Spironolactone as a Potential New Treatment to Prevent Arrhythmias in Arrhythmogenic Cardiomyopathy Cell Model
Abstract
1. Introduction
2. Methods
2.1. Clinical Patient Case
2.2. Cell Culture
2.3. Whole-Cell Current-Clamp Recordings
2.4. Calcium Handling in hiPSC-CMs
2.5. Quantitative Reverse Transcription PCR (RT-qPCR)
2.6. Contraction of hiPSC-CMs’
2.7. Data Analysis and Statistics
3. Results
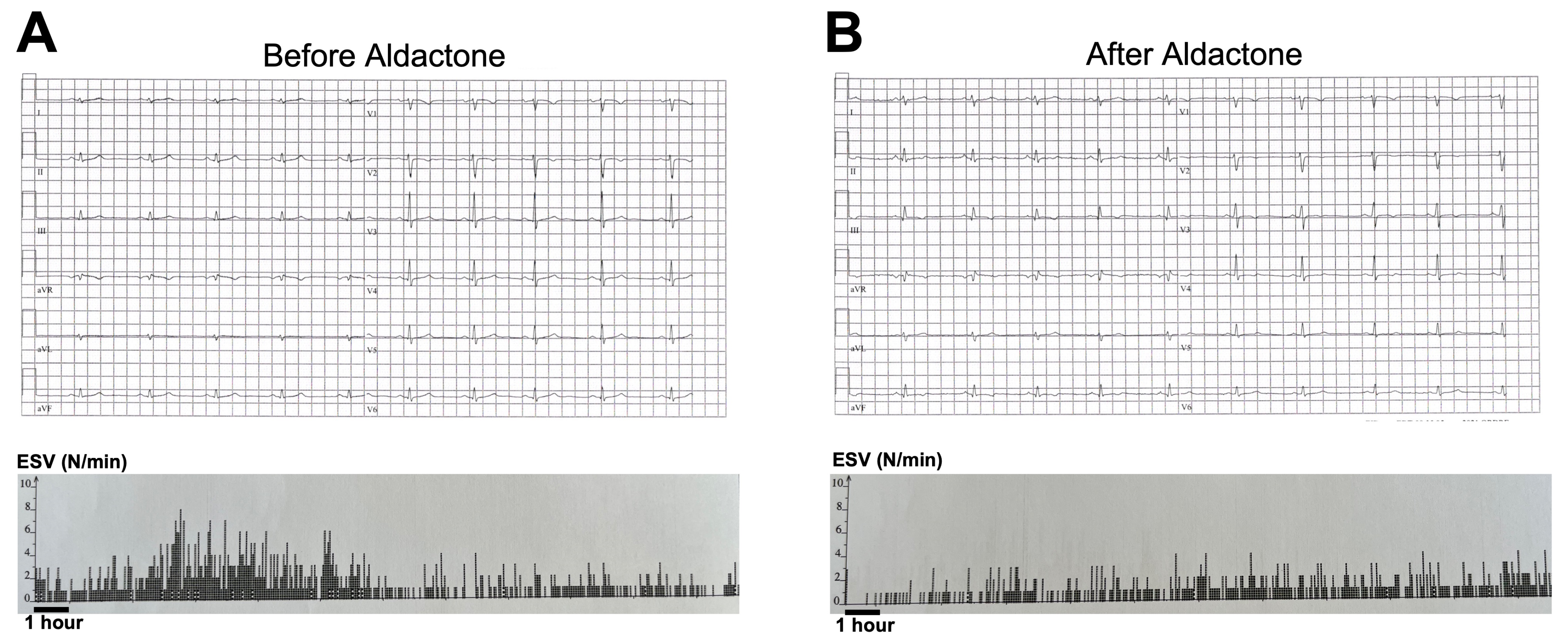
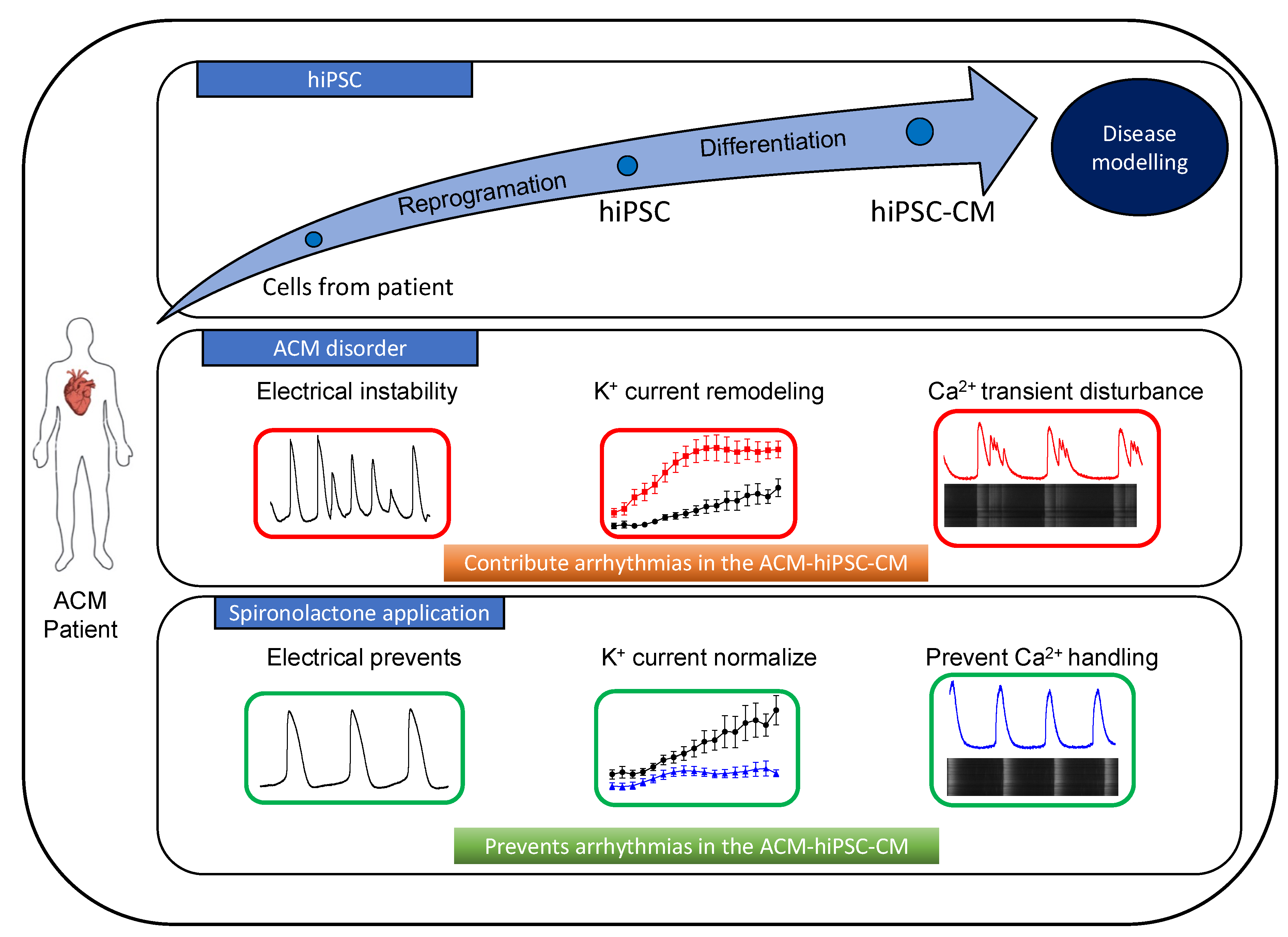
3.1. Aldactone Decreases Arrhythmic Events in an ACM Patient
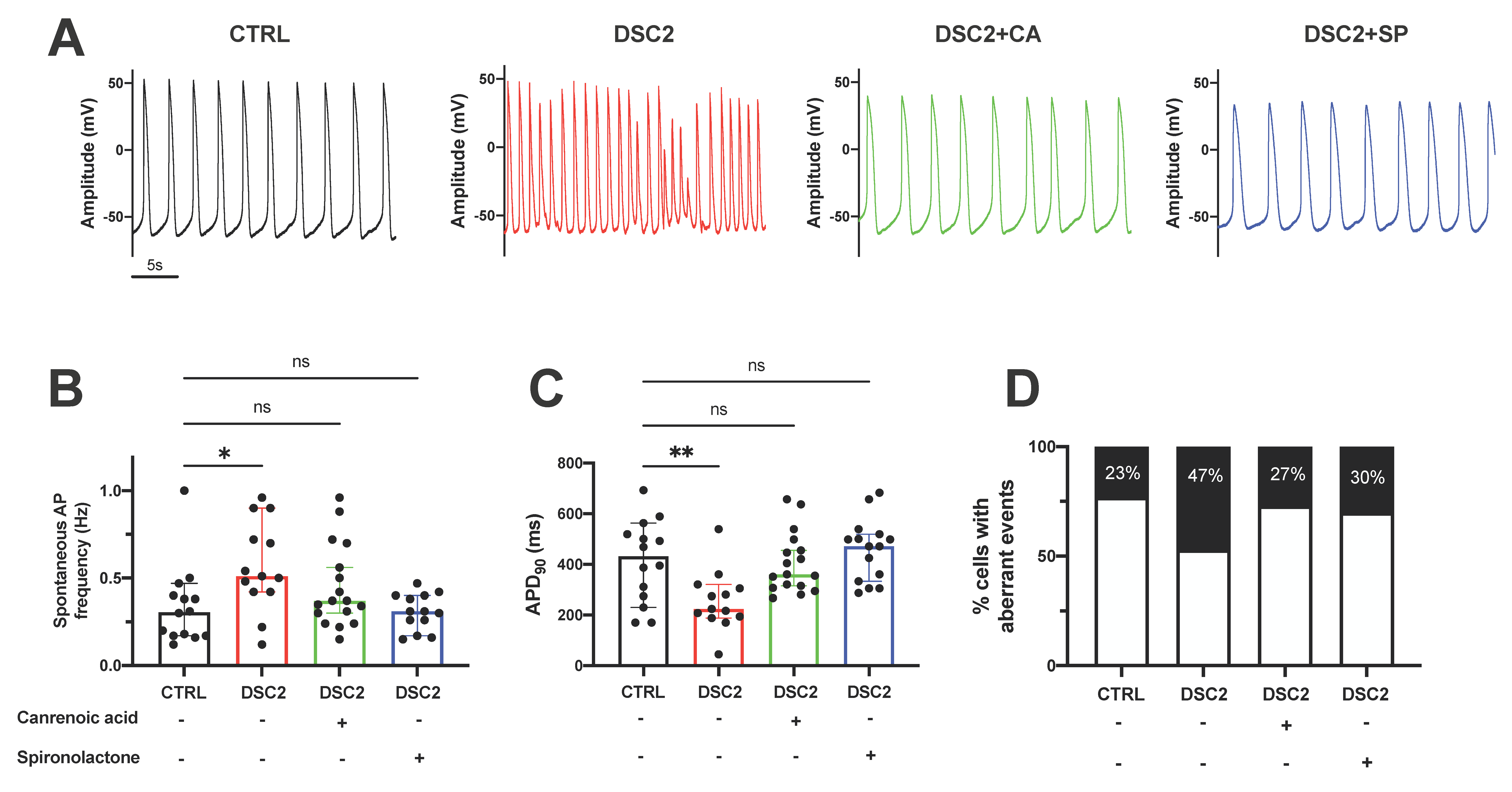
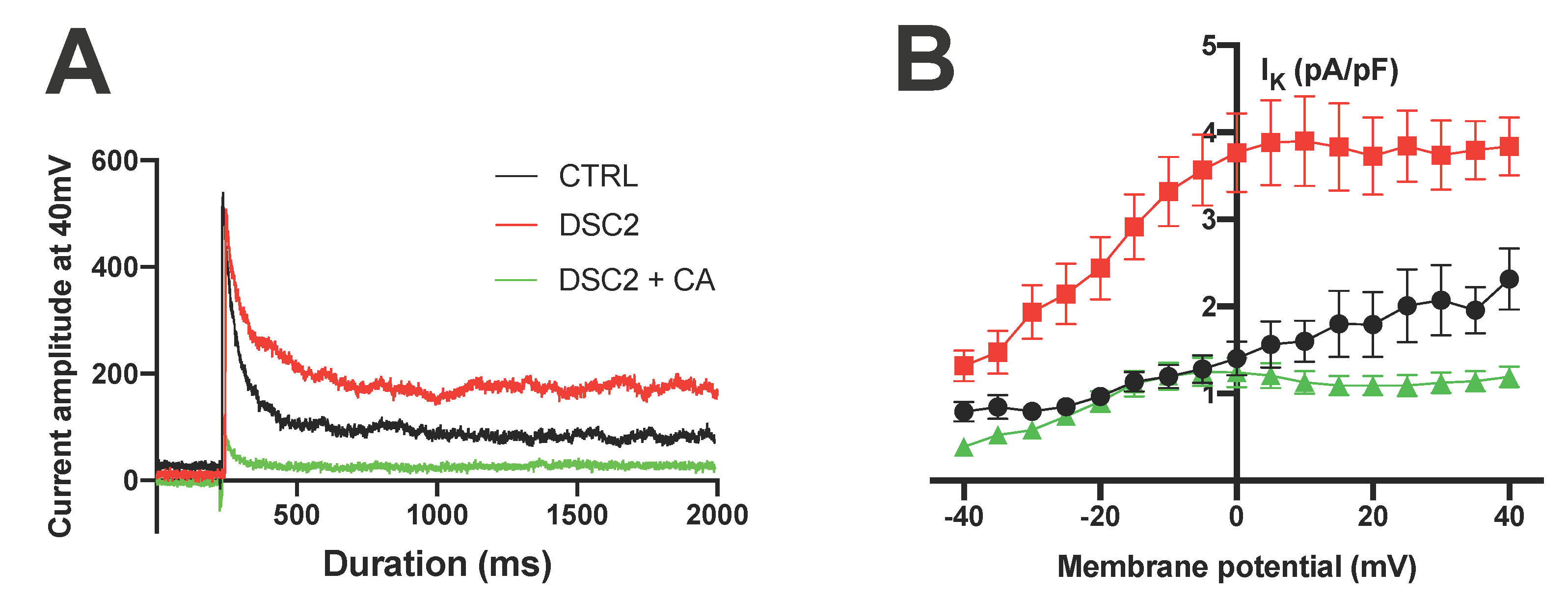
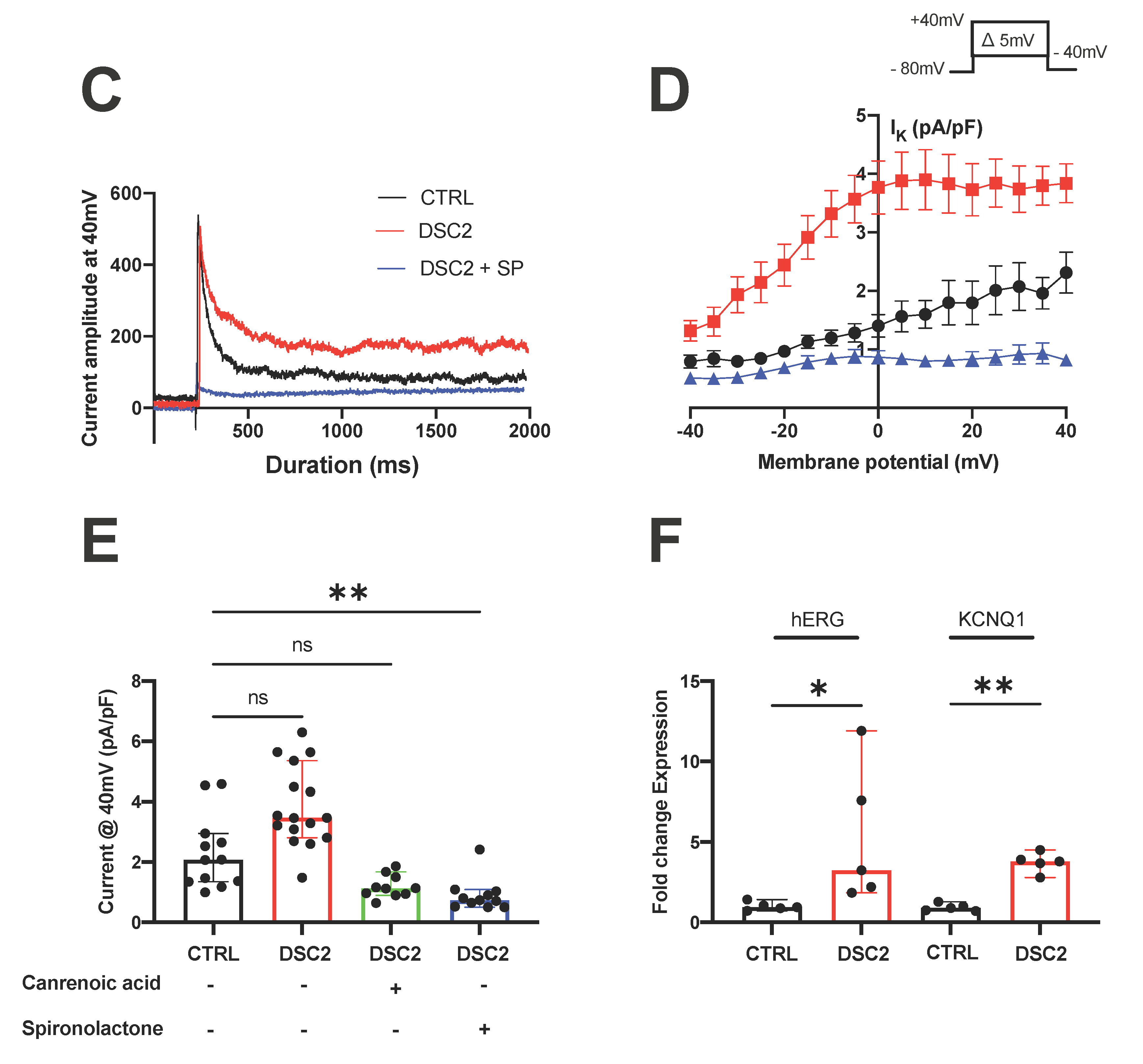
3.2. SP and CA Normalize the AP and Reduce Electrical Instability in ACM-DSC2-hiPSC-CMs
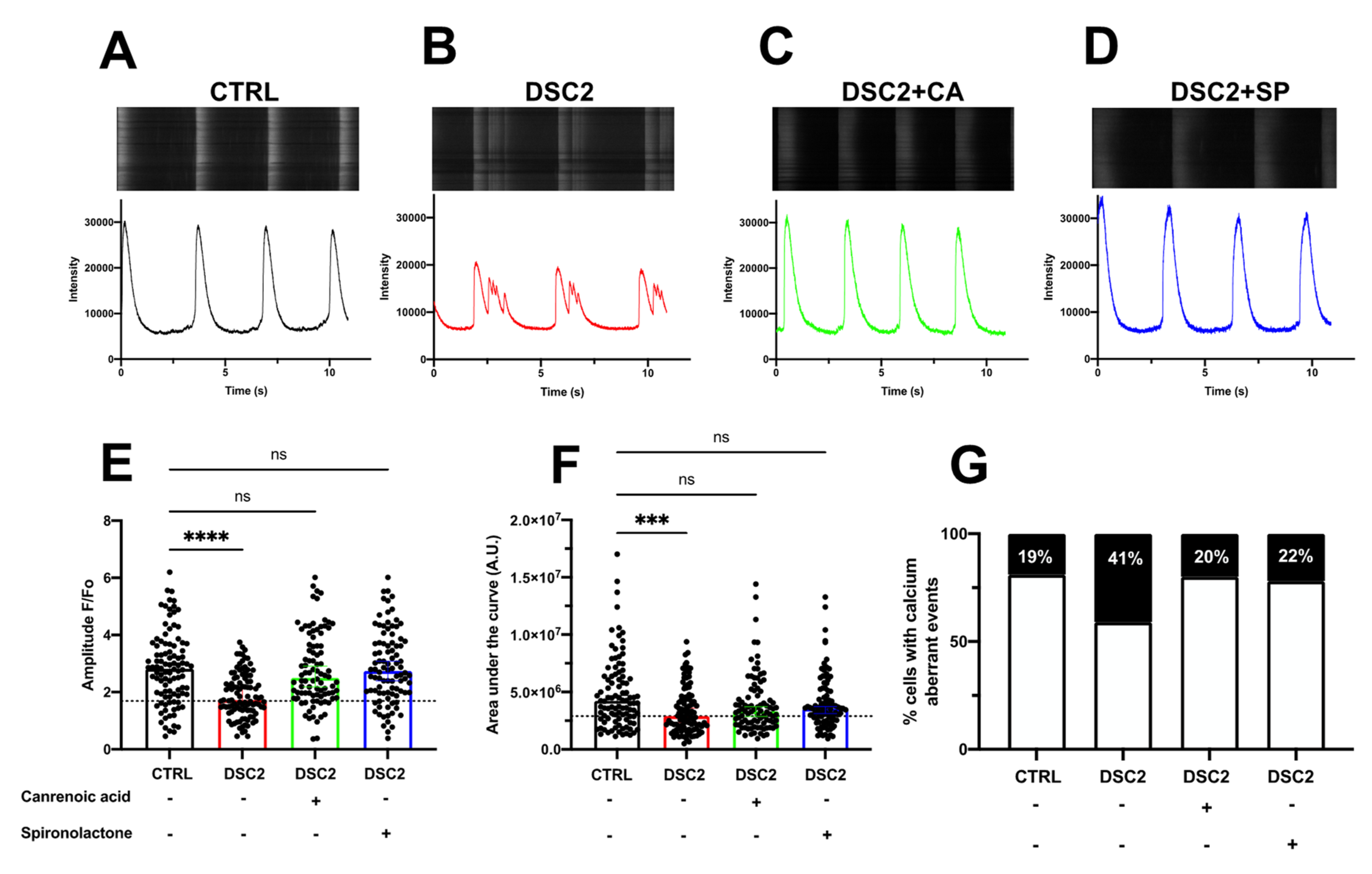
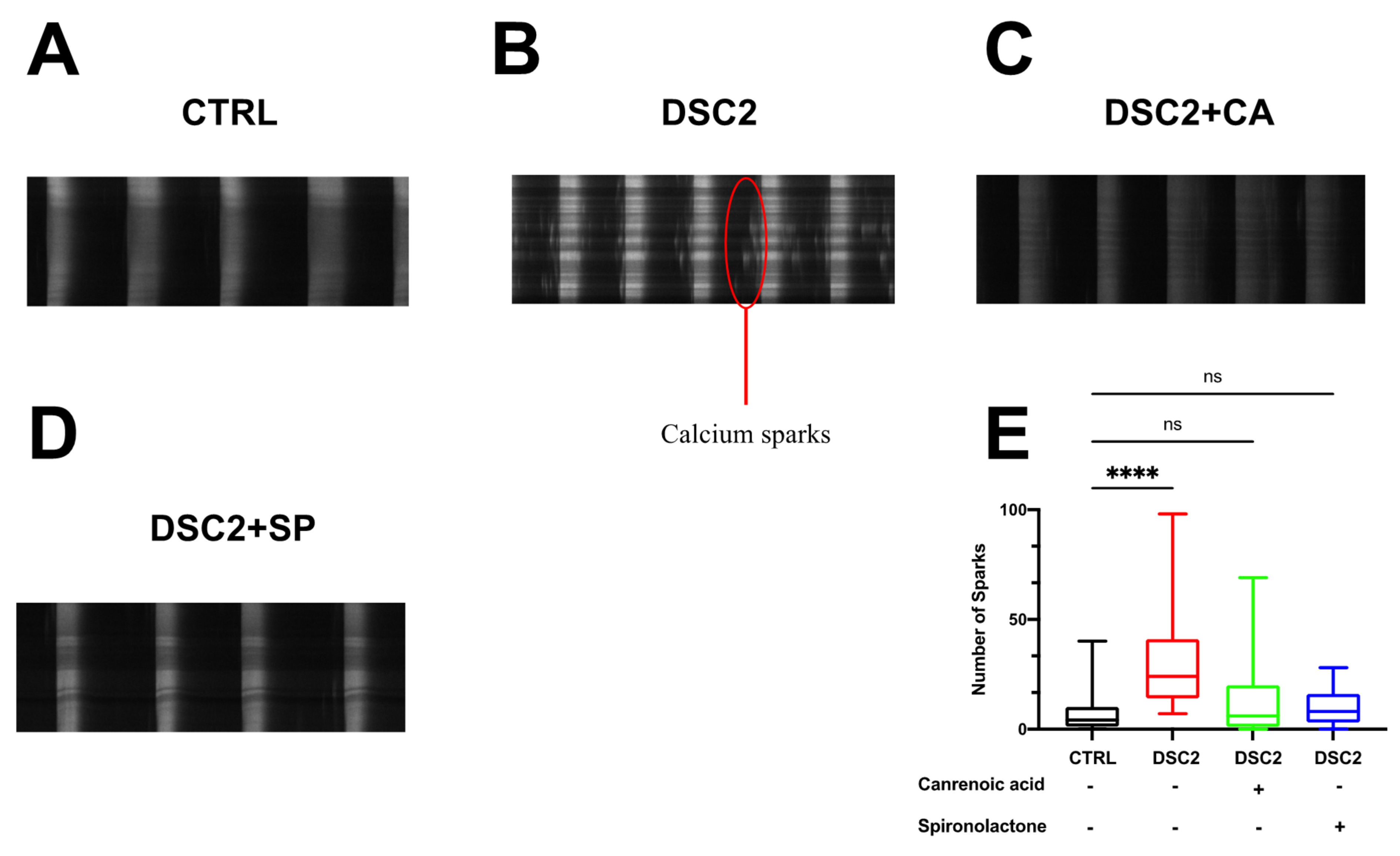
3.3. SP, CA, and Eplerenone Normalize Ca2+ Cycling in ACM-DSC2-hiPSC-CMs
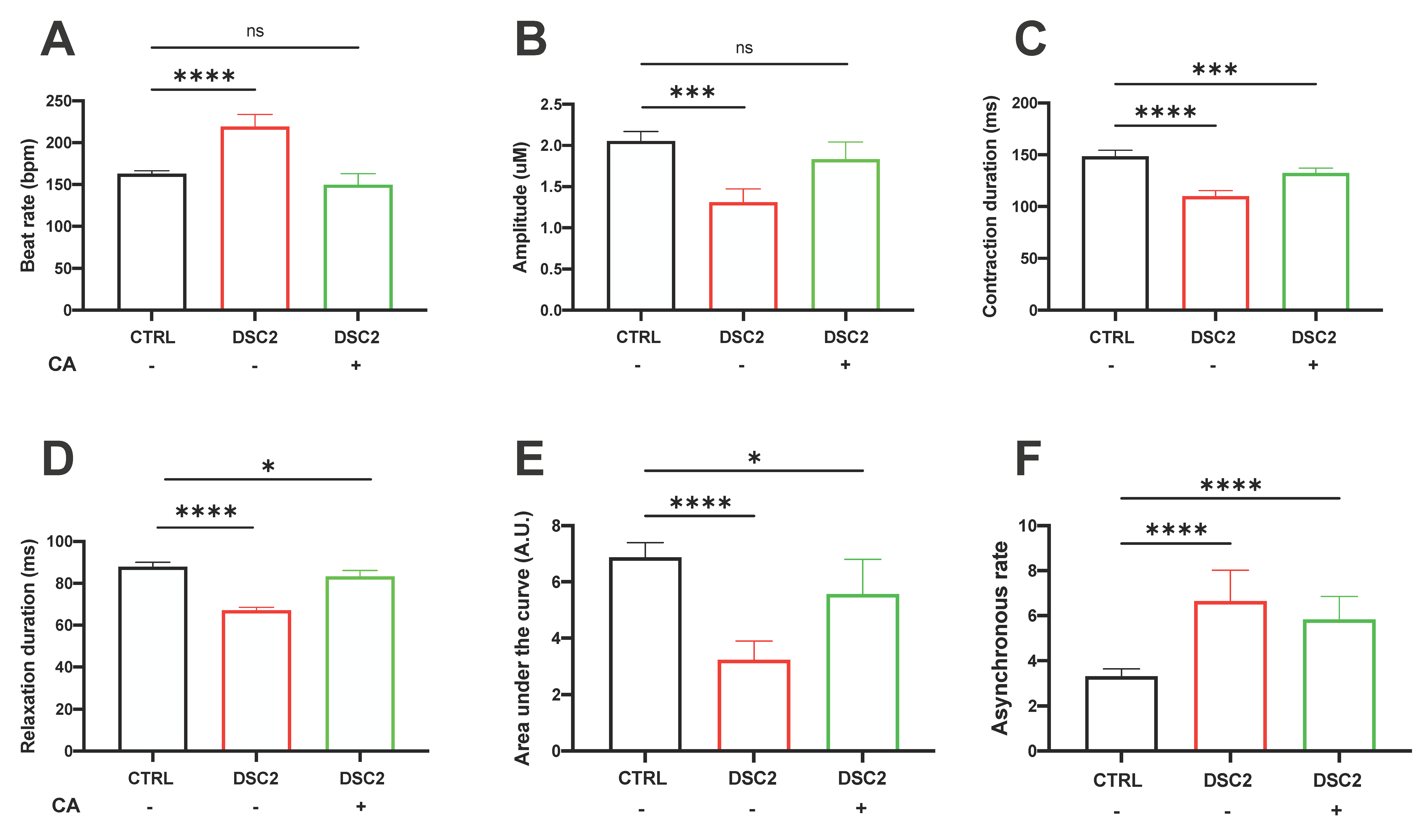
3.4. Effect of CA on Contractile Properties in ACM-DSC2-hiPSC-CMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Awad, M.M.; Calkins, H.; Judge, D.P. Mechanisms of disease: Molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Weiss, J.; Debus, J.D.; Stanasiuk, C.; Klauke, B.; Deutsch, M.A.; Fox, H.; Bax, J.; Ebbinghaus, H.; Gärtner, A.; et al. A homozygous DSC2 deletion associated with arrhythmogenic cardiomyopathy is caused by uniparental isodisomy. J. Mol. Cell Cardiol. 2020, 141, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Caspi, O.; Huber, I.; Gepstein, A.; Arbel, G.; Maizels, L.; Boulos, M.; Gepstein, L. