Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration
Abstract
1. Introduction
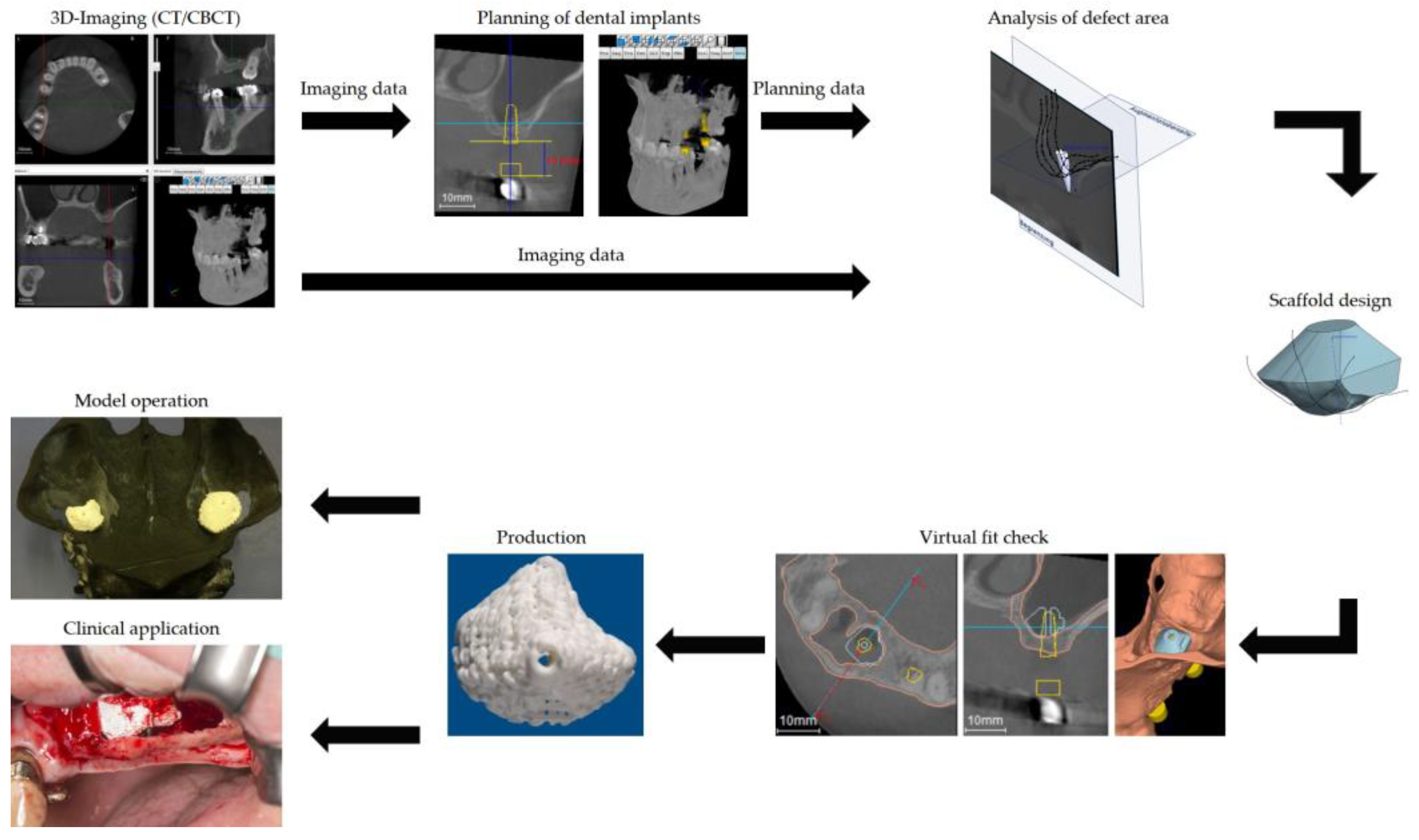
2. Case Presentation
3. Outcome
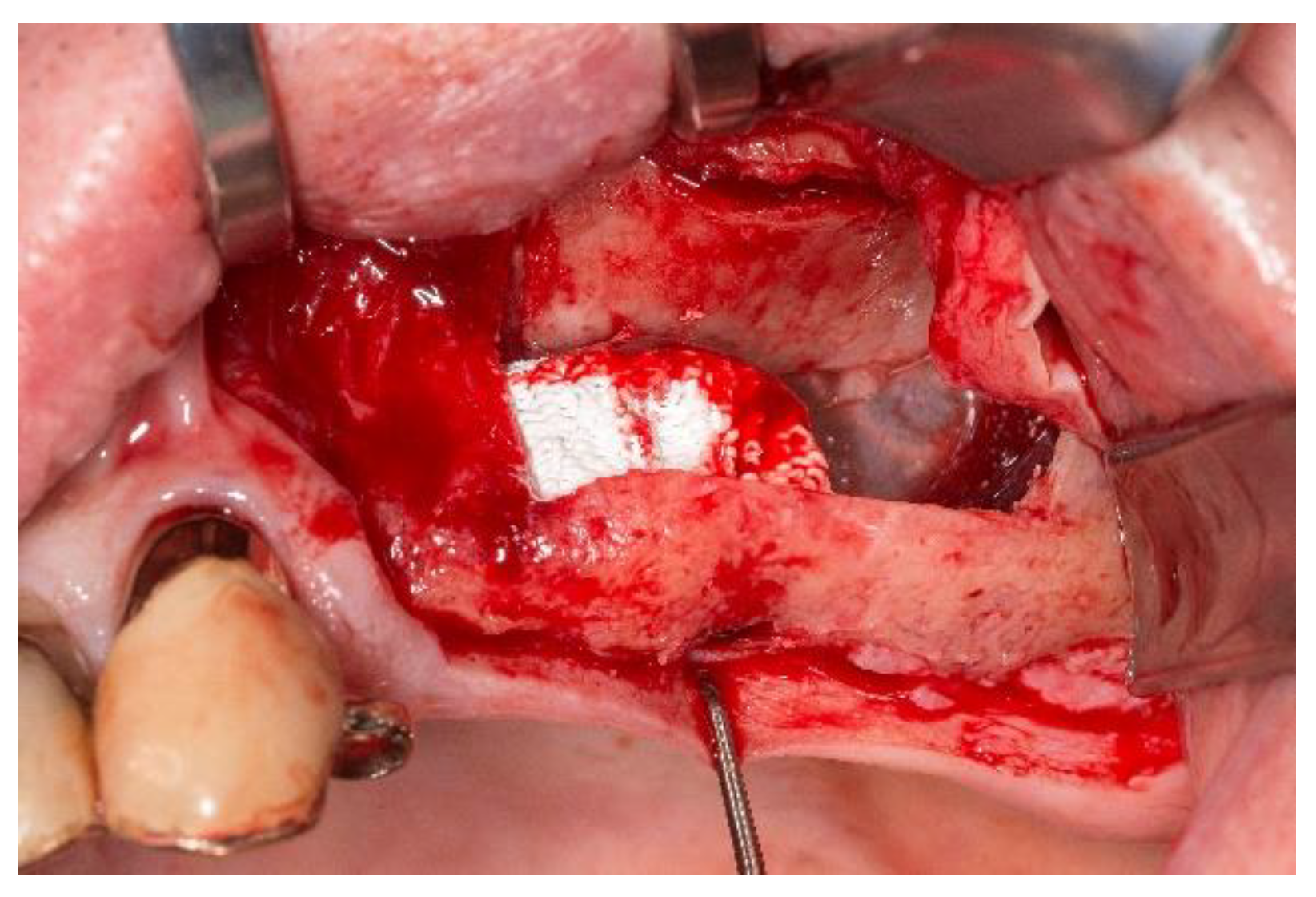
3.1. Handling of the Scaffolds and Clinical Outcome
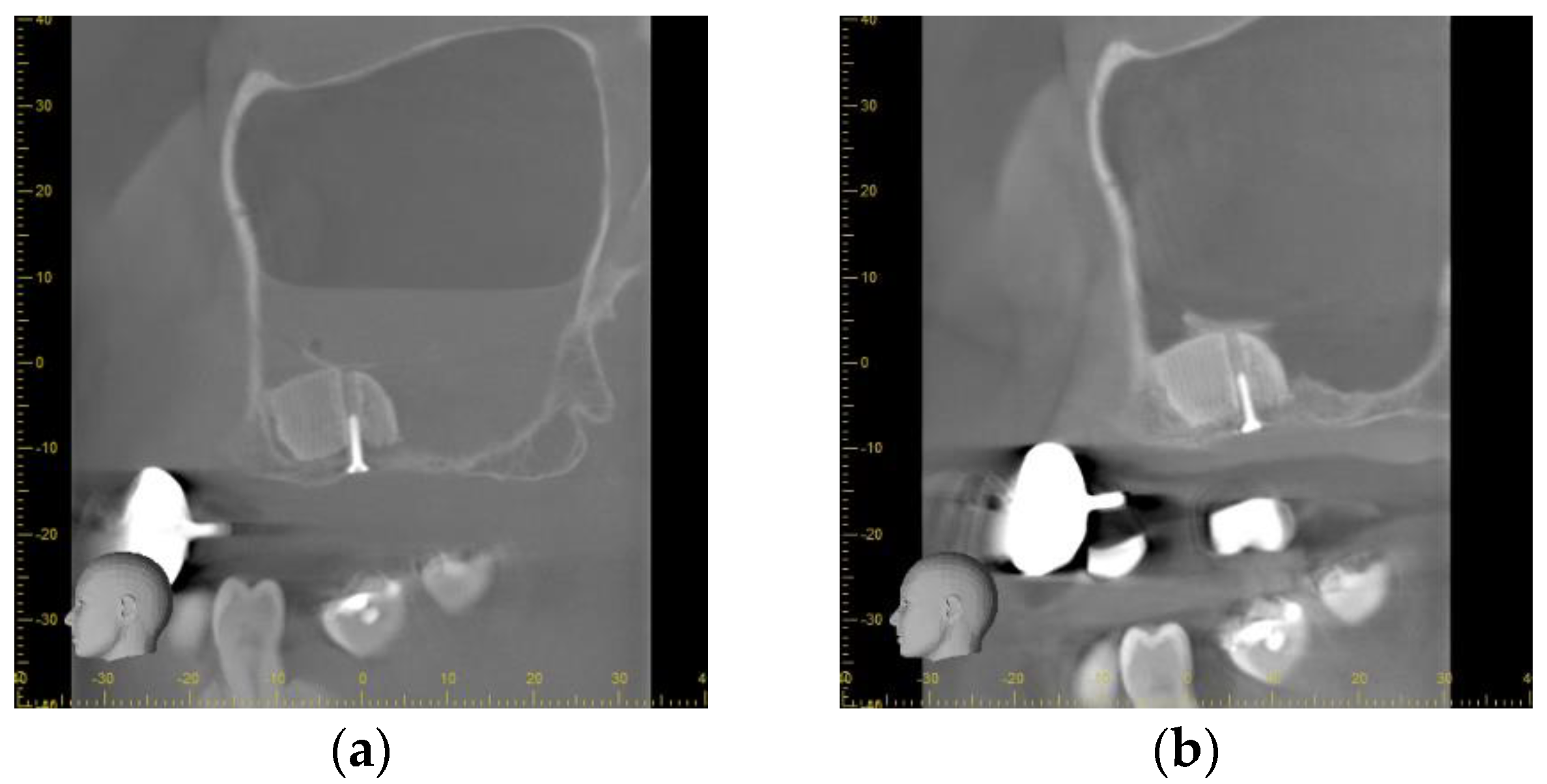
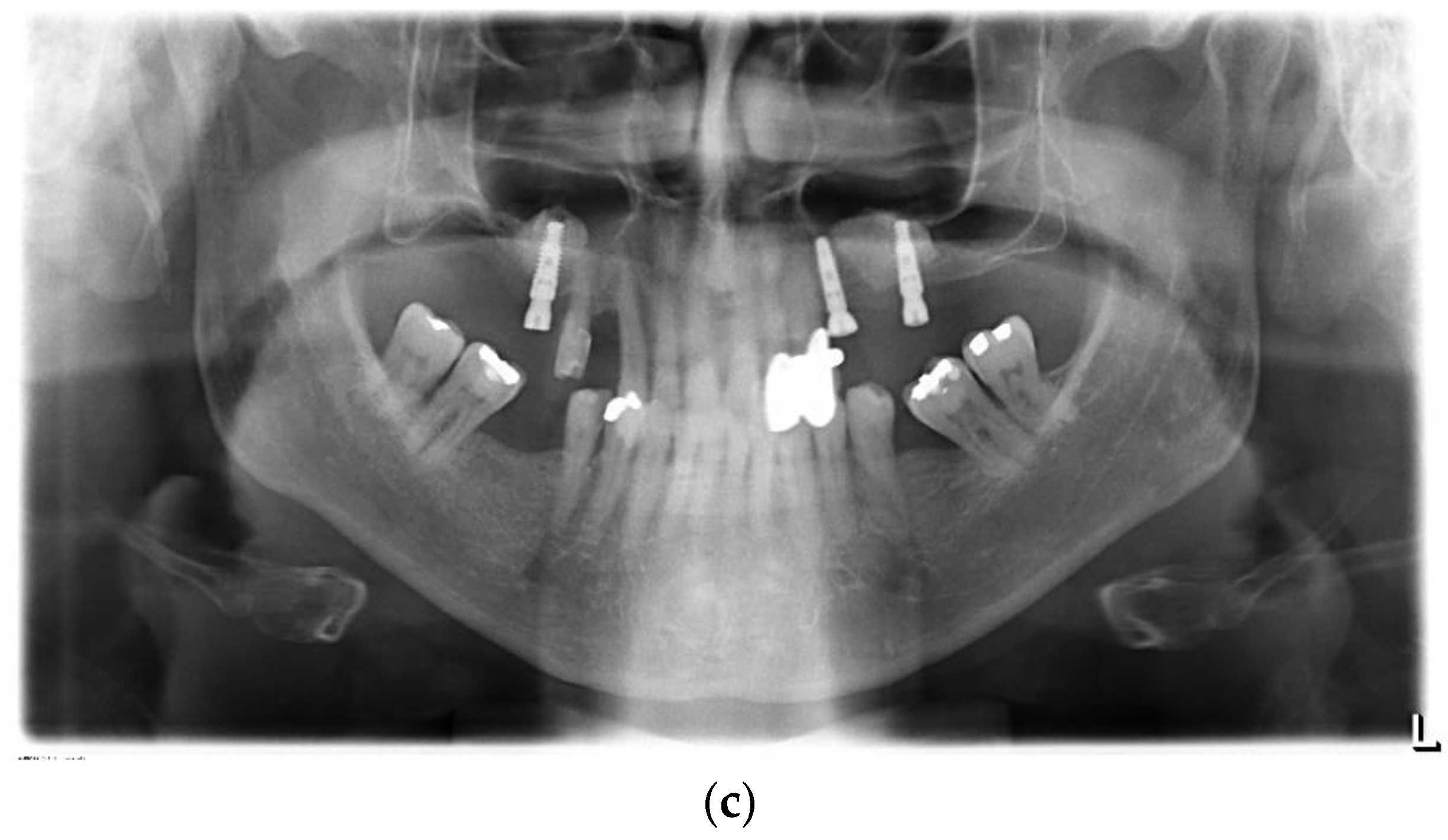
3.2. Radiographical Outcome
3.3. Histological Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Health Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Dubey, N.; Bogie, K.; Cao, C.; Li, J.; Lerchbacker, J.; Mendonça, G.; Kauffmann, F.; Bottino, M.C.; Kaigler, D. Three-dimensional printing of clinical scale and personalized calcium phosphate scaffolds for alveolar bone reconstruction. Dent. Mater. 2022, 38, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Muallah, D.; Sembdner, P.; Holtzhausen, S.; Meissner, H.; Hutsky, A.; Ellmann, D.; Assmann, A.; Schulz, M.; Lauer, G.; Kroschwald, L. Adapting the Pore Size of Individual, 3D-Printed CPC Scaffolds in Maxillofacial Surgery. J. Clin. Med. 2021, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Rasperini, G.; Giannobile, W.V. Clinical and patient-reported outcomes of tissue engineering strategies for periodontal and peri-implant reconstruction. Periodontology 2000 2022. [Google Scholar] [CrossRef]
- Wach, T.; Kozakiewicz, M. Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation. Materials 2020, 13, 3854. [Google Scholar] [CrossRef]
- Shi, J.; Dai, W.; Gupta, A.; Zhang, B.; Wu, Z.; Zhang, Y.; Pan, L.; Wang, L. Frontiers of Hydroxyapatite Composites in Bionic Bone Tissue Engineering. Materials 2022, 15, 8475. [Google Scholar] [CrossRef]
- Prakasam, M.; Silvain, J.-F.; Largeteau, A. Innovative High-Pressure Fabrication Processes for Porous Biomaterials—A Review. Bioengineering 2021, 8, 170. [Google Scholar] [CrossRef]
