Uyarel, H. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio combination can predict prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology 2015, 66, 441–447. [Google Scholar] [CrossRef]
- Yildiz, A.; Yuksel, M.; Oylumlu, M.; Polat, N.; Akyuz, A.; Acet, H.; Aydin, M.; Ülgen, M.S. The Utility of the Platelet-Lymphocyte Ratio for Predicting No Reflow in Patients with ST-Segment Elevation Myocardial Infarction. Clin. Appl. Thromb. Hemost. 2015, 21, 223–228. [Google Scholar] [CrossRef]
- Gu, L.; Xia, Z.; Qing, B.; Chen, H.; Wang, W.; Chen, Y.; Yuan, Y. The Core Role of Neutrophil-Lymphocyte Ratio to Predict All-Cause and Cardiovascular Mortality: A Research of the 2005–2014 National Health and Nutrition Examination Survey. Front. Cardiovasc. Med. 2022, 9, 847998. [Google Scholar] [CrossRef] [PubMed]
- Merdler, I.; Frydman, S.; Sirota, S.; Halkin, A.; Steinvil, A.; Toledano, E.; Konigstein, M.; Litmanowicz, B.; Bazan, S.; Wenkert, A.; et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker in Transcatheter Aortic Valve Implantation (TAVI) Patients. Isr. Med. Assoc. J. 2022, 24, 229–234. [Google Scholar] [PubMed]
- Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Rodzki, M.; Witkowska, A.; Gąsecka, A.; Buczkowski, P.; Perek, B.; Jemielity, M. Neutrophil Counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. Cells 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Olasińska-Wiśniewska, A.; Michalak, M.; Rodzki, M.; Witkowska, A.; Straburzyńska-Migaj, E.; Perek, B.; Jemielity, M. The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology 2021, 11, 34. [Google Scholar] [CrossRef]
- Haran, C.; Gimpel, D.; Clark, H.; McCormack, D.J. Preoperative Neutrophil and Lymphocyte Ratio as a Predictor of Mortality and Morbidity after Cardiac Surgery. Heart Lung Circ. 2021, 30, 414–418. [Google Scholar] [CrossRef]
- Larmann, J.; Handke, J.; Scholz, A.S.; Dehne, S.; Arens, C.; Gillmann, H.J.; Uhle, F.; Motsch, J.; Weigand, M.A.; Janssen, H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 230. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Wang, X. The neutrophil-lymphocyte ratio is associated with postoperative mortality of cardiac surgery. J. Thorac. Dis. 2021, 13, 67–75. [Google Scholar] [CrossRef]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef]