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ. Cardiovasc. Genet. 2013, 6, 557–568. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Zhao, Z.; Lan, H.; Cyganek, L.; Tombers, C.; Li, X.; Buljubasic, F.; Lang, S.; Tiburcy, M.; Zimmermann, W.-H.; et al. Electrical dysfunctions in human-induced pluripotent stem cell-derived cardiomyocytes from a patient with an arrhythmogenic right ventricular cardiomyopathy. EP Eur. 2018, 20, f46–f56. [Google Scholar] [CrossRef]
- Akdis, D.; Medeiros-Domingo, A.; Gaertner-Rommel, A.; Kast, J.I.; Enseleit, F.; Bode, P.; Klingel, K.; Kandolf, R.; Renois, F.; Andreoletti, L.; et al. Myocardial expression profiles of candidate molecules in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia compared to those with dilated cardiomyopathy and healthy controls. Heart Rhythm. 2016, 13, 731–741. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Thiene, G.; McKenna, W.J.; Davies, M.J.; Fontaliran, F.; Nava, A.; Silvestri, F.; Blomstrom-Lundqvist, C.; Wlodarska, E.K.; et al. Spectrum of Clinicopathologic Manifestations of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: A Multicenter Study. J. Am. Coll Cardiol. 1997, 30, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Corrado, D.; Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet Lond Engl. 2009, 373, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.M.; Glidden, D.V.; Polonsky, B.; Zareba, W.; Smith, L.M.; Cannom, D.S.; Estes, N.A.M.; Marcus, F.; Scheinman, M.M.; Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: A report from the North American ARVC Registry. J. Am. Coll Cardiol. 