- Kilian, D.; Holtzhausen, S.; Groh, W.; Sembdner, P.; Czichy, C.; Lode, A.; Stelzer, R.; Gelinsky, M. 3D extrusion printing of density gradients by variation of sinusoidal printing paths for tissue engineering and beyond. Acta Biomater. 2023, 158, 308–323. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Kroczek, K.; Turek, P.; Mazur, D.; Szczygielski, J.; Filip, D.; Brodowski, R.; Balawender, K.; Przeszłowski, Ł.; Lewandowski, B.; Orkisz, S.; et al. Characterisation of Selected Materials in Medical Applications. Polymers 2022, 14, 1526. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2017, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Fernández, E.; De Maeyer, E.; Verbeeck, R.; Boltong, M.; Ginebra, J.; Driessens, F.; Planell, J. Setting Reaction and Hardening of an Apatitic Calcium Phosphate Cement. J. Dent. Res. 1997, 76, 905–912. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Klein, R.; Tetzlaff, R.; Weiss, C.; Schäfer, M.-K.; Tanner, M.; Wiedenhöfer, B.; Grafe, I.; Meeder, P.-J.; Noeldge, G.; Nawroth, P.P.; et al. Osteointegration and Resorption of Intravertebral and Extravertebral Calcium Phosphate Cement. Clin. Spine Surg. A Spine Publ. 2017, 30, E291–E296. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium (II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. 2017, 36, 106–117. [Google Scholar] [CrossRef]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements—Approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-K.; Kim, C.; Pae, H.-C.