- Srivastava, K.; Cockburn, I.A.; Swaim, A.; Thompson, L.E.; Tripathi, A.; Fletcher, C.A.; Shirk, E.M.; Sun, H.; Kowalska, M.A.; Fox-Talbot, K.; et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe 2008, 4, 179–187. [Google Scholar] [CrossRef]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.E. Health Care Ethics: Critical Issues for the 21st Century; Jones & Bartlett Learning: Burlington, MA, USA, 2009. [Google Scholar]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [PubMed]
| N = 179 | Coronary Artery Disease | Heart Valvular Disease | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| Patients (n) | 90 | 89 | 179 | n/a | |||
| Gender (male)-n (%) | 78 (86.7%) | 61 (68.5%) | 139 (77.7%) | 0.004 | |||
| Age (years) | 68.5 | (13.0) | 67.0 | (15.0) | 68.0 | (13.0) | 0.471 |
| Height (meters-m) | 1.69 | (0.10) | 1.70 | (0.11) | 1.70 | (0.10) | 0.524 |
| Weight (kilograms-kg) | 77.2 | (19.1) | 78.3 | (19.2) | 77.6 | (18.5) | 0.824 |
| MUST Score | 1.0 | (1.0) | 1.0 | (1.0) | 1.0 | (1.0) | 0.251 |
| BMI (m/kg2) | 26.4 | (5.1) | 27.8 | (6.4) | 27.2 | (5.8) | 0.308 |
| EuroSCORE II (%) | 2.1 | (2.1) | 3.0 | (1.8) | 2.4 | (2.0) | 0.022 |
| Ejection Fraction (%) | 50 | (10) | 55 | (5) | 55 | (5) | 0.007 |
| ICU Stay (days) | 1.0 | (1.0) | 2.0 | (2.0) | 2.0 | (2.0) | 0.738 |
| Ward Stay (days) | 7.0 | (2.0) | 7.0 | (2.0) | 7.0 | (2.0) | 0.115 |
| Total Hospital Stay (days) | 8.0 | (3.0) | 9.0 | (3.0) | 9.0 | (3.0) | 0.670 |
| Diabetes Mellitus-n (%) | 44 (48.9%) | 38 (42.7%) | 82 (45.8%) | 0.894 | |||
| COPD-n (%) | 27 (30.0%) | 25 (28.1%) | 52 (29.1%) | 0.984 | |||
| Chronic Kidney Failure-n (%) | 24 (26.7%) | 23 (25.8%) | 47 (26.3%) | 0.892 | |||
| CPB time (min) | 78.1 ± 32.5 | 77.9 ± 32.2 | 78.0 ± 32.3 | 0.967 | |||
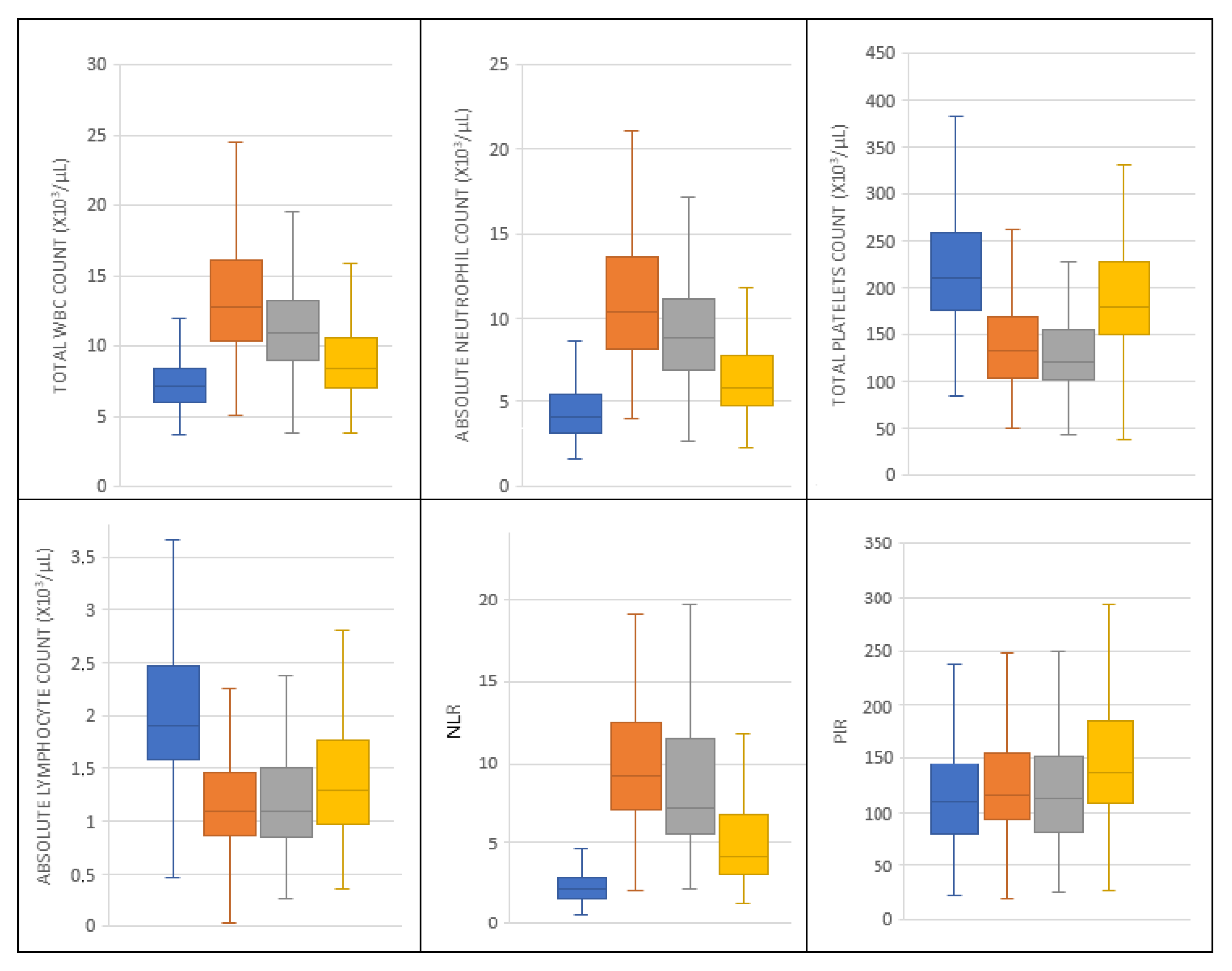
| N = 179 | Blood Components | Median ± SD | Median | Minimum | Maximum | Normal Values |
|---|---|---|---|---|---|---|
| Preoperatively | Total white blood cells | 7.42 ± 1.99 | 7.12 | 3.72 | 14.72 | 3.80–10.50 |
| Absolute neutrophil count | 4.51 ± 1.71 | 4.10 | 2.20 | 10.50 | 1.60–6.50 | |
| Absolute lymphocyte count | 2.07 ± 0.73 | 1.90 | 0.46 | 5.94 | 1.50–3.60 | |