2009, 54, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Belke, D.D.; Garnett, L.; Martens, K.; Abdelfatah, N.; Rodriguez, M.; Diao, C.; Chen, Y.-X.; Gordon, P.M.K.; Nygren, A.; et al. Transgenic mice overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS ONE 2017, 12, e0174019. [Google Scholar] [CrossRef]
- Moreau, A.; Reisqs, J.-B.; Delanoe-Ayari, H.; Pierre, M.; Janin, A.; Deliniere, A.; Bessière, F.; Meli, A.C.; Charrabi, A.; Lafont, E.; et al. Deciphering DSC2 arrhythmogenic cardiomyopathy electrical instability: From ion channels to ECG and tailored drug therapy. Clin. Transl. Med. 2021, 11, e319. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Ackerman, M.J. Potassium-channel mutations and cardiac arrhythmias—Diagnosis and therapy. Nat. Rev. Cardiol. 2012, 9, 319–332. [Google Scholar] [CrossRef]
- Henein, M.Y.; O’Sullivan, C.A.; Coats, A.J.S.; Gibson, D.G. Angiotensin-converting enzyme (ACE) inhibitors revert abnormal right ventricular filling in patients with restrictive left ventricular disease. J. Am. Coll. Cardiol. 1998, 32, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, J.L.; Kapuku, G.; Pelletier, S.; Gosselin, H.; Adam, A.; Gagnon, C.; Lambert, C.; Meloche, S. Cardioprotective Effects of Ramipril and Losartan in Right Ventricular Pressure Overload in the Rabbit: Importance of Kinins and Influence on Angiotensin II Type 1 Receptor Signaling Pathway. Circulation 2001, 104, 939–944. [Google Scholar] [CrossRef]