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Maxillary sinus augmentation using biphasic calcium phosphate: Dimensional stability results after 3–6 years. J. Periodontal Implant. Sci. 2019, 49, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, G.; Verona, M.; Cardoni, G.; Capone, A. Synthetic Bone Substitutes and Mechanical Devices for the Augmentation of Osteoporotic Proximal Humeral Fractures: A Systematic Review of Clinical Studies. J. Funct. Biomater. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Tatum, H. Maxillary and Sinus Implant Reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Canellas, J.V.D.S.; Drugos, L.; Ritto, F.G.; Fischer, R.G.; Medeiros, P.J.D. Xenograft materials in maxillary sinus floor elevation surgery: A systematic review with network meta-analyses. Br. J. Oral Maxillofac. Surg. 2021, 59, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Léonard, A.; Drion, P.; Sourice, S.; Layrolle, P.; Rompen, E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implant. Res. 2010, 22, 538–545. [Google Scholar] [CrossRef]
- Lundgren, S.; Cricchio, G.; Palma, V.C.; Salata, L.A.; Sennerby, L. Sinus membrane elevation and simultaneous insertion of dental implants: A new surgical technique in maxillary sinus floor augmentation. Periodontology 2000 2008, 47, 193–205. [Google Scholar] [CrossRef]
- Kirmeier, R.; Payer, M.; Wehrschuetz, M.; Jakse, N.; Platzer, S.; Lorenzoni, M. Evaluation of three-dimensional changes after sinus floor augmentation with different grafting materials. Clin. Oral Implant. Res. 2008, 19, 366–372. [Google Scholar] [CrossRef]
- Ohayon, L.; Taschieri, S.; Friedmann, A.; Del Fabbro, M. Bone Graft Displacement After Maxillary Sinus Floor Augmentation with or without Covering Barrier Membrane: A Retrospective Computed Tomographic Image Evaluation. Int. J. Oral Maxillofac. Implant. 2019, 34, 681–691. [Google Scholar] [CrossRef]