| Absolute monocyte count | 0.63 ± 0.19 | 0.59 | 0.20 | 1.24 | 0.20–1.00 | |
| Total platelets | 223.89 ± 71.53 | 210.00 | 85.00 | 460.00 | 150.00–450.00 | |
| NLR0 | 2.49 ± 1.75 | 2.11 | 0.59 | 15.65 | ||
| PLR0 | 119.26 ± 53.75 | 109.01 | 21.21 | 359.34 | ||
| Postoperative Day 3 | Total white blood cells | 13.47 ± 4.78 | 12.66 | 5.12 | 35.09 | 3.80–10.50 |
| Absolute neutrophil count | 10.97 ± 4.18 | 10.40 | 3.90 | 28.70 | 1.60–6.50 | |
| Absolute lymphocyte count | 1.21 ± 0.56 | 1.10 | 0.04 | 4.06 | 1.50–3.60 | |
| Absolute monocyte count | 1.24 ± 0.53 | 1.12 | 0.20 | 3.03 | 0.20–1.00 | |
| Total platelets | 140.51 ± 53.94 | 132.00 | 55.00 | 417.00 | 150.00–450.00 | |
| NLR3 | 10.98 ± 11.66 | 8.97 | 2.01 | 142.86 | ||
| PLR3 | 145.09 ± 175.92 | 117.05 | 18.47 | 2142.38 | ||
| Postoperative Day 5 | Total white blood cells | 11.63 ± 3.91 | 11.49 | 3.86 | 25.25 | 3.80–10.50 |
| Absolute neutrophil count | 19.44 ± 3.70 | 8.90 | 2.70 | 23.90 | 1.60–6.50 | |
| Absolute lymphocyte count | 1.21 ± 0.53 | 1.09 | 0.26 | 3.56 | 1.50–3.60 | |
| Absolute monocyte count | 0.80 ± 0.32 | 0.77 | 0.17 | 2.02 | 0.20–1.00 | |
| Total platelets | 135.46 ± 56.47 | 122.50 | 28.00 | 451.00 | 150.00–450.00 | |
| NLR5 | 9.41 ± 6.63 | 7.41 | 2.16 | 55.58 | ||
| PLR5 | 132.04 ± 81.73 | 115.59 | 17.61 | 715.87 | ||
| Postoperative Day 7 | Total white blood cells | 9.40 ± 3.71 | 8.86 | 3.76 | 33.31 | 3.80–10.50 |
| Absolute neutrophil count | 6.88 ± 3.54 | 5.90 | 2.30 | 30.50 | 1.60–6.50 | |
| Absolute lymphocyte count | 1.38 ± 0.56 | 1.29 | 0.36 | 3.94 | 1.50–3.60 | |
| Absolute monocyte count | 0.86 ± 0.34 | 0.82 | 0.27 | 2.23 | 0.20–1.00 | |
| Total platelets | 190.30 ± 72.42 | 182.00 | 38.00 | 460.00 | 150.00–450.00 | |
| NLR7 | 6.14 ± 5.85 | 4.58 | 1.29 | 58.65 | ||
| PLR7 | 155.02 ± 78.75 | 138.19 | 26.21 | 601.43 |
| Score | Patients n (%) | Number of Patients Who Died (% of the Respective Group) |
|---|---|---|
| 0 | 28 | (15.6) |
| 1 | 79 | (44.1) |
| 2 | 46 | (25.7) |
| 3 | 26 | (14.5) |
| Type of Analysis | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Independent Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Gender (F/M) | 0.323 | 0.040–2.604 | 0.289 | 2.264 | 0.090–57.017 | 0.620 |