- Fletcher, R.D.; Cintron, G.B.; Johnson, G.; Orndorff, J.; Carson, P.; Cohn, J.N. Enalapril decreases prevalence of ventricular tachycardia in patients with chronic congestive heart failure. The V-HeFT II VA Cooperative Studies Group. Circulation 1993, 87, VI49–VI55. [Google Scholar] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Shafiq, M.M.; Miller, A.B. Blocking aldosterone in heart failure. Ther. Adv. Cardiovasc. Dis. 2009, 3, 379–385. [Google Scholar] [CrossRef]
- Morel, E.; Manati, A.W.; Nony, P.; Maucort-Boulch, D.; Bessière, F.; Cai, X.; Besseyre des Horts, T.; Janin, A.; Moreau, A.; Chevalier, P. Blockade of the renin-angiotensin-aldosterone system in patients with arrhythmogenic right ventricular dysplasia: A double-blind, multicenter, prospective, randomized, genotype-driven study (BRAVE study). Clin. Cardiol. 2018, 41, 300–306. [Google Scholar] [CrossRef]
- Caballero, R.; Moreno, I.; González, T.; Arias, C.; Valenzuela, C.; Delpón, E.; Tamargo, J. Spironolactone and its main metabolite, canrenoic acid, block human ether-a-go-go-related gene channels. Circulation 2003, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Reisqs, J.-B.; Moreau, A.; Charrabi, A.; Sleiman, Y.; Meli, A.C.; Millat, G.; Briand, V.; Beauverger, P.; Richard, S.; Chevalier, P. The PPARγ pathway determines electrophysiological remodelling and arrhythmia risks in DSC2 arrhythmogenic cardiomyopathy. Clin. Transl. Med. 2022, 12, e748. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, M.; Noorman, M.; Lin, X.; Chkourko, H.; Liang, F.