- Mangano, F.; Zecca, P.; Pozzi-Taubert, S.; Macchi, A.; Ricci, M.; Luongo, G. Maxillary sinus augmentation using computer-aided design/computer-aided manufacturing (CAD/CAM) technology. Int. J. Med. Robot. Comput. Assist. Surg. 2012, 9, 331–338. [Google Scholar] [CrossRef]
- Lode, A.; Meissner, K.; Luo, Y.; Sonntag, F.; Glorius, S.; Nies, B.; Vater, C.; Despang, F.; Hanke, T.; Gelinsky, M. Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J. Tissue Eng. Regen. Med. 2014, 8, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues*. The Sage-Schliff (sawing and grinding) Technique. J. Oral Pathol. Med. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.-H.D.; Ma, Y.-H.D.; Jaiswal, M.S.D.; Hwang, J.-M.D.; Hwang, D.-S.D. Accuracy of Maxillary Positioning During Orthognathic Surgery: A Comparison of Web-based 3-Dimensional Virtual Surgical Planning and Actual Outcomes. J. Craniofacial Surg. 2022, 34, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Reich, K.M.; Beck, F.; Heimel, P.; Lettner, S.; Redl, H.; Ulm, C.; Tangl, S. Bone Graft Packing and Its Association with Bone Regeneration in Maxillary Sinus Floor Augmentations: Histomorphometric Analysis of Human Biopsies. Biology 2022, 11, 1431. [Google Scholar] [CrossRef]
- Iezzi, G.; Scarano, A.; Valbonetti, L.; Mazzoni, S.; Furlani, M.; Mangano, C.; Muttini, A.; Raspanti, M.; Barboni, B.; Piattelli, A.; et al. Biphasic Calcium Phosphate Biomaterials: Stem Cell-Derived Osteoinduction or In Vivo Osteoconduction? Novel Insights in Maxillary Sinus Augmentation by Advanced Imaging. Materials 2021, 14, 2159. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; de Oliveira, P.S.; Padmanabhan, S.K.; Licciulli, A. Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations. Materials 2019, 12, 3079. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Lang, N.P. Sinus floor elevation utilizing the transalveolar approach. Periodontology 2000 2014, 66, 59–71. [Google Scholar] [CrossRef]