| Age (years) | 1.080 | 0.994–1.173 | 0.068 | 0.791 | 0.512–1.224 | 0.124 |
| MUST Score | 1.334 | 0.517–3.443 | 0.551 | N/I | ||
| EuroSCORE II | 1.359 | 1.038–1.779 | 0.025 | 1.065 | 0.538–2.108 | 0.858 |
| Coronary Heart Disease | 0.525 | 0.148–1.861 | 0.318 | N/I | ||
| Heart Valvular Disease | 1.905 | 0.537–6.756 | 0.318 | N/I | ||
| DM | 1.440 | 0.423–4.906 | 0.560 | N/I | ||
| COPD | 0.888 | 0.226–3.488 | 0.865 | N/I | ||
| CKD | 1.905 | 0.537–6.756 | 0.318 | N/I | ||
| ICU Stay (days) | 1.373 | 1.186–1.590 | <0.001 | 1.361 | 1.045–1.774 | 0.022 |
| PLR3 | 0.979 | 0.961–0.997 | 0.026 | 0.896 | 0.798–1.006 | 0.063 |
| NLR5 | 1.110 | 1.034–1.192 | 0.004 | 0.866 | 0.662–1.133 | 0.293 |
| NLR7 | 1.297 | 1.132–1.487 | <0.001 | 2.143 | 1.076–4.267 | 0.030 |
| N = 179 | Pearson Correlation Coefficient | p-Value |
|---|---|---|
| PLR3-NLR3 | 0.916 | <0.001 |
| PLR5-NLR5 | 0.530 | <0.001 |
| PLR7-NLR7 | 0.418 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzikos, G.; Alexiou, I.; Tsagkaropoulos, S.; Menni, A.-E.; Chatziantoniou, G.; Doutsini, S.; Papavramidis, T.; Grosomanidis, V.; Stavrou, G.; Kotzampassi, K. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery. J. Pers. Med. 2023, 13, 473. https://doi.org/10.3390/jpm13030473
Tzikos G, Alexiou I, Tsagkaropoulos S, Menni A-E, Chatziantoniou G, Doutsini S, Papavramidis T, Grosomanidis V, Stavrou G, Kotzampassi K. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery. Journal of Personalized Medicine. 2023; 13(3):473. https://doi.org/10.3390/jpm13030473
Chicago/Turabian StyleTzikos, Georgios, Ioannis Alexiou, Sokratis Tsagkaropoulos, Alexandra-Eleftheria Menni, Georgios Chatziantoniou, Soultana Doutsini, Theodosios Papavramidis, Vasilios Grosomanidis, George Stavrou, and Katerina Kotzampassi. 2023. "Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery" Journal of Personalized Medicine 13, no. 3: 473. https://doi.org/10.3390/jpm13030473
APA StyleTzikos, G., Alexiou, I., Tsagkaropoulos, S., Menni, A.-E., Chatziantoniou, G., Doutsini, S., Papavramidis, T., Grosomanidis, V., Stavrou, G., & Kotzampassi, K. (2023). Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery. Journal of Personalized Medicine, 13(3), 473. https://doi.org/10.3390/jpm13030473