-X.; van der Nagel, R.; Hund, T.; Birchmeier, W.; Mohler, P.; van Veen, T.A.; et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc. Res. 2012, 95, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, P.; Moreau, A.; Richard, S.; Janin, A.; Millat, G.; Bessière, F.; Delinière, A. Short QT interval as a harbinger of an arrhythmogenic cardiomyopathy. HeartRhythm Case Rep. 2021, 7, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T. Aldosterone and spironolactone in heart failure. N. Engl. J. Med. 1999, 341, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Kléber, A.G.; MacRae, C.A.; Saffitz, J.E. Arrhythmogenic Cardiomyopathy—New Insights into Disease Mechanisms and Drug Discovery. Prog Pediatr. Cardiol. 2014, 37, 3–7. [Google Scholar] [CrossRef]
- Delpón, E.; Caballero, R.; Gómez, R.; Núñez, L.; Tamargo, J.; Angiotensin, I.I. angiotensin II antagonists and spironolactone and their modulation of cardiac repolarization. Trends Pharmacol. Sci. 2005, 26, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K(+) channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Núñez, L.; Caballero, R.; Vaquero, M.; Tamargo, J.; Delpón, E. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1+minK channels: Spironolactone and canrenoic acid on K + channels. Br. J. Pharmacol. 2005, 146, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004, 62, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kiyokawa, J.; Tabo, M.; Inoue, T. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. J. Pharmacol. Sci. 2011, 117, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, E.; Mubagwa, K. Antiarrhythmic drugs and cardiac ion channels: Mechanisms of action. Prog Biophys. Mol. Biol. 1998, 70, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Terrenoire, C.; Reiken, S.; Wehrens, X.H.T.; Song, L.-S.; Tillman, E.J.; Mancarella, S.; Coromilas, J.; Lederer, W.J.; Kass, R.S.; et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc. Natl. Acad. Sci. USA 2006, 103, 7906–7910. [Google Scholar] [CrossRef]