- Valentini, P.; Artzi, Z. Sinus augmentation procedure via the lateral window technique—Reducing invasiveness and preventing complications: A narrative review. Periodontology 2000 2022. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Xu, L.-W.; Zhu, H.-Y.; Liu, S.S.-Y. Technical procedures for template-guided surgery for mandibular reconstruction based on digital design and manufacturing. Biomed. Eng. Online 2014, 13, 63. [Google Scholar] [CrossRef]
- Meyer, S.; Hirsch, J.-M.; Leiggener, C.S.; Msallem, B.; Sigron, G.R.; Kunz, C.; Thieringer, F.M. Fibula Graft Cutting Devices: Are 3D-Printed Cutting Guides More Precise Than a Universal, Reusable Osteotomy Jig? J. Clin. Med. 2020, 9, 4119. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Hirsch, J.-M.; Leiggener, C.S.; Zeilhofer, H.-F.; Thieringer, F.M. A simple, effective, universal, and reusable osteotomy tool for jaw reconstructions with microvascular fibula transplants. J. Plast. Reconstr. Aesthetic Surg. 2019, 73, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, D.; Li, K.; Li, H.; Liu, L. Precise locating and cutting of the bone lid with a digital template during the treatment of large mandibular cysts: A case series study. J. Cranio-Maxillofac. Surg. 2021, 49, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chen, F.; Feng, Y.; Xie, C.; Li, D. A reduced healing protocol for sinus floor elevation in a staged approach with deproteinized bovine bone mineral alone: A randomized controlled clinical trial of a 5-month healing in comparison to the 8-month healing. Clin. Implant. Dent. Relat. Res. 2020, 22, 281–291. [Google Scholar] [CrossRef]