- Laitinen, P.J.; Brown, K.M.; Piippo, K.; Swan, H.; Devaney, J.M.; Brahmbhatt, B.; Donarum, E.A.; Marino, M.; Tiso, N.; Viitasalo, M.; et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001, 103, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Roux-Buisson, N.; Gandjbakhch, E.; Donal, E.; Probst, V.; Deharo, J.-C.; Chevalier, P.; Klug, D.; Mansencal, N.; Delacretaz, E.; Cosnay, P.; et al. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Results of a systematic screening. Heart Rhythm. 2014, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Ter Keurs, H.E.D.J.; Boyden, P.A. Calcium and arrhythmogenesis. Physiol. Rev. 2007, 87, 457–506. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Beygui, F.; Puddu, P.-E.; Manrique, A.; Rouet, R.; Milliez, P. Electrophysiological and Antiarrhythmic Properties of Potassium Canrenoate During Myocardial Ischemia—Reperfusion. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Torres, L.; Medei, E.; Ricardo, R.; França, J.; Smaili, S.; Nascimento, J.; Oshiro, M.; Bassani, J.; Ferreira, A.; et al. The negative inotropic action of canrenone is mediated by L-type calcium current blockade and reduced intracellular calcium transients: Negative inotropic action of canrenone. Br. J. Pharmacol. 2009, 158, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Enciso, R.; Guerrero-Hernández, A.; Gómez, A.M.; Benitah, J.-P.; Rueda, A. Aldosterone-Induced Sarco/Endoplasmic Reticulum Ca2+ Pump Upregulation Counterbalances Cav1.2-Mediated Ca2+ Influx in Mesenteric Arteries. Front. Physiol. 2022, 13, 834220. [Google Scholar] [CrossRef] [PubMed]
- He, B.J.; Joiner, M.-L.A.; Singh, M.V.; Luczak, E.D.; Swaminathan, P.D.; Koval, O.M.; Kutschke, W.; Allamargot, C.; Yang, J.; Guan, X.; et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat. Med. 2011, 17, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Curran, J.W.; Shannon, T.R.; Bers, D.M.; Pogwizd, S.M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005, 97, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Uchinoumi, H.; Yang, Y.; Oda, T.; Li, N.; Alsina, K.M.; Puglisi, J.L.; Chen-Izu, Y.; Cornea, R.L.; Wehrens, X.H.T.; Bers, D.M. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J. Mol. Cell. Cardiol. 2016, 98, 62–72. [Google Scholar] [CrossRef]
- Matsumura, K.; Fujii, K.; Oniki, H.; Oka, M.; Iida, M. Role of aldosterone in left ventricular hypertrophy in hypertension. Am. J. Hypertens. 2006, 19, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wei, H.; Lu, J.; Ho, S.; Zhang, G.; Sun, X.; Oh, Y.; Tan, S.H.; Ng, M.L.; Shim, W.; et al. Generation of patient-specific induced pluripotent stem cell-derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2013, 34, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Accession Numbers | Gene Description | Primer Sequence (5’-3’) | Size (pb) |
|---|---|---|---|---|
| RPLP0 | NM_001002.4 | Ribosomal Protein Lateral Stalk Subunit P0 | Forward: AGCAAGTGGGAAGGT Revers: TCATCCAGCAGGTGT | 114 |