- Van Eijden, T. Biomechanics of the Mandible. Crit. Rev. Oral Biol. Med. 2000, 11, 123–136. [Google Scholar] [CrossRef]
- Mangano, C.; Luongo, G.; Luongo, F.; Lerner, H.; Margiani, B.; Admakin, O.; Mangano, F. Custom-made computer-aided-design/ computer-assisted-manufacturing (CAD/CAM) synthetic bone grafts for alveolar ridge augmentation: A retrospective clinical study with 3 years of follow-up. J. Dent. 2022, 127, 104323. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, M.C.; Holtzhausen, S.; Nies, B.; Heinemann, S.; Muallah, D.; Kroschwald, L.; Paetzold-Byhain, K.; Lauer, G.; Sembdner, P. Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration. J. Pers. Med. 2023, 13, 464. https://doi.org/10.3390/jpm13030464
Schulz MC, Holtzhausen S, Nies B, Heinemann S, Muallah D, Kroschwald L, Paetzold-Byhain K, Lauer G, Sembdner P. Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration. Journal of Personalized Medicine. 2023; 13(3):464. https://doi.org/10.3390/jpm13030464
Chicago/Turabian StyleSchulz, Matthias C., Stefan Holtzhausen, Berthold Nies, Sascha Heinemann, David Muallah, Lysann Kroschwald, Kristin Paetzold-Byhain, Günter Lauer, and Philipp Sembdner. 2023. "Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration" Journal of Personalized Medicine 13, no. 3: 464. https://doi.org/10.3390/jpm13030464
APA StyleSchulz, M. C., Holtzhausen, S., Nies, B., Heinemann, S., Muallah, D., Kroschwald, L., Paetzold-Byhain, K., Lauer, G., & Sembdner, P. (2023). Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration. Journal of Personalized Medicine, 13(3), 464. https://doi.org/10.3390/jpm13030464