| KCNQ1 | NM_000218.3 | Kv7.1 (IKS) | Forward: CTCACTCATTCAGACCGCA Reverse: TCTTTACCACAGACTTCTTG | 143 |
| KCNH2 | NM_000238.4 | Kv11.1 (IKr) | Forward: GGAGCCTCTGAACCTGTATG Reverse: GACTGAAGCCACCCTCTAAC | 230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisqs, J.-B.; Moreau, A.; Sleiman, Y.; Charrabi, A.; Delinière, A.; Bessière, F.; Gardey, K.; Richard, S.; Chevalier, P. Spironolactone as a Potential New Treatment to Prevent Arrhythmias in Arrhythmogenic Cardiomyopathy Cell Model. J. Pers. Med. 2023, 13, 335. https://doi.org/10.3390/jpm13020335
Reisqs J-B, Moreau A, Sleiman Y, Charrabi A, Delinière A, Bessière F, Gardey K, Richard S, Chevalier P. Spironolactone as a Potential New Treatment to Prevent Arrhythmias in Arrhythmogenic Cardiomyopathy Cell Model. Journal of Personalized Medicine. 2023; 13(2):335. https://doi.org/10.3390/jpm13020335
Chicago/Turabian StyleReisqs, Jean-Baptiste, Adrien Moreau, Yvonne Sleiman, Azzouz Charrabi, Antoine Delinière, Francis Bessière, Kevin Gardey, Sylvain Richard, and Philippe Chevalier. 2023. "Spironolactone as a Potential New Treatment to Prevent Arrhythmias in Arrhythmogenic Cardiomyopathy Cell Model" Journal of Personalized Medicine 13, no. 2: 335. https://doi.org/10.3390/jpm13020335
APA StyleReisqs, J.-B., Moreau, A., Sleiman, Y., Charrabi, A., Delinière, A., Bessière, F., Gardey, K., Richard, S., & Chevalier, P. (2023). Spironolactone as a Potential New Treatment to Prevent Arrhythmias in Arrhythmogenic Cardiomyopathy Cell Model. Journal of Personalized Medicine, 13(2), 335. https://doi.org/10.3390/jpm13020335







